Abstract
Rationale: Staphylococcus aureus is commonly cultured from the sputum of patients with bronchiectasis; however, little is known about the prevalence of the organism in these patients, the characteristics of patients who have grown the organism, or its implications.
Objectives: Determine the relationship between S. aureus and pulmonary function, frequency of exacerbations, and frequency of hospitalization in patients with bronchiectasis
Methods: The Bronchiectasis Research Registry is a database of adults with non–cystic fibrosis bronchiectasis identified from 13 sites within the United States. Baseline and follow-up demographic, spirometric, microbiologic, and therapeutic data were entered into a central web-based database. Patients were grouped into three cohorts based on their previous respiratory cultures at the time of entry into the Registry: 1) no prior S. aureus or glucose-nonfermenting gram-negative bacilli (NF-GNB) (Pseudomonas, Stenotrophomonas, or Burkholderia spp.); 2) prior S. aureus at least once; or 3) no prior S. aureus but prior NF-GNB at least once. The association between S. aureus isolation and pulmonary function and frequency of exacerbations and hospital admissions was assessed, both at baseline and after 1 year of follow-up.
Results: S. aureus was cultured from 94 of 830 patients (11.3%) included in the analysis. Patients who had grown S. aureus before entry into the Registry had a frequency of prior exacerbations and baseline pulmonary function that was between that of patients who had grown NF-GNB and those who had grown neither NF-GNB or S. aureus. Similarly, at the first follow-up visit after study entry, patients who had grown S. aureus had a frequency of exacerbations and hospitalizations that was between those of patients who had grown NF-GNB and those who had grown neither NF-GNB nor S. aureus. However, in multivariate analysis, S. aureus was not associated with pulmonary function, frequency of exacerbation, or hospital admissions. There were no significant differences in patient characteristics or outcomes between patients who had methicillin-sensitive and methicillin-resistant S. aureus.
Conclusions: Staphylococcus aureus does not appear to be an independent risk factor for severe disease in patients with bronchiectasis enrolled in the Bronchiectasis Research Registry.
Keywords: bronchiectasis, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus
Bronchiectasis is a condition characterized by irreversible dilatation of the airways with chronic cough and sputum production. Most patients have chronic airway infection leading to recurrent exacerbations, thus creating a “vicious cycle” of inflammation, infection, and progressive airway damage (1). It is well known that patients with bronchiectasis and chronic Pseudomonas infection generally have more severe disease and a worse prognosis compared with patients without Pseudomonas infection (2). Less is known about the relationship between infection with other specific organisms and the severity and prognosis of bronchiectasis, although Haemophilus influenzae, the most commonly isolated organism in patients with bronchiectasis, is associated with less severe disease than Pseudomonas (3). Patients who have had isolation of any pathogenic organism are at greater risk of mortality and exacerbations than patients who have never grown a pathogen (4).
Staphylococcus aureus is sometimes isolated from the respiratory secretions of patients with bronchiectasis (3–6). However, there are limited data describing the characteristics of patients infected with S. aureus or the clinical implications of such infection, and the reported frequency of isolation has been variable (6). The limited data suggest that the organism may be associated with more severe disease (4), and unrecognized cystic fibrosis and allergic bronchopulmonary aspergillosis (5); however, no studies have included large numbers of patients with S. aureus. Data regarding the prevalence and effects of methicillin-resistant S. aureus are also limited. The increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in cystic fibrosis (CF) (7, 8), and its correlation with worsened outcomes (9), raises concern regarding this pathogen in bronchiectasis.
The U.S. Bronchiectasis Research Registry (BRR) collects data on patients with non–cystic fibrosis from 13 participating sites across the country (10). To better understand the prevalence and epidemiology of S. aureus in bronchiectasis, as well as the relationship between S. aureus and the severity and prognosis of bronchiectasis, we compared subjects enrolled in the BRR with and without S. aureus infection with respect to severity of disease and short-term prognosis.
Methods
The BRR is a centralized database of patients with non–cystic fibrosis bronchiectasis identified at 13 clinical sites throughout the United States (see https://www.copdfoundation.org/Research/Bronchiectasis-Research-Registry/Learn-More.aspx). The BRR is sponsored by the COPD Foundation, a 501(c) (3) nonprofit organization. Patients were enrolled beginning in 2008 and follow-up data may be submitted yearly for as long as patients consent to remain in the Registry. At the time that the analyses in this article were performed in 2013, 1,467 patients had been enrolled. Adult patients with a physician-established diagnosis of non–cystic fibrosis bronchiectasis were eligible for inclusion. The institutional review board of each participating site approved the study as did an administrative institutional review board for the data-collecting center. After providing informed consent, enrolled subjects and/or their medical records were queried by a study coordinator or principal investigator to obtain demographic, clinical, laboratory, microbiologic, spirometric, imaging, and treatment information, using standardized recording forms. Cystic fibrosis status was established on the basis of clinical history, sweat chloride results, and/or genetic testing results at the time of enrollment into the BRR. Exacerbations were recorded on the basis of historical information and were identified on the basis of the definition of O’Donnell and colleagues (11). Data were entered through a centralized web-based entry system at the University of North Carolina. Spirometry pre- and post-bronchodilator results were abstracted from patient records.
Microbiology
A maximum of three respiratory culture results were abstracted between the 2 years before, and 90 days after, enrollment, defined as the baseline period. We calculated the prevalence of positive microbial cultures for all patients with culture information during the baseline period, and we further stratified these results on the basis of patient nontuberculous mycobacteria (NTM) status. For the purposes of this analysis, we defined “patients with NTM” as those with either a reported history of pulmonary NTM disease before enrollment and/or those with one or more NTM isolates in respiratory specimen cultures within the baseline period.
Study Population
Only patients who had at least one sputum culture during the baseline period were included in the study. Patients were placed into one of three cohorts, based on their baseline sputum culture results: 1) the S. aureus cohort if any baseline culture grew S. aureus; 2) the no Pseudomonas, Burkholderia, Stenotrophomonas, no S. aureus cohort if none of these organisms were isolated from baseline cultures; or 3) the Pseudomonas, Burkholderia, Stenotrophomonas cohort if one of these organisms was cultured, but not S. aureus. Patients with Stenotrophomonas were grouped with patients with Pseudomonas on the basis of studies in cystic fibrosis demonstrating worsened outcomes in patients infected with Stenotrophomonas, similar to what is seen with Pseudomonas (12–14).
Patients with S. aureus were subdivided into MRSA and methicillin-susceptible Staphylococcus aureus (MSSA) for further analyses. Patients not placed into one of the three cohorts were excluded, as well as patients who had at least one sweat chloride result equal to or greater than 60 mEq/L or two cystic fibrosis transmembrane conductance receptor mutations detected by genetic testing. The study population was also restricted to patients who had a follow-up visit within 3 years of their baseline visit.
Statistical Analyses
The three cohorts were compared by χ2 tests for categorical variables and Kruskal–Wallis tests for continuous variables. For those comparisons that revealed a statistically significant difference, Dunn’s nonparametric post hoc test was used for continuous variables, and comparison of standardized residuals was used for categorical variables, to determine which of the three cohorts differed significantly from the others. For the comparison of MRSA versus MSSA, Fisher’s exact tests and Wilcoxon rank-sum tests were used for categorical and continuous variables, respectively. Poisson regression was used to further investigate the relationship between patients’ baseline culture status and the frequency of hospitalizations and exacerbations after enrollment. To control for overdispersion, we included a multiplicative scale factor on the variance. Linear regression was used to analyze the association between baseline culture status and change in FEV1 after enrollment. All three models were adjusted for baseline characteristics including sex, age at enrollment, smoking status, whether the subject had any pulmonary exacerbations in the 2 years before baseline, the prebronchodilator FEV1 measurement (L) at baseline, and the number of days between baseline and either the first follow-up visit (for exacerbations and hospitalizations) or the first follow-up FEV1 measurement.
Results
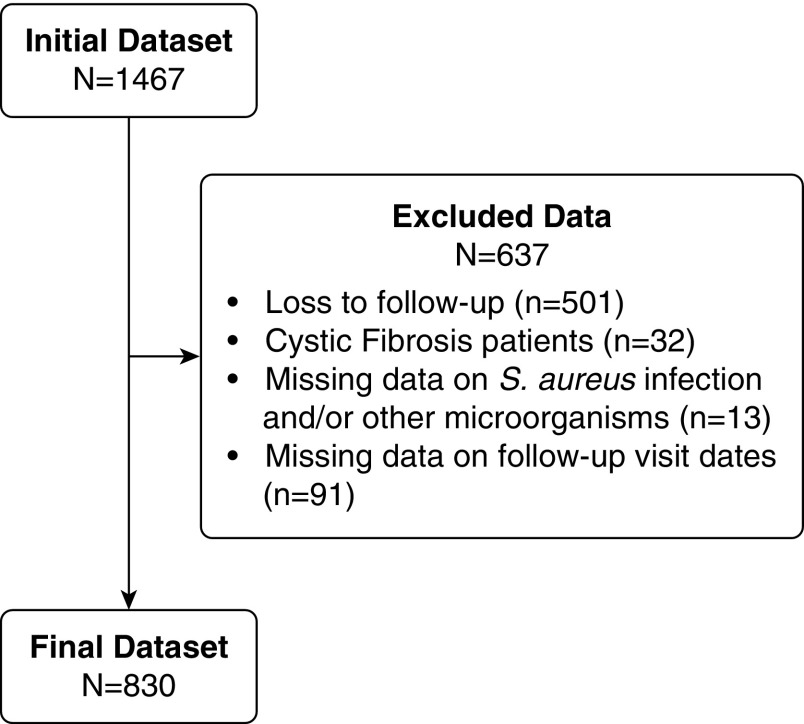
We included 830 subjects in the study (Figure 1). Table 1 demonstrates the demographic data and clinical characteristics of the study population. As can be seen, nontuberculous mycobacterial (NTM) infection was common in this population, reflecting selection bias due to expertise in NTM infection at several of the institutions participating in the BRR. The population is predominantly female with a mean age of 64 ± 14 years. Most patients had suffered from an exacerbation during the 2 years before enrollment, and the mean FEV1 was 70% of predicted.
Figure 1.
Patient inclusions and exclusions. S. aureus = Staphylococcus aureus.
Table 1.
Demographics and baseline characteristics of participants with at least one bacterial culture at baseline
| Total (N) | Overall (N = 830) | |
|---|---|---|
| Sex, n (%) | 829 | |
| Female | 681 (82) | |
| Age, yr | 828 | |
| Mean (SD) | 64 (14) | |
| Age at diagnosis, yr | 734 | |
| Mean (SD) | 56 (17) | |
| Prior diagnoses, n (%) | ||
| Ever had a tuberculosis diagnosis | 806 | 26 (3) |
| Ever had a positive pulmonary nontuberculous mycobacterial culture | 804 | 471 (59) |
| Ever diagnosed with alpha-1 antitrypsin deficiency | 804 | 24 (3) |
| Ever diagnosed with a primary immunodeficiency | 809 | 46 (6) |
| Ever diagnosed with Kartagener’s or primary ciliary dyskinesia | 814 | 42 (5) |
| Ever diagnosed with a rheumatologic disease | 804 | 69 (9) |
| Ever diagnosed with ulcerative colitis or Crohn’s disease | 814 | 22 (3) |
| Ever diagnosed with allergic bronchopulmonary mycosis | 48 | 5 (10) |
| Tobacco use, n (%) | 826 | |
| Past or present | 317 (38) | |
| Never | 509 (62) | |
| Pulmonary exacerbations in the past 2 yr, n (%) | 796 | |
| Yes | 536 (67) | |
| Prebronchodilator FEV1 (L) at entry | 750 | |
| Mean (SD) | 1.79 (0.72) | |
| Prebronchodilator FEV1 (% predicted) at entry | 740 | |
| Mean (SD) | 70 (22) |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; SD = standard deviation.
Table 2 shows the baseline patient characteristics in the three study cohorts. Among the 830 included patients, 94 (11.3%) grew S. aureus at least once during their baseline period. Thirty-two (34%) of these patients also grew either Pseudomonas, Stenotrophomonas, or Burkholderia. Among the 299 patients in the Pseudomonas, Stenotrophomonas, or Burkholderia group, 272 grew Pseudomonas, 39 grew Stenotrophomonas, and 2 grew Burkholderia (sum is greater than 299 as some patients grew more than one of these organisms).
Table 2.
Demographics and baseline characteristics of study sample
| Group 1: No Staphylococcus aureus; No Pseudomonas, Stenotrophomonas, or Burkholderia at Baseline (N = 437) | Group 2: Staphylococcus aureus at Baseline (N = 94) | Group 3: Pseudomonas, Stenotrophomonas, or Burkholderia; No Staphylococcus aureus at Baseline (N = 299) | P Value for χ2 | |
|---|---|---|---|---|
| Sex, n (%) | N = 437 | N = 93 | N = 299 | 0.0375* |
| Female | 373 (85) | 74 (80) | 234 (78) | |
| Age, yr | N = 436 | N = 93 | N = 299 | 0.6941 |
| Mean (SD) | 64 (13) | 63 (14) | 64 (15) | |
| Age at diagnosis, yr | N = 389 | N = 87 | N = 258 | 0.1645 |
| Mean (SD) | 57 (16) | 54 (18) | 54 (18) | |
| Tobacco use, n (%) | N = 434 | N = 93 | N = 299 | 0.6447 |
| Past or present | 166 (38) | 32 (34) | 119 (40) | |
| Never | 268 (62) | 61 (66) | 180 (60) | |
| Pulmonary exacerbations in the past 2 yr, n (%) | N = 418 | N = 91 | N = 287 | <0.0001* |
| Yes | 240 (57) | 69 (76) | 227 (79) | |
| Prebronchodilator FEV1 (L) at entry | N = 392 | N = 87 | N = 271 | 0.0003* |
| Mean (SD) | 1.88 (0.71) | 1.81 (0.71) | 1.67 (0.72) | |
| Prebronchodilator FEV1 (% predicted) at entry | N = 387 | N = 87 | N = 266 | <0.0001* |
| Mean (SD) | 74 (22) | 70 (22) | 64 (22) |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; SD = standard deviation.
P values are based on χ2 tests for categorical variables and Kruskal–Wallis tests for continuous variables.
Denotes statistically significant difference between group 1 and group 3 based on post hoc Dunn’s comparison for continuous variables and using standardized residuals for categorical variables.
Patients who grew S. aureus were less likely to be female than those without growth of the targeted gram-negative pathogens, but similar to the other groups with respect to age at enrollment and age at diagnosis of bronchiectasis. Nonetheless, patients who grew S. aureus were more likely to have had a pulmonary exacerbation during the prior 2 years and had a somewhat lower mean prebronchodilator FEV1 than the group that had not grown any of the targeted pathogens. Antimicrobial sensitivities were available for 67 of the patients who grew S. aureus. In 22 (33%), at least one S. aureus isolate was MRSA. There were no significant differences between patients who grew MRSA versus MSSA with respect to sex distribution, age at enrollment, age at diagnosis, tobacco use, number of exacerbations during the prior 2 years, or prebronchodilator FEV1 (see Table E1 in the online supplement). Among the 94 patients who had grown S. aureus at baseline, 43 (46%) had data from at least one subsequent culture available at the time of the first follow-up visit. Among these 43 patients, 22 (51%) grew S. aureus at least once.
Table 3 demonstrates the frequency of exacerbations and hospitalizations and change in FEV1 after enrollment. Patients who had S. aureus cultured during the baseline period had mean rates of exacerbation and hospitalization that were between those of patients who grew none of the targeted pathogens and those who grew the targeted gram-negative pathogens. There was no significant difference in the amount of decline in FEV1 between the three groups. Multivariable analysis (Table 4) demonstrated that after adjustment for patient characteristics and length of time between the baseline visit and the first follow-up visit, there were no significant differences in outcomes between patients who grew S. aureus and those who grew none of the targeted pathogens, for number of exacerbations (rate ratio, 1.20; 95% confidence interval [CI], 0.82–1.76), for number of hospitalizations (rate ratio, 0.98; 95% CI, 0.49–1.98), or for mean change in FEV1, –0.06 L (95% CI, –0.17 to 0.06). There were no differences in these outcomes between patients with MRSA versus those with MSSA, although our statistical power to detect such differences was limited because of the small numbers (Table E2).
Table 3.
Follow-up data on pulmonary function, exacerbations, and hospitalizations
| Group 1: No Staphylococcus aureus; No Pseudomonas, Stenotrophomonas, or Burkholderia at Baseline (N = 437) | Group 2: Staphylococcus aureus at Baseline (N = 94) | Group 3: Pseudomonas, Stenotrophomonas, or Burkholderia and No Staphylococcus aureus at Baseline (N = 299) | P Value for χ2 | |
|---|---|---|---|---|
| No. of exacerbations between study entry and first F/U visit | N = 365 | N = 81 | N = 234 | <0.0001*† |
| Mean (SD) | 0.7 (1.2) | 0.9 (1.0) | 1.2 (1.6) | |
| No. of hospitalizations between study entry and first F/U visit | N = 364 | N = 81 | N = 235 | <0.0001* |
| Mean (SD) | 0.1 (0.5) | 0.2 (0.5) | 0.3 (0.5) | |
| Change in prebronchodilator FEV1 (L) from study entry to first F/U visit | N = 191 | N = 41 | N = 129 | 0.7369 |
| Mean (SD) | 0.00 (0.37) | −0.02 (0.33) | −0.05 (0.31) |
Definition of abbreviation: FEV1 = forced expiratory volume in 1 second; F/U = follow-up; SD = standard deviation.
P values are based on χ2 tests for categorical variables and Kruskal–Wallis tests for continuous variables.
Denotes statistically significant difference between group 1 and group 3 based on post hoc Dunn’s comparison for continuous variables and using standardized residuals for categorical variables.
Denotes statistically significant difference between group 1 and group 2 based on post hoc Dunn’s comparison for continuous variables and using standardized residuals for categorical variables.
Table 4.
Unadjusted and adjusted estimates* and 95% confidence intervals comparing patients with baseline Staphylococcus aureus infection versus with no baseline S. aureus, Pseudomonas, Stenotrophomonas, or Burkholderia
| Number of Exacerbations | Number of Hospitalizations | Change in FEV1 (L) | |
|---|---|---|---|
| Unadjusted RR (95% CI) | 1.38 (0.95–1.98) | 1.33 (0.61–2.88) | — |
| Adjusted† RR (95% CI) | 1.20 (0.82–1.76) | 0.98 (0.49–1.98) | — |
| Unadjusted difference in mean change (95% CI) | — | — | −0.02 (–0.13 to 0.10) |
| Adjusted‡ difference in mean change (95% CI) | — | — | −0.06 (–0.17 to 0.06) |
Definition of abbreviations: CI = confidence interval; FEV1 = forced expiratory volume in 1 second; RR = rate ratio.
Estimates for the number of exacerbations and number of hospitalizations are incidence rate ratios for group 2 (S. aureus at baseline) versus group 1 (no S. aureus and no Pseudomonas, Stenotrophomonas, or Burkholderia at baseline); the estimate for the change in FEV1 is the difference in the mean change in FEV1 for the same groups.
Adjusted for sex, age, smoking status, whether the subject had any pulmonary exacerbations in the 2 years before baseline, the prebronchodilator FEV1 measurement (L) at baseline, and length of time between the baseline visit and the first follow-up visit.
Adjusted for sex, age, smoking status, whether the subject had any pulmonary exacerbations in the 2 years before baseline, the prebronchodilator FEV1 measurement (L) at baseline, and length of time between the baseline visit and the first follow-up FEV1 measurement.
We repeated the analyses shown in Tables 2 and 3, after excluding from the S. aureus group the 32 patients who had grown both S. aureus and Pseudomonas, Stenotrophomonas, or Burkholderia before enrollment in the Registry and found no significant changes in the results when compared with the analyses presented (results not presented).
Discussion
In this study of 830 BRR patients with non–CF bronchiectasis, we found that 11.3% of the patients had S. aureus isolated from their sputum at least once during the period encompassing 2 years before through 90 days after their initial study visit. Approximately one-third of these isolates were MRSA. The frequency of S. aureus infection we found was similar to some prior reports. Miao and colleagues performed a meta-analysis of studies assessing the frequency of pathogen isolation in bronchiectasis and found that 12% of patients with a pathogen isolated from sputum grew S. aureus, whereas only 5% of positive cultures from bronchoalveolar lavage samples were S. aureus (6). Shah and colleagues isolated S. aureus from 28 of 74 patients (37.8%) (5). These results appear to represent an outlier, perhaps because only patients with multiple sputum cultures were enrolled, likely increasing the chances of detecting temporary infection. Only 12 (16.2%) were chronically infected. Others have reported a lower prevalence of S. aureus isolates, of 4–7% (3, 15, 16). With respect to MRSA, most studies have not distinguished between MSSA and MRSA in their reported data. However, in the multicenter European cohort studied by Chalmers and colleagues, S. aureus was cultured from 51 of 608 (8.4%), of which 8 (15.7%) were MRSA (4).
Although patients in our study who grew S. aureus were demographically similar to patients in the other groups, they were more likely to have had an exacerbation before study enrollment, and had a lower FEV1 than patients who had grown neither S. aureus or Pseudomonas/Stenotrophomonas/Burkholderia. During follow-up, they had a higher unadjusted rate of exacerbation and hospitalization, but these differences were not present after adjustment for baseline characteristics and time between baseline and first follow-up visits. These results suggest that colonization or infection with S. aureus might be a marker for more severe disease, but does not support a causal link between S. aureus and worsened outcomes for patients with bronchiectasis. There were no significant differences in patient characteristics or short-term outcomes between patients who grew MRSA versus those who grew MSSA, although our statistical power to detect such differences was limited by the relatively small sample size.
There are some limitations of our study. Our study population consisted of patients being seen at specialized bronchiectasis centers, and had a large percentage of patients who had previously had a positive nontuberculous mycobacterial culture and thus are likely not representative of all U.S. patients with bronchiectasis . The culture data were obtained from samples that had been obtained for clinical indications; no cultures were done solely for the BRR. The performance of repeated cultures on a scheduled basis might have detected S. aureus more frequently, as there can be transient infection/colonization. A substantial number of patients were lost to follow-up after the baseline visit, and this might have biased the results. Had we monitored patients for a longer time period than just the first follow-up visit, we might have seen more significant differences within the patient population. Because not all patients had cultures performed after the baseline visit, we cannot distinguish between some patients who had transient S. aureus colonization versus chronic infection. Furthermore, documentation of CF testing was not available for a substantial percentage of subjects, although it seems unlikely that CF would be commonly missed in the specialized centers participating in the Registry. Strengths of our study include the large cohort of patients enrolled from 13 sites across the United States, and the collection of data from the enrolling site, other treating physicians, and direct patient interview.
Because of the similarity between CF and non–cystic fibrosis bronchiectasis, and the paucity of high-quality data regarding assessment and treatment decisions for patients with non–cystic fibrosis bronchiectasis, such decisions are often extrapolated from data obtained from patients with CF. Thus, it is appropriate to compare our results with those obtained from patients with CF. S. aureus is often the first bacteria infecting patients with CF, in contrast to our finding that patients with and without S. aureus were similar in age. The effect of S. aureus infection in patients with CF is debated (9). Although such infection can be associated with increased levels of inflammatory markers, and worsened nutritional status, the significance of these findings is unclear, in part because attempts to eradicate the organism can lead to earlier infection with Pseudomonas (9, 17, 18). We noted more frequent prior exacerbations in patients with bronchiectasis with S. aureus, but no evidence of worsened outcomes or more rapid loss of pulmonary function. Finally, although we did not see any evidence of differences in patients with non–cystic fibrosis bronchiectasis infected with MRSA versus MSSA, in CF, MRSA is associated with worse lung function and more frequent hospitalizations (9). The apparent differences between the significance of S. aureus infection in CF versus non–cystic fibrosis bronchiectasis provide further evidence that treatment decisions for patients with non–cystic fibrosis bronchiectasis that are based solely on extrapolation from CF studies may not be appropriate.
In summary, in this study of 830 patients enrolled in the BRR, S. aureus was isolated from 11% of subjects at baseline, and approximately one-third of these isolates were MRSA. Subjects who had S. aureus isolated had a greater number of exacerbations during the year before enrollment and a lower FEV1 than patients who grew neither S. aureus nor Pseudomonas/Stenotrophomonas/Burkholderia. During the follow-up period, after adjustment for patient characteristics, there was no significant difference in the rate of decline in FEV1, or the frequency of exacerbations or hospital admissions between subjects with S. aureus and subjects who grew none of these organisms. These results suggest that in patients with bronchiectasis, isolation of S. aureus might be a marker for more severe disease, but do not support S. aureus being a driver of more severe disease.
Supplementary Material
Footnotes
Supported by the COPD Foundation.
Author Contributions: All authors contributed in each of the following manners: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Moulton BC, Barker AF. Pathogenesis of bronchiectasis. Clin Chest Med. 2012;33:211–217. doi: 10.1016/j.ccm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–1611. doi: 10.1513/AnnalsATS.201506-333OC. [DOI] [PubMed] [Google Scholar]
- 3.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index: an international derivation and validation study. Am J Respir Crit Care Med. 2014;189:576–585. doi: 10.1164/rccm.201309-1575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah PL, Mawdsley S, Nash K, Cullinan P, Cole PJ, Wilson R. Determinants of chronic infection with Staphylococcus aureus in patients with bronchiectasis. Eur Respir J. 1999;14:1340–1344. doi: 10.1183/09031936.99.14613409. [DOI] [PubMed] [Google Scholar]
- 6.Miao XY, Ji XB, Lu HW, Yang JW, Xu JF. Distribution of major pathogens from sputum and bronchoalveolar lavage fluid in patients with noncystic fibrosis bronchiectasis: a systematic review. Chin Med J (Engl) 2015;128:2792–2797. doi: 10.4103/0366-6999.167360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller LR, Waters JH, Provost C. Mechanism of hyperchloremic metabolic acidosis. Anesthesiology. 1996;84:482–483. doi: 10.1097/00000542-199602000-00044. [DOI] [PubMed] [Google Scholar]
- 8.Cystic Fibrosis Foundation Patient Registry. Patient registry annual data report. 2013 Bethesda, MD: Cystic Fibrosis Foundation; 2013 [accessed 2017 Jan 23]. Available from: https://www.cff.org/2013_CFF_Patient_Registry_Annual_Data_Report.pdf.
- 9.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros. 2011;10:298–306. doi: 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, et al. Bronchiectasis Research Registry Consortium. Adult bronchiectasis patients: a first look at the U.S. Bronchiectasis Research Registry. Chest. 2017;151:982–992. doi: 10.1016/j.chest.2016.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell AE, Barker AF, Ilowite JS, Fick RB. rhDNase Study Group. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. Chest. 1998;113:1329–1334. doi: 10.1378/chest.113.5.1329. [DOI] [PubMed] [Google Scholar]
- 12.Waters V, Yau Y, Prasad S, Lu A, Atenafu E, Crandall I, et al. Stenotrophomonas maltophilia in cystic fibrosis: serologic response and effect on lung disease. Am J Respir Crit Care Med. 2011;183:635–640. doi: 10.1164/rccm.201009-1392OC. [DOI] [PubMed] [Google Scholar]
- 13.Cogen J, Emerson J, Sanders DB, Ren C, Schechter MS, Gibson RL, et al. EPIC Study Group. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol. 2015;50:763–770. doi: 10.1002/ppul.23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters V, Atenafu EG, Lu A, Yau Y, Tullis E, Ratjen F. Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients. J Cyst Fibros. 2013;12:482–486. doi: 10.1016/j.jcf.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Li AM, Sonnappa S, Lex C, Wong E, Zacharasiewicz A, Bush A, et al. Non–CF bronchiectasis: does knowing the aetiology lead to changes in management? Eur Respir J. 2005;26:8–14. doi: 10.1183/09031936.05.00127704. [DOI] [PubMed] [Google Scholar]
- 16.Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108:955–961. doi: 10.1378/chest.108.4.955. [DOI] [PubMed] [Google Scholar]
- 17.Ahlgren HG, Benedetti A, Landry JS, Bernier J, Matouk E, Radzioch D, et al. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm Med. 2015;15:67. doi: 10.1186/s12890-015-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.