Abstract
Animal K+ channel α- (pore-forming) subunits form native proteins by association with β-subunits, which are thought to affect channel function by modifying electrophysiological parameters of currents (often by inducing fast inactivation) or by stabilizing the protein complex. We evaluated the functional association of KAT1, a plant K+ channel α-subunit, and KAB1 (a putative homolog of animal K+ channel β-subunits) by co-expression in Xenopus laevis oocytes. Oocytes expressing KAT1 displayed inward-rectifying, non-inactivating K+ currents that were similar in magnitude to those reported in prior studies. K+ currents recorded from oocytes expressing both KAT1 and KAB1 had similar gating kinetics. However, co-expression resulted in greater total current, consistent with the possibility that KAB1 is a β-subunit that stabilizes and therefore enhances surface expression of K+ channel protein complexes formed by α-subunits such as KAT1. K+ channel protein complexes formed by α-subunits such as KAT1 that undergo (voltage-dependent) inactivation do so by means of a “ball and chain” mechanism; the ball portion of the protein complex (which can be formed by the N terminus of either an α- or β-subunit) occludes the channel pore. KAT1 was co-expressed in oocytes with an animal K+ channel α-subunit (hKv1.4) known to contain the N-terminal ball and chain. Inward currents through heteromeric hKv1.4:KAT1 channels did undergo typical voltage-dependent inactivation. These results suggest that inward currents through K+ channel proteins formed at least in part by KAT1 polypeptides are capable of inactivation, but the structural component facilitating inactivation is not present when channel complexes are formed by either KAT1 or KAB1 in the absence of additional subunits.
The first (animal) K+ channel was cloned in 1988 by extensive genetic analysis and chromosome mapping of a Drosophila mutant with a phenotype displaying uncontrolled shaking (Pongs, 1992). Expression of the translation product of this Shaker gene in Xenopus laevis oocytes resulted in voltage-gated, K+-selective currents (Timpe et al., 1988), leading to the supposition that functional K+ channels are formed by tetramers of four membrane-spanning Shaker polypeptides (i.e. α-subunits). In 1992, a plant homolog of Shaker proteins (KAT1) was cloned by complementation of a K+-uptake-deficient yeast (Anderson et al., 1992). Functional expression in oocytes confirmed that KAT1 formed voltage-gated, inward-rectifying K+ channels (Schachtman et al., 1992). Interestingly, although KAT1 has striking sequence similarity to animal Shaker K+ channel polypeptides, channels formed by KAT1 do not function in a similar manner. Animal K+ channels formed by the Shaker family of polypeptides are outward-rectifying (Jan and Jan, 1994). Voltage-gating of currents through channels formed by KAT1 results in inward rectification (Schactman et al., 1992; Cao et al., 1995).
Purification of a native animal K+ channel protein in 1994 identified a polypeptide with a primary sequence and molecular mass similar to that deduced from the corresponding Shaker cDNA (Scott et al., 1994). That study identified a hydrophilic, approximately 40-kD polypeptide that bound tightly to (i.e. co-purified with) the hydrophobic, membrane-spanning Shaker polypeptide. Library screening with probes based on the partial sequence of the low-molecular-mass polypeptide led to the cloning of a corresponding full-length cDNA (Kvβ2) and a second cDNA encoding a homolog (Kvβ1) (Rettig et al., 1994). Co-expression of Kvβ1 with cDNAs encoding Shaker polypeptides (Kv1.1 and Kv1.4) resulted in increased inactivation of K+ currents (Rettig et al., 1994). This work established that (at least some) K+ channel proteins are formed in native membranes by both membrane-spanning, pore-forming Shaker polypeptides (i.e. α-subunits), and “regulatory” β-subunits.
Prior work has led to the cloning of two plant homologs of animal K+ channel β-subunits from Arabidopsis (KAB1) (Tang et al., 1995) and rice (Oryza sativa) (KOB1) (Fang et al., 1998). A physical association was demonstrated between the plant K+ channel β-subunit and a plant α-subunit (KAT1) (Tang et al., 1996). Expression patterns of a plant β-subunit have been correlated with K+ nutrition in plants (Fang et al., 1998). Immunocytochemical studies have documented the presence of (native) plant β-subunits in cellular membranes known to contain K+ channels (Tang et al., 1998). However, the functional relationship between cloned plant K+ channel α- and β-subunits has yet to be examined. Here, we report preliminary investigation of this relationship. Our approach was to examine the functional interaction of α- and β-subunits by co-expression in oocytes.
MATERIALS AND METHODS
Molecular Biology
KAT1 and hKv1.4 cDNAs were obtained from L. Kochian (Cornell University, Ithaca, NY) and B. Rudy (New York University Medical Center), respectively. KAB1 was cloned as described previously (Tang et al., 1995). For KAT1:KAB1 co-expression experiments, the KAT1 and KAB1 cDNA templates used for cRNA synthesis were subcloned into the pBS-KS II plasmid (Tang et al., 1995). KAT1:HKv1.4 co-expression was undertaken using constructs subcloned into the pSP64-poly(A+) plasmid (Promega, Madison, WI). Deletion and chimeric constructs of KAT1 were generated using PCR as follows. To ensure high fidelity during cDNA generation, the DNA polymerase VentR (New England BioLabs, Beverly, MA), which contains a 3′ → 5′ proofreading exonuclease activity, was used in place of Taq DNA polymerase, the concentration of template (plasmid) DNA was increased to 100 ng, and PCR cycles were limited to 10 for a reaction.
PCR products were size-fractionated on 1% (w/v) agarose gels, and bands of the expected size were isolated and purified (QIAQuick gel extraction kit, Qiagen USA, Valencia, CA) prior to restriction digestion and subcloning. After subcloning, PCR-generated cDNAs were sequenced to ensure fidelity of the constructs. The HindIII and XbaI restriction fragment of KAT1 in the pSP64 plasmid was subcloned into the corresponding restriction sites of the pBS-SK II (Stratagene, La Jolla, CA) plasmid. PCR was undertaken with this KAT1 construct as template and with the primers; 5′-CCGGATCCATATGCTCTCTGCCGATC-3′ and 5′-AATTAACCCTCACTAAAGGG-3′. The product of this reaction was subjected to restriction digestion with BamHI and SacI, and subsequently subcloned into the pSP64-poly(A+) plasmid, resulting in a pSP64 construct containing the N-terminal deletion mutant KAT1Δ1–28, which was used to generate cRNA. PCR was undertaken with hKv1.4 as a template and with the primers 5′-GCAAGCTTATGCGTGTGGTGATAAATGTGTC-3′ and 5′-GCGGATCCGAAACTTCAACAGGGCCTC-3′;this fragment of the hKv1.4 cDNA encodes amino acids 177 to 270, which includes the NAB domain identified as facilitating α:α-subunit binding (Li et al., 1992; Xu et al., 1995). The PCR product was subjected to restriction digestion with HindIII and BamHI and ligated into the corresponding restriction sites of the pSP64 plasmid containing KAT1Δ1–28, resulting in the NAB-KAT1 construct.
Oocyte Preparation
Stage V to VI ooctyes (1–1.2 mm in diameter) were harvested from ovarian lobes of anesthetized (immersion in 1 L of 1.5 g/L tricaine [Sigma, St. Louis] for approximately 1 h) mature female Xenopus laevis (Xenopus I, Madison, WI) frogs, and defolliculated by a 1- to 2-h incubation in a 60-mm Petri dish at room temperature with gentle shaking in Barth's solution (88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 0.82 mm MgSO4·7H2O, 0.41 mm CaCl2·2H2O, 0.33 mm Ca[NO3]2·4H2O, and 10 mm HEPES, pH 7.4, filter-sterilized through a 0.2-μm membrane) supplemented with 5 mg/mL type A collagenase (Boehringer Mannheim, Basel). After defolliculation, healthy (i.e. clearly delineated hemispheres) oocytes were immersed in a solution containing 100 mm K2HPO4, pH 6.5, and 0.1% (w/v) BSA for 1 h at room temperature on a rotary shaker (60 rpm), followed by washing (approximately five times) in Barth's solution supplemented with 0.1% (w/v) BSA. Oocytes were then transferred to fresh Barth's solution containing 0.1% (v/v) gentamycin (Gibco-BRL, Cleveland), and incubated for 24 h at 18°C in the dark prior to cRNA microinjection. After microinjection, oocytes were incubated in ND96 solution (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2·6H2O, and 5 mm HEPES, pH 7.5) at 18°C in the dark for two to 5 d prior to use in voltage clamp studies. During the period after oocytes were harvested, the incubation solution was replaced every 12 h, and dead oocytes were removed whenever solutions were replaced.
In Vitro Transcription
High-yield transcription kits (AmpliScribe, Epicentre Technologies, Madison, WI) with T7, SP6, and T3 initiation sites (as appropriate for each plasmid construct) were used according to the manufacturer's protocol to generate cRNA for microinjection into oocytes. For KAT1:KAB1 co-expression experiments, plasmids were linearized with XhoI. For KAT1:HKv1.4 co-expression experiments, plasmids were linearized with PvuII. After linearization, DNA was phenol extracted three times. About 1 μg of linearized plasmid DNA was used for each transcription reaction, and synthesized cRNAs were capped concomitantly to transcription using the methylated Cap analog m7[5′]ppp[5′]G (Epicentre Technologies). Products from in vitro transcription reactions were extracted with phenol, precipitated with ethanol, washed once in 70% (v/v) ethanol, and resuspended in RNase-free double-distilled water. For KAT1:KAB1 co-expression experiments, KAT1 cRNA was dissolved at different concentrations. The quality of cRNA preparations was monitored by electrophoretic size fractionation on agarose gels containing formaldehyde and stained with ethidium bromide. The cRNA concentration was determined spectrophotometrically (A260) (Sambrook et al., 1989). Aliquots (50 nL) of cRNA were microinjected into oocytes 1 d after removal from frogs. Microinjection of cRNA was undertaken with a manual microdispenser (Drummond Scientific, Broomall, PA) under a stereomicroscope.
Electrophysiology
Voltage clamp methods were used to characterize K+ currents of heterologous ion channel proteins expressed in X. laevis oocytes. All recordings were made in the two-electrode, whole-cell configuration using an amplifier (GeneClamp 500, Axon Instruments, Los Angeles). Voltage stimuli were generated and currents were recorded with a Digidata 1200 interface and Micron PC using PClamp 6.02 software (Axon Instruments) for data acquisition and analysis. Current recordings were filtered at 2 kHz and on-line P/4 leak subtraction was performed on all recordings as described by Bezanilla and Armstrong (1997). Bath solutions were continuously perfused during recordings. When hyperpolarizing step voltages were applied during current recordings, the bath solution contained 140 mm KCl, 2 mm CaCl2·2H2O, and 10 mm HEPES, pH 7.5. When depolarizing step voltages were applied, the bath solution was changed to 115 mm NaCl, 2.5 mm KCl, 1.8 mm CaCl2·2H2O, 1 mm NaHCO3, 1 mm MgCl2·6H2O, and 10 mm HEPES, pH 7.5. For each treatment of each experiment, recordings were obtained from a minimum of 10 separate oocytes and repeated in at least two independent experiments. Data in figures that portray time-dependent currents at a series of step voltages are from an individual oocyte representative of recordings from other replicate oocytes.
Immunoblot Analysis
Immunoreactivity of oocyte (water-injected controls and those injected with KAB1 cRNA) polypeptides with anti-KAB1 rabbit antiserum was evaluated as described in detail by Tang et al. (1996). Oocytes were dissolved in SDS-PAGE sample buffer. Denatured polypeptides were size-fractionated on SDS-PAGE, transferred to nitrocellulose membrane, and exposed to anti-KAB1 antiserum (1:3,000 dilution). Immunoreactive bands were visualized with horseradish peroxidase secondary antibody (goat anti-rabbit IgG) conjugate and enhanced chemiluminescence detection (ECL, Amersham, Uppsala).
RESULTS AND DISCUSSION
Prior research (Schactman et al., 1992) has demonstrated that the K+ channel α-subunit KAT1 forms voltage-gated, inward-rectifying K+-selective ion channels in the X. laevis oocyte heterologous expression system. As shown in Figure 1, A and E, currents recorded from oocytes injected with KAT1 cRNA displayed similar channel characteristics in our studies. K+ currents through channels formed by KAT1 had voltage-activation thresholds at about −100 to −120 mV, inward rectification, and no voltage-dependent inactivation. In addition, with a similar bath medium composition and cRNA level, currents were of a similar magnitude (at corresponding command potentials) as those found in previous studies (Schactman et al., 1992; Hoshi, 1995; Ward, 1997).
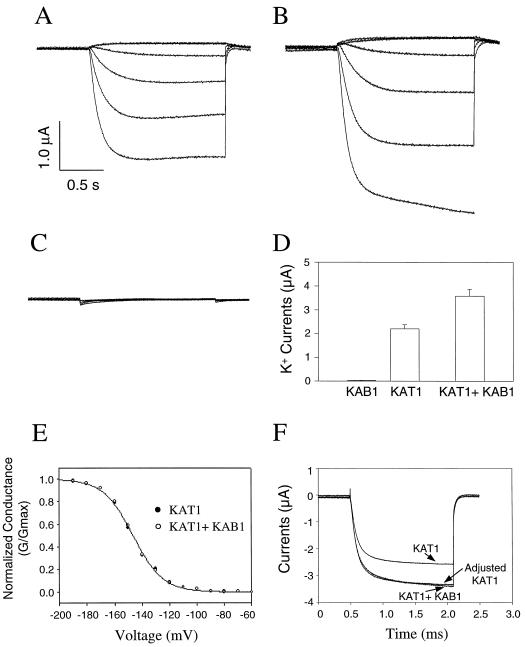
Figure 1.
Analysis of KAB1 co-expression effects on KAT1 currents. Time-dependent K+ currents are shown for oocytes injected with approximately 10 μg of KAT1 cRNA (A), approximately 10 μg each of KAT1 and KAB1 cRNA (B), or water (C). During recordings shown in A, B, and C, the holding potential was −60 mV, the current was recorded at −160-, −120-, −80-, −40-, 0-, and +40-mV step voltages, and the current and time axes are indicated by vertical and horizontal bars, respectively. D, K+ currents (±se) recorded from oocytes (n ≥ 13 for each of the treatments shown) maintained at a holding potential of −60 mV during a step voltage to −160 mV. Pooled data are presented for recordings of K+ currents from oocytes injected with either KAB1 (alone), KAT1 (alone), or KAT1 and KAB1 (together) cRNA. E, G/Gmax at varying command potentials for K+ currrents recorded from oocytes injected with KAT1 cRNA or with KAT1 and KAB1 cRNA. In many cases, the data points for the two treatments overlap; only one data point is visible at many of the step voltages. F, Time-dependent currents (−60-mV holding potential, −160-mV step voltage). Data for KAT1 alone and in the presence of KAB1 are shown, along with a superimposition of the two currents by adjusting the KAT1 data.
As mentioned above, prior studies have found the most pronounced effect K+ channel β-subunits have on the current parameters of co-expressed animal α-subunits is the induction of or the increase in the rate of voltage-dependent inactivation. However, not all animal β-subunits have been demonstrated to have this effect (Rettig et al., 1994; Majumder et al., 1995; Morales et al., 1995; Fink et al., 1996). In some cases, co-expression resulted in similar inactivation rates, but increased currents (Chouinard et al., 1995; McCormack et al., 1995; Fink et al., 1996). The experiments shown in Figures 1 and 2 were undertaken to evaluate the functional interaction between the plant β-subunit KAB1 and α-subunit KAT1 by examining K+ currents in oocytes expressing the corresponding cRNAs.
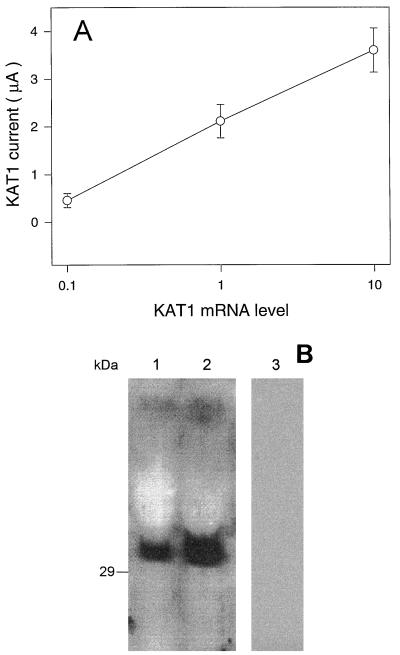
Figure 2.
A, Relationship between the amount of KAT1 cRNA injected into oocytes and current amplitude. Means (±se; n ≥ 13) of currents recorded at a −160-mV step voltage (−60-mV holding potential) are shown for oocytes injected with the standard (approximately 10 ng) amount of cRNA and also for oocytes injected with RNA prepared at either a 10-fold higher concentration (designated as level 10) or a 10-fold dilution (designated as level 0.1). The standard level of cRNA (level 1) was chosen because this treatment typically results in maximal currents of approximately 2 μA at a step voltage of −160 mV. B, Immunoblot analysis of KAB1 protein expressed in oocytes. Three oocytes were dissolved in SDS-PAGE sample buffer after injection of either approximately 10 ng (lane 1), approximately 50 ng (lane 2), or no KAB1 cRNA (lane 3, control oocytes injected with water).
To determine the effect of KAB1 on the expression level of KAT1, it was critical to provide KAT1 cRNA to oocytes at a level that would allow for increased expression of functional K+ channel complexes to be evidenced by increased currrents. For this set of experiments, a “standard” (i.e. 1×) level of KAT1 cRNA (approximately 10 μg) was chosen for oocyte injection, which allowed for the induction of K+ currents at a magnitude of approximately 2 μA (e.g. Figs. 1A and 2A) at a step voltage of −160 mV. As shown in Figure 2A, at this level of KAT1 cRNA, current amplitute was proportional to RNA dose. Of course, K+ channel β-subunits would not be expected to form functional channels alone (i.e. without the presence of α-subunits). Therefore, the expression level of KAB1 protein in oocytes was monitored immunologically using an anti-KAB1 antibody. Immunoblot analysis (Fig. 2B) documented the presence of varying amounts of KAB1 protein in oocytes injected with different levels of KAB1 cRNA, indicating that this K+ channel subunit is also translated in the heterologous expression system. No protein immunoreactive with anti-KAB1 antibody was present in water-injected control oocytes (Fig. 2B). Results presented in Figure 2 indicate that both KAT1 and KAB1 cRNA are translated in oocytes, and that the altered level of functional KAT1 assembly and insertion into the oocyte plasma membrane can be monitored as altered level of current.
The effect of KAB1 co-expression on KAT1 currents was evaluated in the experiments shown in Figure 1. Representative time-dependent currents recorded from oocytes injected with KAT1 cRNA alone, KAT1 and KAB1 cRNA, and water-injected controls are shown in Figure 1, A through C, respectively, as a function of varying command voltage. It is evident from these recordings that in the presence or absence of KAB1, inward KAT1 currents show no inactivation during the duration of the recordings. Data in Figure 1, A and B, portray (for each treatment) current recordings from single representative oocytes. Time-dependent currents were recorded from a total of 13 oocytes injected with KAT1 cRNA alone and from 13 other oocytes injected with KAT1 and KAB1 cRNA. As portrayed in Figure 1, A and B, in no case did inactivation occur in any of the other oocytes tested (not shown); no quantitative analysis of KAB1-dependent changes in current inactivation is presented since none occurred under either treatment.
Some (animal) β-subunits alter K+ current activation kinetics, or conductance profiles at varying command potentials (G/Gmax) upon co-expression with α-subunits (e.g. Chouinard et al., 1995). An evaluation of KAB1 effects on these parameters of KAT1 currents is presented in Figure 1. As shown in Figure 1E, analysis of G/Gmax for KAT1 currents in the presence or absence of KAB1 indicated that co-expression of KAB1 did not affect relative KAT1 conductance at varying command potentials or voltage thresholds for activation. Chouinard et al. (1995) demonstrated a shift in the midpoint for activation of a Shaker K+ channel α-subunit upon co-expression with the Hk β-subunit. The G/Gmax curves for KAT1 in the presence and absence of KAB1 are superimposable; KAB1 does not have the same effect.
The effects of KAB1 on activation kinetics of KAT1 currents were also evaluated (Fig. 1F). When time-dependent KAT1 currents in the presence and absence of KAB1 are normalized, the time course for current activation is also superimposable. The analysis shown in Figure 1F for KAT1 activation in the presence and absence of KAB1 includes data for one command potential. The half-time for attainment of maximal current (i.e. the τ for activation) can be analyzed for currents recorded at a range of step voltages. Calculated τ for KAT1 activation in the presence and absence of KAB1 was essentially identical: 174 and 170 ms, respectively, at −180 mV; 213 and 210 ms, respectively, at −160 ms; and 543 and and 540 ms, respectively, at −120 mV. These differences are relatively minor compared with other studies (e.g. Chouinard et al., 1995).
In contrast to this lack of an effect on current gating parameters, co-expression with KAB1 did increase the magnitude of KAT1 currents (Fig. 1D, also compare Fig. 1, A and B). No inward K+ currents were evident in oocytes injected with KAB1 cRNA alone (Fig. 1D) or in water-injected control oocytes (Fig. 1C). However, co-expression of KAB1 with the channel-forming α-subunit KAT1 resulted in an increase (approximately 64%, from a mean of 2.2 to 3.6 μA at −160 mV) in the amplitude of whole-cell currents recorded from oocytes (Fig. 1D). This effect of KAB1 on KAT1 current amplitude was of a similar extent as has been found upon co-expression of some animal K+ channel α- and β-subunits (e.g. Chouinard et al., 1995).
Prior studies examining this effect with animal β-subunits have found that such increases in K+ currents recorded from oocytes expressing heterologous α- and β-subunits were attibutable to an increase in the level of functional K+ channel complexes formed (Fink et al., 1996; Shi et al., 1996). Direct evidence from these studies has shown that this effect of K+ channel β-subunits on current is due to a structural stabilization of the multimeric K+ channel complex formed by α-subunits, resulting in an increase in the concentration of functional channels formed in the target membrane, which leads to increased currents. In no case has the increased current obtained upon co-expression of a β-subunit been attributed to increased single-channel conductance. We conclude, therefore, that the translation product of the Arabidopsis cDNA KAB1 does have a functional interaction with the Arabidopsis K+ channel α-subunit KAT1, and that this interaction results in increased channel complex stability and/or functional assembly in the plasma membrane of this heterologous expression system. This finding is consistent with the following other studies of KAB1:KAT1 interaction.
KAB1 has been shown to form a physical association with KAT1 protein in vitro (Tang et al., 1996). Immunocytological studies have shown that KAB1 is present in the plasmalemma of Arabidopsis leaf cells (Fang et al., 1998), as was KAT1 (Ichida et al., 1997). Expression levels of both KAB1 (Tang et al., 1996, in which a KAB1 homolog in fava bean was monitored) and KAT1 (Nakamura et al., 1995) are greater in guard cells than that found in the leaf mesophyll. Finally, a recent study monitoring the conductance parameters of a mutated KAT1 construct expressed in transgenic Arabidopsis plants indicated that in planta, KAT1 may be present in native membrane systems as the pore-forming component of K+ channel complexes that also contain other subunits (Ichida et al., 1997). No direct evidence was presented in that paper; their hypothesis was based on the observation that native KAT1 currents recorded from voltage-clamped guard cell protoplasts differed from KAT1 currents recorded from oocytes. The demonstration in our study of a functional interaction between KAT1 and KAB1, when taken in context of these other studies, continues the development of the hypothesis that in situ, at least some plant K+ channel complexes may be heteromultimers comprised of both α- and β-subunits.
Our evaluation of the functional interaction between KAT1 and KAB1 suggests that, in situ, KAB1 may function only to increase the total cell K+ current. This effect likely results from the stabilization of the channel complex by the β-subunit, leading to an increase in surface expression. Analysis of the expression pattern of K+ channel β-subunits suggests that this contribution to K+ channel function may be critical in situ. Immunocytochemical studies document the presence of KAB1 (and KOB1, a KAB1 homolog from rice) in a wide variety of plant organs (Tang et al., 1996; Fang et al., 1998). KAB1 protein has also been found to be associated with numerous cell membrane systems, including the plasmalemma, tonoplast, chloroplast envelope, and mitochondrial inner membrane (Tang et al., 1998). Expression of such plant K+ channel β-subunits is increased in tissues and cell types that accumulate K+, and depletion of K+ from specific plant organs is associated with a loss of β-subunit protein (Tang et al., 1996, 1998; Fang et al., 1998). This ubiquitous expression pattern of a plant K+ channel β-subunit that apparently does not act to modulate channel gating or other aspects of single channel currents is similar to the situation in animals. Intriguingly, of the total K+ channel β-subunit protein (i.e. including subunits that modulate gating and those that have only been shown to increase current) expressed in mammalian brain, the greatest proportion (>90%) is of the type (like KAB1) that does not modulate channel gating parameters (Rhodes et al., 1997).
Despite the demonstration in this report of a functional interaction between KAB1 and KAT1, and the evolving model of plant K+ channels as possibly being composed of both α- and β-subunits, the molecular basis for putative inactivation of voltage-gated, inward-rectifying K+ channels in native plant cell membranes remains an unresolved issue. Not much work has been done to date examing the inactivation of plant K+ channels. In a recent review, Schroeder et al. (1994) pointed out that K+ channels (in native plant membranes) remain open for many minutes when voltage clamped at hyperpolarizing potentials. In addition, the inward-rectifying plant K+ channel α-subunits that have been studied in heterologous expression systems maintain maximal currents for many minutes (e.g. Fig. 1), displaying no voltage-dependent inactivation.
Schroeder et al. (1994) suggested that plant K+-selective channels, in providing a pathway for long-term K+ flux across plant cell membranes, could be expected to lack the mechanism that typically facilitates voltage-dependent inactivation of animal K+ channels. The few studies published to date generally support this model of plant K+ channel function without a mechanism corresponding to that which induces voltage-dependent inactivation in animal K+ channels. Native plant K+ channels facilitating inward currents have been shown to have inactivation time constants that are independent of voltage (Schroeder et al., 1984; White and Tester, 1992), indicating the lack of a voltage-dependent inactivation mechanism. However, there are some reports (Kolb et al., 1987; Schauf and Wilson, 1987; G. Findlay, personal communication) demonstrating a significant voltage dependence of the time constant for inactivation of K+ currents across native plant membranes. The possibility exists that future studies at the molecular level, and the expression analysis of cDNAs encoding K+-selective channels will reveal the presence in plants of a voltage-dependent inactivation mechanism similar to those characterized for animal systems.
Current models of structure:function aspects of animal K+ channel inactivation (e.g. Jan and Jan, 1994) portray the most predominant means of voltage-dependent inactivation as occurring via the N-terminal ball and chain mechanism. A portion of the protein on the inner portion of the channel complex (the “ball”) can move freely (i.e. “tethered” to the rest of the protein by the “chain”) and can physically occlude the inner portion of the channel pore, thus facilitating inactivation of ion currents. The animal K+ channel complex is formed by four α- and four β-subunits (Dolly et al., 1994). Experimental evidence supporting this inactivation model indicates that the ball and chain portion of the channel complex can be formed by the N terminus of either a single α- or β-subunit.
Native animal K+ channel complexes can be formed by different members of α- and β-subunit families (Jan and Jan, 1994; Xu et al., 1995). Thus, a remarkable diversity in K+ channel function, including inactivation parameters (Xu and Li, 1997), can be generated by the formation of functional channel complexes that include different α- and β-subunits. Some animal K+ channel α- and β-subunit polypeptides are known to have N-terminal regions that form the ball and chain structure, while others do not. The presence of either an α- or a β-subunit in a K+ channel that contains the N-terminal ball and chain appears to be sufficient to facilitate voltage-dependent inactivation. Co-expression in oocytes of different α- and β-subunits (i.e. one α- or β-polypeptide that can facilitate ball and chain inactivation with other subunits lacking the inactivation mechanism) has been shown to generate heteromeric channels that do inactivate, despite containing subunits that would not form channels with inactivating currents if they were expressed alone and formed homomeric channels (Isacoff et al., 1990; Ruppersberg et al., 1990; Xu and Li, 1997).
The plant K+ channel α- and β-subunits cloned to date, including KAT1 and KAB1 used in the studies reported here, all have short (relative to some animal homologs that contain N-terminal ball and chain segments) N termini. Thus, it is not known whether any plant K+ channel subunit can assemble with other subunits to form channels with inactivating currents. We undertook the series of experiments shown in Figures 3 and 4 to address this hypothesis. The cDNA encoding the plant α-subunit KAT1 was modified so that it could form heteromeric channels with animal α-subunits. In addition to (in some cases) the ball and chain structure, the N termini of animal K+ channel α-subunits such as hKv1.4 are known to contain a region, referred to as the NAB domain, which facilitates α-α-subunit assembly (Li et al., 1992; Xu et al., 1995).
Figure 3.
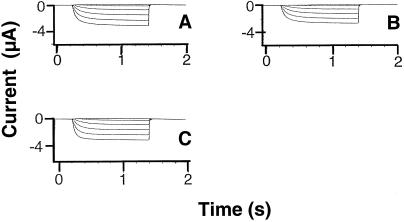
Time-dependent K+ currents recorded from oocytes injected with cRNA generated from various KAT1 constructs. In this experiment, the holding potential was −40 mV, and currents were recorded at step voltages ranging from −60 to −180 mV in 20-mV increments on oocytes injected with (10 ng) KAT1 (A), KAT1Δ1–28 (B), or NAB-KAT1Δ1–28 (C) cRNA. In all cases, currents were recorded from a minimum of 10 oocytes and, for a given treatment, reproducible results were obtained from at least two independent experiments (i.e. two batches of oocytes). Currents shown are representative of all recordings for a particular treatment.
Figure 4.
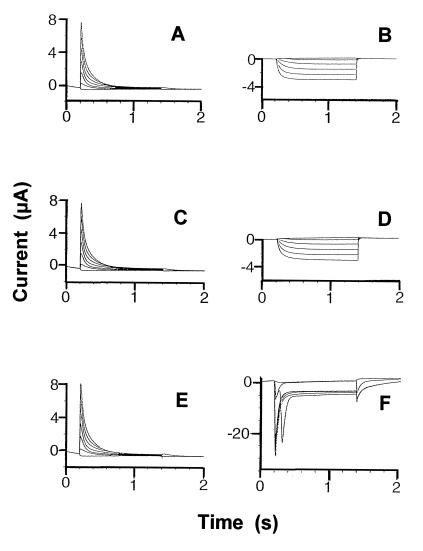
Time-dependent K+ currents recorded from oocytes injected with hKv1.4 (10 ng) and/or various constructs of KAT1 cRNA (10 ng). Treatments (i.e. cRNA) were as follows: hKv1.4 (A), KAT1Δ1–28 (B), hKv1.4 and KAT1Δ1–28 (C and D), and hKv1.4 and NAB-KAT1Δ1–28 (E and F). For A, C, and E, the voltage-clamp protocol generated outward currents, the holding potential was −70 mV, and the step voltage was varied between −80 and +40 mV in 20-mV increments. For B, D, and F, the voltage-clamp protocol generated inward currents, the holding potential was −40 mV, and the step voltage was varied between −60 and −180 mV in 20-mV increments. In all cases, currents were recorded from a minimum of 10 oocytes and, for a given treatment, reproducible results were obtained from at least two independent experiments (i.e. two batches of oocytes). Curents shown are representative of all recordings for a particular treatment.
The N-terminal 28 amino acids (i.e. a portion of the polypeptide N-terminal to S1, the first membrane-spanning region) were deleted from the KAT1 sequence. As shown in Figure 3, this did not prevent functional expression in oocytes or substantially alter the kinetics, voltage dependence, or amplitude of currents formed by the mutated KAT1 α-subunit when expressed in oocytes. The NAB portion of the human α-subunit hKv1.4 was added to the KAT1Δ1–28 construct. The chimeric NAB-KAT1Δ1–28 construct, upon expression in oocytes, also formed functional channels with current parameters similar to the inward-rectifying, non-inactivating currents formed by native KAT1 (Fig. 3). However, co-expression of the chimeric NAB-KAT1Δ1–28 with native hKv1.4 generated a population of functional channels with highly unique current parameters.
A comparison of oocyte currents generated from expression of various constructs of hKv1.4 and KAT1 is shown in Figure 4. Homomeric channels formed by hKv1.4 display outward-rectifying, fast-inactivating K+ currents (Fig. 4A). Hyperpolarizing voltage steps did not result in inward currents when hKv1.4 was expressed alone (data not shown, also see Philipson et al., 1991). Co-expression of hKv1.4 with the deletion mutant KAT1Δ1–28 apparently resulted only in the formation of homomeric channels formed by self-assembly of each of the α-subunits. The fast-inactivating outward currents induced at depolarizing voltages (Fig. 4C) are similar to those found when hKv1.4 was expressed alone (Fig. 4A). Hyperpolarizing step voltages induced non-inactivating inward currents in oocytes expressing both constructs (Fig. 4D) that were similar to currents induced upon expression of KAT1Δ1–28 alone (Fig. 4B).
Currents recorded from oocytes co-injected with KAT1 mutated to contain the N-terminal NAB domain, which facilitates α-α-subunit assembly, along with the human outward-rectifier hKv1.4, displayed unique properties. At depolarizing step voltages, the outward currents recorded from these oocytes were again fast-inactivating (Fig. 4E). However, hyperpolarizing step voltages resulted in “hybrid” currents (Fig. 4F) that displayed characteristics consistent with the assembly of mutated plant α-subunit NAB-KAT1Δ1–28 with at least one animal α-subunit hKv1.4 to form heteromeric channel complexes. The inward currents shown in Figure 4F could only have occurred through channel complexes with the mutated KAT1 subunit present. hKv1.4 is an outward rectifier (Philipson et al., 1991); homomeric channels formed by just hKv1.4 subunits would not display such inward currents.
The current amplitude displayed by oocytes expressing these hybrid channels is substantially greater than that which occurs in oocytes expressing either α-subunit alone (Fig. 4); we do not know why this occurs, but speculate that the hybrid channel's single channel conductance may be greater. The fast inactivation of these inward currents could only occur if the N-terminal ball of a hKv1.4 subunit was occluding the inner pore of the hybrid channel complex. Thus, the results shown in Figure 4F indicate that inward currents through a K+ channel formed (at least in part) by KAT1 α-subunits can undergo N-type ball and chain inactivation, but the physical mechanism facilitating this inactivation is not present when channel complexes are formed by KAT1 alone or, presumably, by KAT1 and KAB1.
The studies shown in Figures 3 and 4 were undertaken so as to provide an experimental context to evaluate the potential for N-terminal inactivation of currents through K+ channel complexes that contain KAT1 as a structural component. However, these results also provide a new understanding of structural components of K+ channel α-subunits that contribute to channel assembly. Apparently, the N terminus (amino acids 1–28) of KAT1 is not required for either assembly or function. The NAB domain found in animal channels (but not present in native plant K+ channel α-subunits cloned to date) is sufficient for chimeric assembly of animal and plant α-subunits, as well as for the co-assembly of subunits of inward- and outward-rectifying channels.
In this study, a functional interaction was demonstrated between the plant (Arabidopsis) K+ channel α-subunit KAT1 and β-subunit KAB1. Results suggest that co-assembly of KAB1 with KAT1 does not alter the gating kinetics of the multimeric channel complex. Rather, the presence of the β-subunit may enhance stability and therefore surface expression of channels formed by the α-subunit. Compared with studies of animal systems, relatively few K+ channel cDNAs have currently been cloned from plants, and none can be deduced to contain a functional domain corresponding to that which facilitates N-type inactivation of K+ channels native to animal membranes.
We cannot know from the published literature if α-subunits such as KAT1 are present in native plant membranes as part of a multimeric K+ channel complex containing either α- or β-subunits capable of facilitating N-type inactivation. However, the functional expression studies reported here demonstrating voltage-dependent inactivation of currents through multimeric channels containing KAT1 and hKv1.4 raise the possibility that plant α-subunits could form channels that undergo this type of gating behavior if any of the subunits forming the channel contributed the correct functional domain.
The recent work of Xu and Li (1997) raises an intriguing point regarding the functional analysis of the KAB1 and KAT1 interaction and putative N-type inactivation of KAT1. That study demonstrated that an animal K+ channel β-subunit (Kvβ2) that lacked the N-terminal ball (i.e. as is the case with KAB1) did play an important role in the gating behavior of native K+ channel complexes. They concluded that some animal α-subunits are present in situ in heteromeric K+ channel complexes containing both Kvβ1 (which does have the N-terminal inactivation domain) and Kvβ2. The presence of Kvβ2 in the native channel complex is functionally significant because the β2-subunit prevents the channel from being inactivated by the ball domain of the β1-subunit. KAB1 could serve the same function in native plant K+ channel complexes.
Footnotes
This material is based on work supported by the National Science Foundation (grant nos. MCB–9513921 and BIR–9512977). This is Storrs Agricultural Experiment Station publication no. 1,883.
LITERATURE CITED
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel I: sodium current experiments. J Gen Physiol. 1997;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Crawford NM, Schroeder JI. Amino terminus and the first four membrane-spanning segments of the Arabidopsis K+ channel KAT1 confer inward-rectification property of plant-animal chimeric channels. J Biol Chem. 1995;270:17697–17701. [PubMed] [Google Scholar]
- Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B. A potassium channel β-subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc Natl Acad Sci USA. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolly JO, Rettig J, Scott VES, Parcej DN, Wittka R, Sewing S, Pongs O. Oligomeric and subunit structures of neuronal voltage-sensitive K+ channels. Biochem Soc Trans. 1994;22:473–478. doi: 10.1042/bst0220473. [DOI] [PubMed] [Google Scholar]
- Fang Z, Kamasani U, Berkowitz GA. Molecular cloning and expression characterization of a rice K+ channel β subunit. Plant Mol Biol. 1998;37:597–606. doi: 10.1023/a:1005913629485. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Heurteaux C, Romey G, Barhanin J, Lazdunski M. A new K+ channel β subunit to specifically enhance Kv2.2 (CDRK) expression. J Biol Chem. 1996;271:26341–26348. doi: 10.1074/jbc.271.42.26341. [DOI] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida AM, Pei Z-M, Baizabal-Aguirre VM, Turner KJ, Schroeder JI. Expression of a Cs+-resistant guard cell K+ channel confers Cs+-resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell. 1997;9:1843–1857. doi: 10.1105/tpc.9.10.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff EY, Jan YN, Jan LY. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990;345:530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Potassium channels and their evolving gates. Nature. 1994;371:119–122. doi: 10.1038/371119a0. [DOI] [PubMed] [Google Scholar]
- Kolb HA, Kohler K, Martinoia E. Single potassium channels in membranes of isolated mesophyll barley vacuoles. J Membr Biol. 1987;95:163–169. [Google Scholar]
- Li M, Jan YN, Jan LY. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992;257:1225–1230. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- Majumder K, De Biasi M, Wang Z, Wible BA. Molecular cloning and functional expression of a novel potassium channel α-subunit from human atrium. FEBS Lett. 1995;361:13–16. doi: 10.1016/0014-5793(95)00120-x. [DOI] [PubMed] [Google Scholar]
- McCormack K, McCormack T, Tanouye M, Rudy B, Stuhmer W. Alternative splicing of the human Shaker K+ channel β1 gene and functional expression of the β2 gene product. FEBS Lett. 1995;370:32–36. doi: 10.1016/0014-5793(95)00785-8. [DOI] [PubMed] [Google Scholar]
- Morales MJ, Castellino RC, Crews AL, Rasmusson RL, Strauss HC. A novel β subunit increases rate of inactivation of specific voltage-gated potassium channel α subunits. J Biol Chem. 1995;270:6272–6277. doi: 10.1074/jbc.270.11.6272. [DOI] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL, Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson LH, Hice RE, Schaefer K, LaMendola J, Bell GI, Nelson DJ, Steiner DF. Sequence and functional expression in Xenopus oocytes of a human insulinoma and islet potassium channel. Proc Natl Acad Sci USA. 1991;88:53–57. doi: 10.1073/pnas.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol Rev. 1992;72:S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Rettig J, Helnemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of β-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvβ1 and Kvβ2 β subunits with Kv1 α subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg JP, Schroter KH, Sakmann B, Stocker M, Sewing S, Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990;345:535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. pp. 9.31–9.58. [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schauf CL, Wilson KJ. Properties of single K+ and Cl− channels in Asclepias tuberosa protoplasts. Plant Physiol. 1987;85:413–418. doi: 10.1104/pp.85.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hedrich R, Fernandez JM. Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature. 1984;312:361–362. [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Scott VES, Rettig J, Parcey DN, Kleen JN, Findlay JBC, Pongs O, Dolly JO. Primary structure of a β subunit of α-dendrotoxin-sensitive K+ channels from bovine brain. Proc Natl Acad Sci USA. 1994;91:1637–1641. doi: 10.1073/pnas.91.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. β subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- Tang H, Vasconcelos AC, Berkowitz GA. First evidence that plant K+ channel proteins have two different types of subunits. Plant Physiol. 1995;109:327–330. doi: 10.1104/pp.109.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Vasconcelos AC, Berkowitz GA. Physical association of KAB1 with plant K+ channel α subunits. Plant Cell. 1996;8:1545–1553. doi: 10.1105/tpc.8.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Vasconcelos AC, Ma J, Berkowitz GA. In vivo expression pattern of a plant K+ channel β subunit protein. Plant Sci. 1998;134:117–128. [Google Scholar]
- Timpe LC, Scharz TL, Tempel BL, Papazian DM, Jan YN, Jan LY. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature. 1988;331:143–145. doi: 10.1038/331143a0. [DOI] [PubMed] [Google Scholar]
- Ward JM. Patch-clamping and other molecular approaches for the study of plasma membrane transporters demystified. Plant Physiol. 1997;114:1151–1159. doi: 10.1104/pp.114.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Tester M. Potassium channels from the plasma membrane of rye roots characterized following incorporation into planar lipid bilayers. Planta. 1992;186:188–202. doi: 10.1007/BF00196248. [DOI] [PubMed] [Google Scholar]
- Xu J, Li M. Kvβ2 inhibits the Kvβ1-mediated inactivation of K+ channels in transfected mammalian cells. J Biol Chem. 1997;272:11728–11735. doi: 10.1074/jbc.272.18.11728. [DOI] [PubMed] [Google Scholar]
- Xu J, Yu W, Jan YN, Jan LY, Li M. Assembly of voltage-gated potassium channels: conserved hydrophilic motifs determine subfamily-specific interactions between the α-subunits. J Biol Chem. 1995;270:24761–24768. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]