Abstract
There is compelling evidence that a number of neurodegenerative diseases share common pathogenic mechanisms. Better understanding these mechanisms will allow us to develop new therapeutic strategies. This commentary follows up on our recent findings that tau pathology can be found in healthy fetal tissue transplanted into the brain of patients with either Huntington or Parkinson disease. We will examine how tau appears to be shared in a number of different conditions and how its expression relates to cognitive decline and disease progression. We will further review pathogenic mechanisms and especially the relevance of the possible prion-like behavior of tau. We will conclude by discussing how all this work opens up novel therapeutic approaches to treating the cognitive impairments related to neurodegenerative diseases using a common strategy.
Keywords: Tau, neurodegenerative disease, prion, Huntington disease, antibody-based therapy
Comment on: Cisbani G, Maxan A, Kordower JH, Planel E, Freeman TB, Cicchetti F. Presence of tau pathology within foetal neural allografts in patients with Huntington’s and Parkinson’s disease. Brain. 2017 Nov 1;140(11):2982-2992. doi: 10.1093/brain/awx255. PubMed PMID: 29069396.
Premise
Through the postmortem examination of human patients with Huntington (HD) and Parkinson disease (PD) who have been in receipt of fetal allografts, we have identified various pathological forms of the tau protein that appear to be present not only in the host brain but also in the grafted tissue.1 In total, we have examined 4 cases and found hyperphosphorylated tau in transplants in both patients with HD who had come to autopsy 9 and 12 years posttransplantation and the patient with PD who had come to autopsy 16 years posttransplantation. The patient with PD who passed away 18 months posttransplantation did not show tau hyperphosphorylation within grafts,1 implying that time is a critical factor in the manifestation of this pathology in the healthy grafted tissue. As mounting evidence accumulates that abnormal tau is common to several neurodegenerative diseases, our observations bring into question whether hyperphosphorylated tau is not only a significant feature of these diseases but also has the ability to be passed from diseased to healthy cells. If true, this raises important questions regarding whether this could be targeted for novel therapeutic intervention across a large number of neurodegenerative disorders.
Tau Is Associated to Cognitive Impairments in Multiple Neurodegenerative Contexts
While normal tau phosphorylation is essential to neuronal homeostasis, hyperphosphorylated tau accumulates as neurofibrillary tangles and/or neuropil threads2 that interfere with fundamental cell functions. Hyperphosphorylated tau has been classically associated with Alzheimer disease (AD); however, a number of human brain disorders have now been recognized as depicting primary tau pathology. These include primary age-related tauopathy, chronic traumatic encephalopathy, progressive supranuclear palsy, corticobasal degeneration, frontotemporal dementia and parkinsonism linked to chromosome 17, meningioangiomatosis, subacute sclerosing panencephalitis, tangle-only dementia, argyrophilic grain disease, Pick’s disease, and PD. In many of these pathologies, the amount of hyperphosphorylated tau tangles correlates with cognitive deficits. This has been revealed, in part, by assessment of tau levels in both the blood and the cerebrospinal fluid (CSF). In fact, phosphorylated tau in the CSF has been shown to be one of the strongest predictive factors to determine who will progress to mild cognitive impairment from normal aging across a 5-year span.3 Supporting these findings are recent studies demonstrating that increased age correlated positively with phosphorylated tau levels in CSF and negatively with global cognitive performance.4 Tau’s association with cognitive decline, however, persists beyond age-related dementias with common neurodegenerative disorders, such as PD5 and amyotrophic lateral sclerosis (ALS),6 also demonstrating a relationship between phosphorylated tau and cognitive deficits and/or decline.
Monogenic disorders of the central nervous system, such as HD, have also been reported to have a number of pathological features of tauopathies. A series of recent papers have brought forward compelling evidence that patients with HD have aggregated tau inclusions across a number of brain sites7,8 and that more rapid cognitive decline can be seen in patients with HD with the H2 haplotype of the tau gene (MAPT) compared with those of the H1 haplotype.9 This is of particular interest because the H1 MAPT haplotype has been shown to be a risk haplotype in other neurodegenerative disorders, such as PD and the dementia associated with it. In support of these pathological and genetic findings is data from imaging in which positron emission tomography (PET) tau deposition has been demonstrated to be more closely associated with cognitive function than amyloid β (Aβ) PET imaging in patients with AD.10 Furthermore, both PET and CSF measures of tau, but not amyloid pathology, have been associated with worsening cognition in AD.11 The almost ubiquitous presence of tau pathology in such a diverse range of brain disorders suggests that tau is either an early marker of dysfunction or a central component to the pathological process. In either case, tau is a common feature of many neurodegenerative conditions and as such, its role needs to be better understood given the obvious therapeutic implications that such an understanding would bring.
The Spreading and Seeding Capacities of Tau
Our recent findings on the presence of various forms of hyperphosphorylated tau in genetically unrelated and healthy allografted tissue transplanted to brains of patients with either HD or PD1 are the most recent in a series of studies on cell transplantation that indicates the potential ability of pathological proteins to spread from diseased to healthy tissue in neurodegenerative conditions.12 Disease pathology paired with evidence of protein aggregation in grafted cells has been well-characterized in patients with PD, where Lewy bodies have been described in ventral mesencephalic tissue allografts,13–15 providing some of the first evidence that pathological proteins may spread between diseased and healthy cellular elements. Work from our group has also reported on a number of HD cases where the mutant huntingtin protein (mHtt), the genetic product of HD, was found within genetically unrelated grafted tissue.12 Cell transplantation, which was envisioned in part as a means to rebuild circuits in the diseased brain, served to demonstrate that pathogenic proteins could possibly transynaptically propagate into the nondiseased tissue. Of significance, recent work assessing tau burden in patients with AD using the ligand AV-1451 for PET imaging revealed that highly connected neuronal networks depicted greater tau pathology. The distribution of tau appeared to differ between disease conditions as similar conclusions were not reached in subjects having supranuclear palsy—another disease characterized by tau pathology—undergoing identical imaging protocols.16 The properties of certain proteins to propagate between cells and thereby contribute to pathogenesis may be specific to certain disease contexts. However, one needs to be careful in drawing conclusions from such human studies given that the tau signal measured may be equally attributable to the fact that tightly connected networks have higher metabolic demands and this may in turn stress cells, precipitating tau pathology. Preclinical work is needed to discard such possibilities.
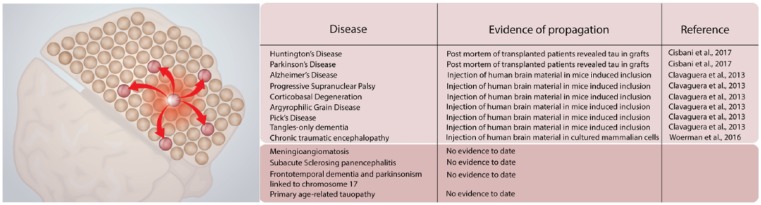
Beyond its ability to be transferred between cells, tau seems to behave in a prion-like manner, as observed both in vitro and in animal studies (Figure 1). For example, the use of sarkosyl-insoluble tau from the brains of mice transgenic for human P301S tau and the induction of aggregation of soluble human P301S tau in HEK293T cells have been observed.17 Further characterization allowed to conclude that initiation of aggregation was dependent on the form of tau that was taken up, as monomeric tau was internalized but failed to seed.17 Clavaguera et al18 investigated the prionic properties of tau using brain homogenates derived from human patients with nonfamilial AD, tangle-only dementia, argyrophilic grain disease, progressive supranuclear palsy, corticobasal degeneration, and Pick’s disease. Argyrophilic tau inclusions formed with all of these different types of homogenates when injected in both transgenic and nontransgenic mice. These findings suggest that once the aggregates have formed, they become self-propagating and spread in a prion-like manner.18 Tau aggregates derived from patients with chronic traumatic encephalopathy were able to replicate in HEK293T cells expressing both 3R and 4R isoforms of tau, where 4R isoforms facilitated the propagation of the protein19 (Figure 1). Taken together, the prion-like properties of tau seem secure.
Figure 1.
Spreading and seeding properties of tau. Left panel: schematic highlighting the production of one cell expressing hyperphosphorylated tau and the ability for that protein to spread to nearby cells and throughout the brain. Right panel: summary table of key experiments that have reported the ability of tau to spread and seed pathology in a number of disease contexts.
There are numerous mechanisms, in addition to transynaptic transport, that may account for protein spread in neurodegenerative diseases and which could be common to tau, Aβ, mHtt, and/or α-synuclein. Tunneling nanotubes are small entities that serve as a communication bridge between cells.20 Various cell types, including neurons, have the ability to produce these retractable protrusions, allowing direct transfer of biological material, such as tau, between adjacent cells.21 Nonsynaptic endocytosis is a mechanism that controls various cellular functions such as internalization and recycling of plasma membrane components/ligands as well as the uptake and degradation of macromolecules and extracellular particles. Pathological proteins can hijack this pathway to access healthy neurons.22 Encapsulated and free forms of pathological proteins are particularly prone to spreading within peripheral organs and into the central nervous system, which raises important clinical issues relating to human-to-human transfer of disease. Remarkably, Duffy et al23 published a case report of a corneal graft from a patient with Creutzfeldt-Jakob disease causing the appearance of the disease in the recipient and subsequently this has been shown for a variety of tissues including dural grafts.24 More recently, younger patients (36-51 years old) who developed iatrogenic Creutzfeldt-Jakob disease following the administration of cadaveric growth hormone 30 years previously, and without predisposition to juvenile AD, have been shown to have, at postmortem, extensive Aβ pathology in the parenchyma along with cortical and leptomeningeal cerebral Aβ angiopathy, something that is rarely seen at that age.25 Familial amyloid polyneuropathy, an autosomal dominant progressive peripheral sensorimotor neuropathy, can also be transmitted to healthy patients following liver transplant which produces mutated transthyretin amyloid fibrils26—also seen in ALS.27
Another key clinical question in HD, and similar diseases, is to better understand how the extent and type of pathology predict clinical phenotype and course. Cognitive deficits associated with HD are the greatest predictor of quality of life in patients. While clinical variability in dementia development may have genetic causes (eg, tau haplotype in HD), another possibility relates to the propagation of pathological proteins, as recently shown for synucleinopathies.28–30 For example, characterizing the number and types of extracellular vesicles according to disease progression and cognitive profiles could provide a way by which to better stratify patients with HD. This will, in turn, open up new therapeutic approaches with the advantage that we also have the means by which to look at target engagement using this therapeutic intervention. Overall, studies highlighting the exact mechanisms of release and reuptake will prove to be valuable in understanding how therapies targeting protein spread and load could be developed.
Recent work has shown that antibodies directed against tau can block seeding in vitro and markedly decrease pathology with improved cognition in vivo.31 Prolonged antibody treatment has indeed been shown to reduce hyperphosphorylated, aggregated, and insoluble tau in mice. Blocked tau seeding activity detected in brain lysates using a biosensor assay further reduced microglial activation and improves cognitive deficits.31 There are a plethora of tau targets for which specific antibodies have been designed: hyperphosphorylated tau, total tau, specific tau conformations, multimeric tau, or tau fragments, each providing advantages and disadvantages. Targeting hyperphosphorylated tau may represent a desirable approach given that various phosphorylated tau epitopes are selectively and/or specifically associated with disease pathology. The field of AD—the disease in which tau tangles were first identified—is much further along in understanding the role of tau in cognition. Consequently, numerous clinical trials of tau immunotherapy have already been initiated.32
Given the established correlation between cognitive decline and reduced quality of life in patients with HD, a treatment targeting cognition would be of great clinical relevance—clinical relevance that is underscored by the current lack of symptomatic management of this devastating aspect of the disease. Pairing this type of approach to other treatment options that are being investigated, such as gene therapy to prevent the translation of DNA into protein, should be envisioned. For example, it is very likely that just targeting mHtt with antisense oligonucleotides, as currently being tested in HD, will not be sufficient to address and treat all aspects of the disease. Tau also needs to be a focus.
The presence of tau in diverse neurological disorders, spanning from acute to chronic conditions implies a common pathological signature associated with neuronal dysfunction. Such shared genetic and pathological feature may open up important new therapeutic targets not only for AD and tauopathies but also for a whole range of neurodegenerative conditions.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: F.C. is a recipient of a Researcher Chair from the Fonds de Recherche du Québec en santé (FRQS) providing salary support and operating funds, and receives fudning from the Canadian Institutes of Health Research (CIHR) to conduct her HD-related research. A.M. is supported by an FRQS doctoral research award as well as an O’Brien Foundation Fellowship.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AM and FC wrote this commentary together.
References
- 1. Cisbani G, Maxan A, Kordower JH, Planel E, Freeman TB, Cicchetti F. Presence of tau pathology within foetal neural allografts in patients with Huntington’s and Parkinson’s disease. Brain. 2017;140:2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mudher A, Brion JP, Avila J, Medina M, Buee L. EuroTau: towing scientists to tau without tautology. Acta Neuropathol Comm. 2017;5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert M, Zhu Y, Moghekar A, et al. Predicting progression from normal cognition to mild cognitive impairment for individuals at 5 years. Brain. 2018;141:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haapalinna F, Kokki M, Jaaskelainen O, et al. Subtle cognitive impairment and Alzheimer’s disease-type pathological changes in cerebrospinal fluid are common among neurologically healthy subjects. J Alzheimers Dis. 2018;62:165–174. [DOI] [PubMed] [Google Scholar]
- 5. Liu C, Cholerton B, Shi M, et al. CSF tau and tau/Aβ42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rusina R, Ridzon P, Kulist’ak P, et al. Relationship between ALS and the degree of cognitive impairment, markers of neurodegeneration and predictors for poor outcome. A prospective study. Eur J Neurol. 2010;17:23–30. [DOI] [PubMed] [Google Scholar]
- 7. Gratuze M, Cisbani G, Cicchetti F, Planel E. Is Huntington’s disease a tauopathy? Brain. 2016;139:1014–1025. [DOI] [PubMed] [Google Scholar]
- 8. Gratuze M, Noel A, Julien C, et al. Tau hyperphosphorylation and deregulation of calcineurin in mouse models of Huntington’s disease. Hum Mol Genet. 2015;24:86–99. [DOI] [PubMed] [Google Scholar]
- 9. Vuono R, Winder-Rhodes S, de Silva R, et al. The role of tau in the pathological process and clinical expression of Huntington’s disease. Brain. 2015;138:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:338–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koychev I, Gunn RN, Firouzian A, et al. PET tau and amyloid-β burden in mild Alzheimer’s disease: divergent relationship with age, cognition, and cerebrospinal fluid biomarkers. J Alzheimers Dis. 2017;60:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cicchetti F, Lacroix S, Cisbani G, et al. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann Neurol. 2014;76:31–42. [DOI] [PubMed] [Google Scholar]
- 13. Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. [DOI] [PubMed] [Google Scholar]
- 14. Kordower JH, Goetz CG, Chu Y, et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann Neurol. 2017;81:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. [DOI] [PubMed] [Google Scholar]
- 16. Cope TE, Rittman T, Borchert RJ, et al. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain. 2018;141:550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falcon B, Cavallini A, Angers R, et al. Conformation determines the seeding potencies of native and recombinant Tau aggregates. J Biol Chem. 2015;290:1049–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clavaguera F, Akatsu H, Fraser G, et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci U S A. 2013;110:9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woerman AL, Aoyagi A, Patel S, et al. Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc Natl Acad Sci U S A. 2016;113:E8187–E8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abounit S, Zurzolo C. Wiring through tunneling nanotubes—from electrical signals to organelle transfer. J Cell Sci. 2012;125:1089–1098. [DOI] [PubMed] [Google Scholar]
- 21. Abounit S, Wu JW, Duff K, Victoria GS, Zurzolo C. Tunneling nanotubes: a possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion. 2016;10:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu JW, Herman M, Liu L, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288:1856–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duffy P, Wolf J, Collins G, DeVoe AG, Streeten B, Cowen D. Letter: possible person-to-person transmission of Creutzfeldt-Jakob disease. N Engl J Med. 1974;290:692–693. [PubMed] [Google Scholar]
- 24. Heath CA, Barker RA, Esmonde TF, et al. Dura mater-associated Creutzfeldt-Jakob disease: experience from surveillance in the UK. J Neurol Neurosurg Psychiatry. 2006;77:880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ritchie DL, Adlard P, Peden AH, et al. Amyloid-β accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 2017;134:221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Llado L, Baliellas C, Casasnovas C, et al. Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl. 2010;16:1386–1392. [DOI] [PubMed] [Google Scholar]
- 27. Holmes BB, Diamond MI. Amyotrophic lateral sclerosis and organ donation: is there risk of disease transmission? Ann Neurol. 2012;72:832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Melki R. Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J Parkinsons Dis. 2015;5:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peelaerts W, Bousset L, Van der Perren A, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. [DOI] [PubMed] [Google Scholar]
- 30. Prusiner SB, Woerman AL, Mordes DA, et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015;112:E5308–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yanamandra K, Kfoury N, Jiang H, et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer’s disease. Trends Mol Med. 2015;21:394–402. [DOI] [PubMed] [Google Scholar]