Abstract
Background
Plasma neurofilament light (NFL) is a promising biomarker for Alzheimer disease (AD), which increases in the early stage of AD and is associated with the progression of AD. We performed a genome-wide association study (GWAS) of plasma NFL in Alzheimer’s Disease Neuroimaging Initiative 1 (ADNI-1) cohort to identify novel variants associated with AD.
Methods
This study included 179 cognitively healthy controls (HC), 176 patients with mild cognitive impairment (MCI), and 172 patients with AD. All subjects were restricted to non-Hispanic Caucasian derived from the ADNI cohort and met all quality control (QC) criteria. Association of plasma NFL with the genetic variants was assessed using PLINK with an additive genetic model, i.e.dose-dependent effect of the minor alleles. The influence of a genetic variant associated with plasma NFL (rs7943454) on brain structure was further assessed using PLINK with a linear regression model.
Results
The minor allele (T) of rs7943454 in leucine zipper protein 2 gene (LUZP2) was associated with higher plasma NFL at suggestive levels (P = 1.39 × 10− 6) in a dose-dependent fashion. In contrast, the minor allele (G) of rs640476 near GABRB2 was associated with lower plasma NFL at suggestive levels (P = 6.71 × 10− 6) in a dose-dependent effect in all diagnostic groups except the MCI group. Furthermore, the minor allele (T) of rs7943454 within LUZP2 increased the onset risk of AD (odds ratio = 1.547, confidence interval 95% = 1.018–2.351) and was associated with atrophy of right middle temporal gyrus in the whole cohort in the longitudinal study (P = 0.0234).
Conclusion
GWAS found the associations of two single nucleotide polymorphisms (rs7943454 and rs640476) with plasma NFL at suggestive levels. Rs7943454 in LUZP2 was associated with the onset risk of AD and atrophy of right middle temporal gyrusin the whole cohort. Using an endophenotype-based approach, we identified rs7943454 as a new AD risk locus.
Electronic supplementary material
The online version of this article (10.1186/s12920-018-0364-8) contains supplementary material, which is available to authorized users.
Keywords: Genome-wide association study, Plasma NFL, Alzheimer disease, LUZP2, GABRB2, Genetic factors
Background
Alzheimer disease (AD) is the main cause of dementia and one of the major challenges for health care across the world, which is characterized pathologically by extracellular accumulation of amyloid-β (Aβ), intracellular deposition of neurofibrillary tangles (NFT), neuronal loss and synaptic dysfunction [1]. Well-established cerebrospinal fluid (CSF) biomarkers including Aβ42, total-tau (t-tau), phosphorylated tau (p-tau) have been used for the diagnosis of AD and monitoring its progression [2], but the their application is hampered by a high degree of invasiveness, complex operations and high costs. Biomarkers in peripheral blood are more appropriate screening tools for AD among old individuals to monitor AD progression. Interestingly, recent studies using ultra-sensitive assay showed that plasma neurofilament light (NFL), the main component of neurofilaments (cytoskeletal protein of neurons), increased in patients with AD dementia and was associated with other established CSF and neuroimaging biomarkers of AD [3, 4]. Plasma NFL is a noninvasive biomarker for neuronal injury in AD compared with CSF biomarkers. Thus, it has the potential for monitoring AD progression [3].
AD is a clinically heterogeneous neurodegenerative disease with a strong genetic component. Genetic risk factors of AD impact the CSF or neuroimaging biomarkers through which they might modulate the process of AD [5]. Thus, biomarkers for AD may be used as endophenotypes to explore the genetic factors that impact their metabolism [6–8]. Based on the association between plasma NFL and AD, we performed a genome-wide association study (GWAS) using plasma NFL as an endophenotype of AD to explore genetic factors involved in plasma NFL metabolism. We hypothesized that these genetic factors may influence pathological change in AD.
Methods
Subjects
In this study, 172 AD patients, 176 subjects with mild cognitive impairment (MCI), and 179 healthy controls (HC) whose data met all quality control (QC) criteria were included from the Alzheimer’s Disease Neuroimaging Initiative 1 (ADNI-1) cohort. The full cohort with plasma NFL and genotype data included 578 subjects. All the subjects were restricted to non-Hispanic Caucasian checked with their pedigree information checked to reduce potential bias of population stratification that might confound GWAS results. This step removed 40 subjects. After QC of the plasma NFL levels and removal of 11 outliers, there were 527 subjects with plasma NFL data left. The detailed demographic information and plasma NFL data have been shown in Table 1.
Table 1.
The demographic information of participants with plasma NFL data
| Baseline diagnosis | AD | MCI | HC | Total |
|---|---|---|---|---|
| n | 172 | 176 | 179 | 527 |
| Age (years), mean ± SD (range) | 76 ± 7 (56–91) | 75 ± 8 (54–89) | 76 ± 5 (62–90) | 75 ± 7 (54–91) |
| Gender, male/female | 90/82 | 117/59 | 103/76 | 310/217 |
| APOE ε4 carrier (%) | 66.9 | 52.8 | 26.8 | 48.6 |
| Plasma NFL (pg/ml), mean ± SDa | 48.7 ± 20.9 | 39.9 ± 17.7 | 32.8 ± 15.5 | 40.4 ± 19.3 |
Abbreviations: AD Alzheimer’s disease, APOE Apolipoprotein E; HC healthy control, MCI mild cognitive impairment, NFL neurofilament light, SD standard deviation
aPlasma NFL levels were different across the 3 diagnostic groups (P < 0.0001). Tukey’s multiple comparisons test showed that AD patients had higher plasma NFL levels compared with MCI group and healthy controls (P < 0.001). MCI group also had higher plasma NFL levels compared to healthy controls (P = 0.0007)
ADNI dataset
ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and nonprofit organizations. ADNI was established to develop serial magnetic resonance imaging (MRI), positron emission tomography (PET), and a combination of biomarkers, neuropsychological and clinical assessment to improve early diagnosis and measure the progression of AD [9]. The ADNI database has three protocols (ADNI 1, ADNI 2 and ADNI Grand Opportunities (ADNI GO)) at present and recruited more than 1500 participants including normal older subjects, MCI and early AD in this research. More information is available on the website of ADNI (www.loni.ucla.edu/ADNI).
Plasma measurements and quality control
Plasma NFL was analyzed using the ultrasensitive Single Molecule array (Simoa) technique as previously described [10]. The assay used a combination of monoclonal antibodies and purified bovine NFL as a calibrator. Analytical sensitivity was < 1.0 pg/mL, and the NFL levels in all tested sample were above the detection limit. Further QC was performed to reduce the potential influence of extreme outliers on statistical results. Mean and standard deviations (SD) of baseline plasma NFL were calculated. Subjects who had a value which is 3-fold SD greater or smaller than the mean value (< 42.8–3 × 26.8 pg/mL or > 42.8 + 3 × 26.8 pg/mL) were removed from the analysis. This step removed 11 subjects.
Genotyping and quality control
The ADNI 1 samples involved in this study were genotyped by Human 610-Quad BeadChip (Illumina, Inc., San Diego, CA). PLINK software (version 1.07) was used to explore the association of plasma NFL with the genetic variants using the following stringent criteria: minimum call rate for single nucleotide polymorphisms (SNPs) and individuals > 98%; minimum minor allele frequencies (MAF) > 0.20, Hardy-Weinberg equilibrium test P > 0.001. The restriction to SNPs with a minor allele frequency > 0.20 served to reduce the potential for false-positive results and enhance statistical power. An apolipoprotein E (APOE) genotyping kit was used to identify APOE alleles, which were defined by rs7412 and rs429358 [7].
Brain structure on MRI
The data of MRI brain structure were derived from UCSF FreeSurfer datasets, which were used to conduct association test of rs7943454in leucine zipper protein2 gene (LUZP2) with brain structure. The cerebral image segmentation and analysis were performed with the FreeSurfer version5.1 (http://surfer.nmr.mgh.harvard.edu/) based on the2010 Desikan-Killanyatlas [11]. The technical details of these procedures have been described in prior publications [12]. Brian regions have been reported to be closely associated with AD such as hippocampus, parahippocampus, middle temporal gyrus, posterior cingulate, precuneus and entorhinal cortex, which were selected as our regions of interest (ROI) to analyze their its associations with rs7943454.
Statistical analyses
Association studies of plasma NFL with the genetic variants were performed using PLINK (version 1.07) with the additive genetic model, i.e. dose-dependent effect of the minor allele. The analysis included a total of 30,1687 genotyped variants. To adjust for multiple testing, Bonferroni correction was applied and SNPs with corrected p < 0.01 (uncorrected p < 3.31 × 10–8, i.e., 0.01/301687 markers) were considered genome-wide significant. And we secondarily examined SNPs with uncorrected p values less than 10–5 to identify potential candidates. Age, gender and diagnosis were included as covariates. Bonferroni correction of the P values by the total number of acceptable quality SNPs was used for multiple test correction. Differences in continuous variables (plasma NFL levels, volume of regional brain) were examined using one-way analysis of variance (ANOVA), and Tukey’s multiple comparisons test was used to perform the pairwise analysis after ANOVA. Genome-wide associations were visualized using a software program (R, version 3.4.0; The R Foundation). Regional associations were visualized with the Locus Zoom web tool (http://locuszoom.org/). Moreover, a multiple linear regression model was applied using PLINK to estimate coefficients for testing a possible correlation between brain structure and rs7943454. Age, gender, education, and APOE ε4status were used as covariates.
Results
Characteristics of included subjects
The information about these included subjects has been shown in Table 1. Briefly, 172 AD (82 women, 76 ± 7 years), 176 MCI (59 women, 75 ± 8 years) and 179 HC (76 women, 76 ± 5 years) subjects were recruited in this study. AD group had the highest frequency of ε4 allele within APOE gene (66.9%). AD patients (48.7 ± 20.9 pg/ml) had higher plasma NFL levels compared with MCI group (39.9 ± 17.7 pg/ml) and HC group (32.8 ± 15.5 pg/ml) (P < 0.001). MCI group also had higher plasma NFL levels compared to HC group (P = 0.0007). The sensitivity and specificity of plasma NFL used for the diagnosis of AD were 0.73 and 0.84 respectively. The area under the curve (AUC) of the model containing plasma NFL, age at baseline, gender, educational level and APOE ε4 genotype was 0.86 in predicting the onset of AD among HC controls group. By comparison, the AUROCs were 0.84 to 0.87 for CSF Aβ42, CSF t-tau, and CSF p-tau (Additional file 1: Figure S1).
SNPs associated with plasma NFL levels
There were 527 individuals with plasma NFL data as mentioned above. After adjusting for age, gender and diagnosis, two SNPs (rs7943454, rs640476) were identified associated with plasma NFL at suggestive levels of P < 10− 5 (Fig. 1a, Table 2). No SNPs with genome-wide significant association with plasma NFL levels was identified in this study.
Fig. 1.
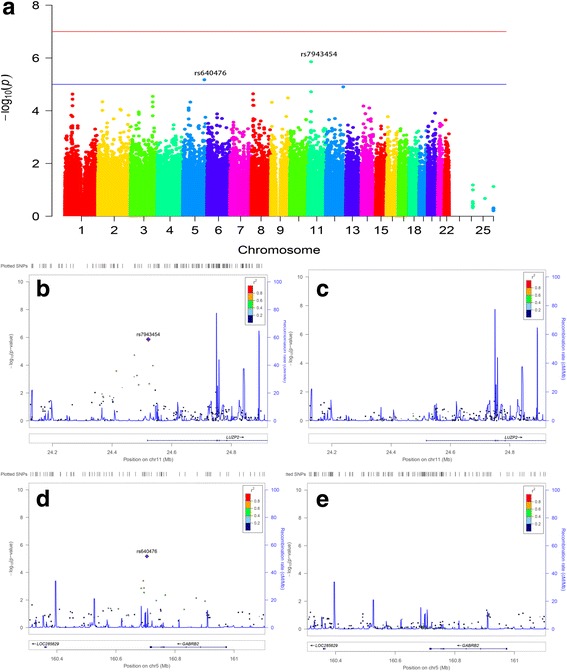
Manhattan and regional plots for associations with plasma NFL levels. a Genome-wide signal intensity (Manhattan) plots showing the –log10 (p-value) for individual SNPs. b Regional association results for the 24.2 Mb to 24.8 Mb region of chromosome 11. c Association results for 24.2 Mb to 24.8 Mb region of chromosome 11 controlling for rs7943454. d Regional association results for the 160.4 Mb to 161 Mb regions of chromosome 5. e Association results for 160.4 Mb to 161 Mb regions of chromosome 5 controlling for rs640476
Table 2.
Top SNPs associated with plasma NFL levels (P values < 10− 5)
| CHR | SNP | MAF | Closest Gene | SNP Type/Location | P values |
|---|---|---|---|---|---|
| 11 | rs7943454 | 0.460 | LUZP2 | intron | 1.39 × 10−6 |
| 5 | rs640476 | 0.297 | GABRB2 | intergenic | 6.71 × 10−6 |
Abbreviations: CHR chromosome, LUZP2 leucine zipper protein 2, MAF minor allele frequency, SNP single nucleotide polymorphism, GABRB2 gamma-aminobutyric acid type A receptor beta2 subunit
The minor allele of rs7943454 (T) was associated with higher plasma NFL levels in a dose-dependent effect in all diagnostic groups (Fig. 2a). In contrast, the minor allele of rs640476 (G) showed association with lower plasma NFL levels in a dose-dependent effect in all diagnostic groups except the MCI group (Fig. 2b).
Fig. 2.
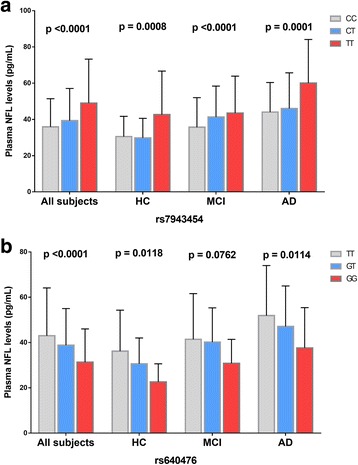
Mean plasma NFL levels of different diagnostic groups and genotypes. Mean and standard errors of plasma NFL levels are shown for groups defined by baseline diagnosis and genotypes. P < 0.05 was considered statistically significant after examination with a multiple linear regression model using age, gender and the diagnosis as covariates. a The minor alleleofrs7943454 (T) showed association with higher plasma NFL levels in a dose-dependent effect in all diagnostic groups. b The minor allele of rs640476 (G) showed association with lower plasma NFL levels in a dose-dependent effect in all diagnostic groups except the MCI group
SNPs mapped closely to the two suggestive SNPs (rs7943454, rs640476) regions were also analyzed. These nearby SNPs showed association with plasma NFL levels at P levels lower than 0.01. However, these SNPs associated with plasma NFL also disappeared after controlling the genotype of the two suggestive SNPs (Fig. 1b and c, Fig. 1d and e). The results indicated that these nearby SNPs were driven by the two suggestive SNPs.
Rs7943454 and onset risk of AD
The International Genomics of Alzheimer’s Disease Project (IGAP) is the largest genetic epidemiology investigation of AD risk to date. In 2013, the IGAP reported a grand-scale meta-analysis and identified 11 new susceptibility loci for AD [13]. The IGAP research was divided into a discovery step (stage 1) and a replication step (stage 2). We checked the two loci associated with plasma NFL in IGAP database in the stage 1 meta-analysis and identified rs7943454 as a risk locus for AD (P = 0.03476). The minor allele of rs7943454 (T) increased the onset risk of AD 1.547-fold in our analysis (odds ratio = 1.547, confidence interval 95% = 1.018–2.351).
Impact of rs7943454 on brain structure
Several cortical areas including middle temporal gyrus, posterior cingulate, precuneus, parahippocampal gyrus, and hippocampus were chosen as the ROI of the AD related MRI measures analysis. We analyzed the association of rs7943454 with AD related brain structures in a linear model using age, gender, education years, APOE ε4 status and intracranial volume (ICV) as covariates. There was no regional cortical volume associated with rs7943454 at baseline in the hybrid population (AD, MCI, and HC subjects) (Fig. 3a). However, rs7943454 increased the percentage of atrophy of right middle temporal gyrus in the hybrid population in the one-year follow-up research (P = 0.0234, Fig. 3b). Subjects with TT genotype had greater atrophy rate of right middle temporal gyrus than those with CC genotype (CC: 0.9880 ± 0.0622, CT: 0.9783 ± 0.0377, TT: 0.9537 ± 0.0433) (P = 0.05). There was no significant difference in the volume of right middle temporal gyrus between CT genotype and CC genotype or TT genotype.
Fig. 3.
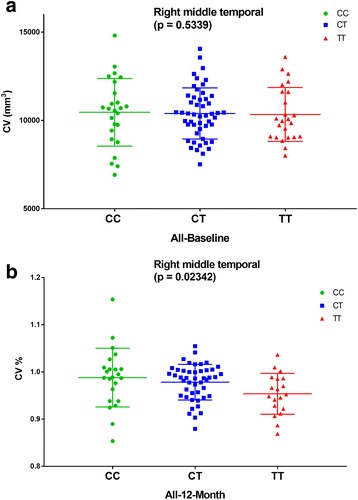
Rs7943454 and right middle temporal gyrus. a The volume of right middle temporal gyrus was not associated with rs7943454 at baseline in the whole cohort (P = 0.5339). b Rs7943454 increase the percentage of atrophy of right middle temporal gyrus in the whole cohort in the following-up research of 1 year (P = 0.0234). Subjects with TT genotypes had greater atrophy rate of right middle temporal gyrus than those with CC genotypes (P = 0.05)
Discussion
To our knowledge, this study was the first one using plasma NFL as an endophenotype of AD for GWAS. The use of quantitative traits in GWAS has been shown to increase statistical power over case-control designs [8]. We identified two SNPs (rs7943454, rs640476) associated with plasma NFL at suggestive levels. The minor allele (T) of rs7943454 within LUZP2 increased the onset risk of AD and was associated with atrophy of right middle temporal gyrus in the entire cohort of the one-year longitudinal study. The use of plasma NFL as an endophenotype of AD for GWAS enabled us to identify a novel AD candidate gene in addition to examining the influence of well-known AD genes on AD biomarkers. We also found the minor allele (T) of rs7943454 in LUZP2 was associated with higher plasma NFL levels in a dose-dependent fashion. In contrast, the minor allele (G) of rs640476 near GABRB2 was associated with lower plasma NFL at suggestive levels in a dose-dependent effect in all diagnostic groups except the MCI group.
Plasma NFL showed significant increase in AD patients than in MCI and healthy controls (P < 0.001). Similar with CSF NFL, plasma is not disease-specific and even more marked increases are found in several other neurodegenerative disorders. Increasing evidence indicates that plasma NFL is a potential biomarker for the progression of AD but not for the diagnosis [4, 14–16]. Plasma NFL is noninvasive and has diagnostic accuracy for AD in the same range as established CSF biomarkers [3]. Plasma NFL may be widely used as a biomarker in clinical studies and drug development of AD, although there is still a long way to go. The elevated NFL levels in plasma also indicate that the degeneration of large-caliber axons plays an important role in the progression of AD [14].
The variation rs7943454 is located on chromosome 11p14.3 within LUZP2 region. LUZP2 is a leucine zipper protein coding gene that has been reported to be deleted in some patients with Wilms tumor-Aniridia-Genitourinary anomalies-mental Retardation (WAGR) syndrome [17]. It has also been reported that LUZP2 was associated with prostate cancer and hypereosinophilic syndrome [18, 19]. The function of LUZP2 is still unclear and inhibition of its expression did not show any obvious abnormal phenotypes in mice [20]. Rs7943454 in LUZP2 has been reported as a risk locus of AD in IGAP, which has been validated in our analysis. The minor allele (T) of rs7943454 increased plasma NFL levels in a dose-dependent fashion and was associated with atrophy of right middle temporal gyrus in the hybrid population. Neuroimaging changes occur years before cognitive decline and middle temporal gyrusis identified as a critical region of memory that changes in the early stage of AD, followed by progressive neocortical damage. Atrophy of middle temporal gyrus is now considered to be a valid diagnostic marker in the early stage of AD [21]. Our study showed that the minor allele (T) of rs7943454 increased the atrophy rate of right middle temporal gyrus in the one-year follow-up study and the C-allele remarkably prevented its atrophy. This result further proved that rs7943454 was associated with the onset risk of AD and LUZP2 may be a new susceptibility gene of AD. However, we didn’t find any relation between rs7943454 and right middle temporal gyrus in subgroups due to the limited sample size.
The variation rs640476 is located on chromosome 5 q34, the intergenic region between GABRB2 (gamma-aminobutyric acid type A receptor beta2 subunit) and LOC285629 (also known as LINC02159, long intergenic non-protein coding RNA 2159). GABRB2 is the gene coding β2 subunit of γ-aminobutyric acid receptor type A (GABAA receptor), which is the major mediator of fast inhibitory synaptic transmission in the central nervous system. Mutations in GABRB2 genes have been reported to be associated with intellectual disability and epilepsy [22, 23]. A missense mutation in GABRB2 was also reported to be associated with early myoclonic encephalopathy [24]. Mutations in GABRB2 may reduce the expression of GABAA receptor and change the channel function, which could perturb GABA ergic inhibition in the brain. Disruption of excitatory-inhibitory (E/I) balance may be an important mechanism contributing to AD cognitive decline. Interestingly, despite vast neuronal loss in AD patients, GABA ergic neurons and receptors are relatively spared [25]. We have conducted a linkage disequilibrium analysis in order to annotate the identified functional variants. We found five loci which has strong linkage disequilibrium with rs7943454 (rs1509601: r2 = 0.81, D’ = 1; rs7927899: r2 = 0.81, D’ = 1; rs6484052: r2 = 0.99, D’ = 1; rs6484053: r2 = 0.8, D’ = 0.98; rs4922682: r2 = 0.91, D’ = 0.96). All these loci are also located in an intron region. We detected only one locus which has strong linkage disequilibrium with rs640476 (rs587875: r2 = 0.98, D’ = 1) and was also in an intergenic region.
Replication studies with independent, larger samples will be important to confirm these findings. In this study, we used a stringent MAF threshold (MAF > 0.20) and stringent Bonferroni corrections. These restrictions can improve statistical power to avoid false positive result but may miss less common SNPs. The modest number of subjects restricts stratified analyses for the three diagnostic groups. Besides, a two-year follow-up may be too short to observe the influence of rs7943454 on brain structure changes.
Conclusions
In summary, we identified that two SNPs (rs7943454 in LUZP2 and rs640476 near GABRB2) were associated with plasma NFL at suggestive levels. Rs7943454 in LUZP2 was associated with the onset risk of AD and atrophy of right middle temporal gyrus in the whole cohort. Using endophenotype-based approach, we identified rs7943454 as a new AD risk locus.
Additional file
Figure S1. Plasma neurofilament light for AD diagnosis. Receiver operating cuves of logistic regression model are controlled for age at baseline, gender, educational level and APOE ε4 genotype. (PDF 6 kb)
Acknowledgements
The authors thank scientists contributed in developing the clinical and genetic resources necessary to collect these data and complete this project. The authors also gratefully thank the efforts of hundreds of individuals whose help and participation made this work possible. Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).
Funding
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
This work was supported by grants from the Taishan Scholars Program of Shandong Province (ts201511109 and tsqn20161079), Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Qingdao Innovation and Entrepreneurship Leading Talent Program.
Availability of data and materials
Data are available to researchers by applying to the respective organizations, ADNI and IGAP. The ADNI data are available at (http://adni.loni.usc.edu/). The IGAP data are available at (http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php).
Abbreviations
- AD
Alzheimer disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- ANOVA
One-way analysis of variance
- APOE
Apolipoprotein E
- Aβ
amyloid-β
- CSF
Cerebrospinal fluid
- GABRB2
Gamma-aminobutyric acid type A receptor beta2 subunit
- GWAS
Genome-wide association study
- ICV
Intracranial volume
- IGAP
The International Genomics of Alzheimer’s Disease Project
- LUZP2
Leucine zipper protein 2 gene
- MAF
Minimum minor allele frequencies
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- NFL
Neurofilament light
- NFT
Neurofibrillary tangles
- PET
Positron emission tomography
- p-tau
Phosphorylated tau
- t-tau
Total-tau
- QC
Quality control
- ROI
Regions of interest
- SD
Standard deviations
- SNPs
Single nucleotide polymorphisms
- WAGR
Wilms tumor-Aniridia-Genitourinary anomalies-mental Retardation
Authors’ contributions
JQL, XZY, LT, WAC and JTY: design and conceptualization, analysis and interpretation of the data, drafting and revision of the manuscript. HYL: experimental implementation, data collection and analysis. JQL: experimental implementation, data analysis and revision of the manuscript. XPC: revision of the manuscript. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Regional ethical committees of all participating institutions approved the ADNI (https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf). All study participants or authorized representatives provided written informed consent. Ethics approval was obtained from the institutional review boards of each institution involved: Oregon Health and Science University; University of Southern California; University of California—San Diego; University of Michigan; Mayo Clinic, Rochester; Baylor College of Medicine; Columbia University Medical Center; Washington University, St. Louis; University of Alabama at Birmingham; Mount Sinai School of Medicine; Rush University Medical Center; Wien Center; Johns Hopkins University; New York University; Duke University Medical Center; University of Pennsylvania; University of Kentucky; University of Pittsburgh; University of Rochester Medical Center; University of California, Irvine; University of Texas Southwestern Medical School; Emory University; University of Kansas, Medical Center; University of California, Los Angeles; Mayo Clinic, Jacksonville; Indiana University; Yale University School of Medicine; McGill University, Montreal-Jewish General Hospital; Sunnybrook Health Sciences, Ontario; U.B.C.Clinic for AD & Related Disorders; Cognitive Neurology—St. Joseph’s, Ontario; Cleveland Clinic Lou Ruvo Center for Brain Health; Northwestern University; Premiere Research Inst (Palm Beach Neurology); Georgetown University Medical Center; Brigham and Women’s Hospital; Stanford University; Banner Sun Health Research Institute; Boston University; Howard University; Case Western Reserve University; University of California, Davis—Sacramento; Neurological Care of CNY; Parkwood Hospital; University of Wisconsin; University of California, Irvine—BIC; Banner Alzheimer’s Institute; Dent Neurologic Institute; Ohio State University; Albany Medical College; Hartford Hospital, Olin Neuropsychiatry Research Center; Dartmouth-Hitchcock Medical Center; Wake Forest University Health Sciences; Rhode Island Hospital; Butler Hospital; UC San Francisco; Medical University South Carolina; St. Joseph’s Health Care Nathan Kline Institute; University of Iowa College of Medicine; Cornell University; and University of South Florida: USF Health Byrd Alzheimer’s Institute.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Data used in preparation for this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at:http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Electronic supplementary material
The online version of this article (10.1186/s12920-018-0364-8) contains supplementary material, which is available to authorized users.
Contributor Information
Jie-Qiong Li, Email: qdljq891124@163.com.
Xiang-Zhen Yuan, Email: yuanxiangzhen@163.com.
Hai-Yan Li, Email: udahai007@163.com.
Xi-Peng Cao, Email: SophieCao1992@163.com.
Jin-Tai Yu, Phone: +86-532-8890-5659, Email: yu-jintai@163.com.
Lan Tan, Phone: +86-532-8890-5659, Email: dr.tanlan@163.com.
Wei-An Chen, Phone: +86-532-8890-5659, Email: wzanan@126.com.
References
- 1.Alzheimer's Assoc 2015 Alzheimer's disease facts and figures. Alzheimer’s Dement. 2015;11(3):332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer's disease: an update. Annu Rev Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- 3.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Alzheimer's disease neuroimaging I: Association of Plasma Neurofilament Light with Neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8(9):e75091. doi: 10.1371/journal.pone.0075091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang HF, Wan Y, Hao XK, Cao L, Zhu XC, Jiang T, Tan MS, Tan L, Zhang DQ, Tan L, et al. Bridging integrator 1 (BIN1) genotypes mediate Alzheimer's disease risk by altering neuronal degeneration. J Alzheimers Dis. 2016;52(1):179–190. doi: 10.3233/JAD-150972. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Yu JT, Wojta K, Wang HF, Zetterberg H, Blennow K, Yokoyama JS, Weiner MW, Kramer JH, Rosen H, et al. Genome-wide association study identifies MAPT locus influencing human plasma tau levels. Neurology. 2017;88(7):669–676. doi: 10.1212/WNL.0000000000003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, Shaw LM, Trojanowski JQ, Potkin SG, Huentelman MJ, et al. Genome-wide association study of CSF biomarkers Abeta1-42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76(1):69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruchaga C, Kauwe JS, Harari O, Jin SC, Cai Y, Karch CM, Benitez BA, Jeng AT, Skorupa T, Carrell D, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer's disease. Neuron. 2013;78(2):256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, et al. The Alzheimer's disease neuroimaging initiative: a review of papers published since its inception. Alzheimers Dement. 2013;9(5):e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R, Cardoso MJ, Ourselin S, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87(13):1329–1336. doi: 10.1212/WNL.0000000000003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, LW J, Ward C, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K. Alzheimer's disease neuroimaging I: association of cerebrospinal fluid Neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73(1):60–67. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, Blennow K, Hansson O. Alzheimer's disease neuroimaging I: cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO Mol Med. 2016;8(10):1184–1196. doi: 10.15252/emmm.201606540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 17.Fischbach BV, Trout KL, Lewis J, Luis CA, Sika M. WAGR syndrome: a clinical review of 54 cases. Pediatrics. 2005;116(4):984–988. doi: 10.1542/peds.2004-0467. [DOI] [PubMed] [Google Scholar]
- 18.Kjeldsen E. A novel acquired cryptic three-way translocation t(2;11;5)(p21.3;q13.5;q23.2) with a submicroscopic deletion at 11p14.3 in an adult with hypereosinophilic syndrome. Exp Mol Pathol. 2015;99(1):50–55. doi: 10.1016/j.yexmp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Zhao Y, Wang L, Zhang J, Karnes RJ, Kohli M, Wang G, Huang H. Alterations of androgen receptor-regulated enhancer RNAs (eRNAs) contribute to enzalutamide resistance in castration-resistant prostate cancer. Oncotarget. 2016;7(25):38551–38565. doi: 10.18632/oncotarget.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Michaud EJ, Johnson DK. Cloning, functional study and comparative mapping of Luzp2 to mouse chromosome 7 and human chromosome 11p13-11p14. Mamm Genome. 2003;14(5):323–334. doi: 10.1007/s00335-002-2248-6. [DOI] [PubMed] [Google Scholar]
- 21.Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava S, Cohen J, Pevsner J, Aradhya S, McKnight D, Butler E, Johnston M, Fatemi A. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am J Med Genet A. 2014;164A(11):2914–2921. doi: 10.1002/ajmg.a.36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirose S. Mutant GABA(a) receptor subunits in genetic (idiopathic) epilepsy. Prog Brain Res. 2014;213:55–85. doi: 10.1016/B978-0-444-63326-2.00003-X. [DOI] [PubMed] [Google Scholar]
- 24.Ishii A, Kang JQ, Schornak CC, Hernandez CC, Shen W, Watkins JC, Macdonald RL, Hirose S. A de novo missense mutation of GABRB2 causes early myoclonic encephalopathy. J Med Genet. 2017;54(3):202–211. doi: 10.1136/jmedgenet-2016-104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rissman RA, Mobley WC. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer's disease. J Neurochem. 2011;117(4):613–622. doi: 10.1111/j.1471-4159.2011.07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Plasma neurofilament light for AD diagnosis. Receiver operating cuves of logistic regression model are controlled for age at baseline, gender, educational level and APOE ε4 genotype. (PDF 6 kb)
Data Availability Statement
Data are available to researchers by applying to the respective organizations, ADNI and IGAP. The ADNI data are available at (http://adni.loni.usc.edu/). The IGAP data are available at (http://web.pasteur-lille.fr/en/recherche/u744/igap/igap_download.php).


