Abstract
Objectives:
Ninety percent of head and neck cancers are squamous cell carcinoma which develops in the oral cavity. Metastasis is the main causative factor for death in 90% of all cancer-related deaths and begins with the invasion of tumor cells through the walls of small blood vessels or lymph vessels. A growing body of evidence has shown that vasculogenic mimicry (VM) facilitates tumor growth and cancer metastasis. The current study aimed to present the role of vascular endothelial (VE)-cadherin, CD44, and vimentin in inducing VM and epithelial-mesenchymal transition (EMT) and to identify the cancer stem cell (CSC) niche in different grades of oral squamous cell carcinoma (OSCC).
Materials and Methods:
A total of 63 OSCC samples (21 samples each grade) were collected from the archive of Pathology Department of Besat educational hospital, Hamadan, Iran, from 2000 to 2015. VE-cadherin, CD44, and vimentin/periodic acid–Schiff (PAS) double-staining were used to validate VM. VM was identified by the detection of PAS-positive loops surrounded by tumor cells. Chi-square test was used to examine the differences between the variables. Significant level was set at 0.05. Pearson's correlation was used to assess the co-localization of the markers.
Results:
There were statistically significant differences between tumor grade and the expression levels of VE-cadherin, CD44, and vimentin (P = 0.000). In addition, significant differences were found between tumor grade and microvessel density (P = 0.000) and between tumor grade and VM (P = 0.000).
Conclusion:
Our results may disclose a definite relationship between VE-cadherin, CD44 and vimentin expression levels, VM formation, EMT, CSCs, and microvessel count in OSCC samples. For this reason, it is suggested that VE-cadherin, CD44, and vimentin are related to angiogenesis and VM formation in OSCC, therefore, in tumor progression and metastasis. Recently, antitumor angiogenic therapies have been challenged. The presence of VM may explain the failure of antiangiogenic treatments.
Keywords: CD44, oral squamous cell carcinoma, vasculogenic mimicry, vascular endothelial-cadherin, vimentin
INTRODUCTION
Head and neck cancer is the sixth most common cancer in the world.[1] Ninety percent of head and neck cancers are squamous cell carcinoma which develops in the oral cavity.[2,3] Metastasis is the main causative factor for death in 90% of all cancer-related deaths[4,5,6] and begins with the invasion of tumor cells through the walls of small blood vessels or lymph vessels.[7,8] Then, cancer cells settle into a niche to promote proliferation and vasculogenesis.[7,9] Folkman first proposed a theory regarding tumor angiogenesis. According to this theory, a tumor forms new vasculature from existing blood vessels.[10] Maniotis et al. indicated that the vascular-like channels which function as tumor blood vessels were formed in melanoma. This phenomenon was called “vasculogenic mimicry” (VM).[11] A growing body of evidence has shown that VM formation facilitates tumor growth and cancer metastasis.[12] Previously published works have shown that VM indicates a poor prognosis in oral squamous cell carcinoma (OSCC).[13] Vascular endothelial-cadherin (VE-cadherin), an adhesive protein, promotes cell-to-cell interaction. Recently, VE-cadherin has been demonstrated in both endothelial cells and highly aggressive melanoma cells.[14] Overexpression level of VE-cadherin enhances the cancer neovascularization, growth, and progression.[15,16]
On the other hand, a small subset of tumor cells involves in cancer development. These cells, cancer stem cells (CSCs), not only are able to reproduce the whole phenotype of the original tumor but also are capable of self-renewal.[17] It is suggested that CSCs may also be involved in vascular formation in cancers.[18] Previous publications have shown that CSCs can induce epithelial-mesenchymal transition (EMT) to promote tumor cell invasion and metastasis.[19] CD44, a cell-surface glycoprotein, involves in cell-cell interactions, cell migration, and adhesion. For this reason, it plays a major role in tumorigenesis and metastasis.[20] Furthermore, CD44 has been described as a CSC marker in head and neck squamous cell carcinoma (HNSCC) and can re-establish the tumor heterogeneity.[21] Increased CD44 expression level has been detected in cancers such as HNSCC.[22] There are controversial results regarding to CD44 expression level in OSCC and lymph node metastasis. While Fonseca et al, and Lindquist et al. indicated a positive relationship between high CD44 expression and lymph-node metastasis,[23,24] Mostaan et al., and Rodrigo et al. found a correlation between low CD44 expression and capability for metastasis.[25,26]
Vimentin, a mesenchymal marker, plays a crucial role in EMT.[16,27] In some cancers such as esophageal squamous cell carcinoma, the increased expression level of vimentin is associated with a higher incidence of lymph node metastasis.[28,29] Recently, studies have shown that CSC and EMT enhance VM formation through stimulating cancer cell plasticity, remodeling the extracellular matrix, and connecting VM channels with host blood vessels,[30] but the regulatory mechanism is still unclear. The identification of biomarkers related to EMT, CSCs, and VM may provide a chance to develop drugs targeting EMT, CSC, and VM formation.[31,32] VM can be identified by the detection of periodic acid–Schiff (PAS)-positive loops surrounded by tumor cells (not endothelial cells), with or without red blood cells in it.[12] The current study aimed to present the role of VE-cadherin, CD44, and vimentin in inducing VM and EMT and to identify the CSC niche in different grades of OSCC.
MATERIALS AND METHODS
PATIENTS AND TISSUE SAMPLES
PASS software (power analysis and sample size) software (version 11.0.7; PASS, NCSS, LLC) was used to calculate the sample size using the following information: DF = 4, effect size = 0.5, power (1−β) = 0.9, and alpha (significance level) = 0.05.
A total of 63 OSCC samples (21 samples each grade) were collected from the archive of Pathology Department of Besat Educational Hospital, Hamadan, Iran, from 2000 to 2015. Institutional Review Board approval number was 9409034804.
There were 25 cases from the tongue, 15 cases from the buccal mucosa and 13 cases from gingiva, and 10 cases from floor of mouth. Adjacent normal oral mucosa served as control group. Hematoxylin and eosin staining was performed to confirm the previous diagnosis. Histologically, OSCCs were classified as low, intermediate, or high grade on the basis of the presence of cytological atypia, keratin pearl, mitotic activity, and other criteria.[33]
DOUBLE IMMUNOHISTOCHEMISTRY/PERIODIC ACID–SCHIFF STAINING
The specimens were processed for immunohistochemistry analysis. Monoclonal anti-mouse IgG antibodies used in the immunohistochemistry assay were vimentin (Novocastra™ ready to use) and CD44 (1:200; Thermo Scientific, Std./HCAM Ab-4). Polyclonal anti-rabbit VE-cadherin antibody (1:170; Abcam; 33168) was used as well. Then, the sections were stained with PAS. Briefly, tissue sections were cut by 4 mm thickness. Then, the sections were deparaffinized and dehydrated with graded alcohol. The antigen retrieval was done in citrate buffer (pH = 6) for CD44 and vimentin and in EDTA/Tris (pH = 9) for VE-cadherin. Using Leica detection kit, endogenous peroxidase activity was blocked. After 3 washes in Tris-buffered saline (TBS), the samples were incubated with primary antibodies for 1 h. Negative controls were prepared by omitting the primary antibody. The positive control staining was also performed (reactive lymph node according to the manufacturer instructions). After TBS washing, the slides were developed in freshly prepared diaminobenzidine solution for 6 min. Then, PAS staining was performed, followed by counterstaining with hematoxylin, dehydration, and mounting.
DETECTION AND SCORING
VE-cadherin and CD44 expression was detected in the membrane of the tumor cells. Vimentin expression was detected in the cytoplasm of cancer cells. Microvessel density (MVD) was determined by light microscopy examination of stained sections at the “hot spot.” Fields of the greatest neovascularization were identified by light microscope at low power (×100). The average vessel count of the five fields (×400) was regarded as the MVD count. The MVD was classified as either high (≥15) or low (<15); 15 was considered as the median value of MVD in our study. VM was also assessed. VE-cadherin, CD44, and vimentin/PAS double-staining were used to validate VM. VM was identified by the detection of PAS-positive loops surrounded by tumor cells (not endothelial cells), with or without red blood cells in it. The abundance of positive cells for biomarkers was graded as follows: 1 (weak) for <20% positive cells, 2 (moderate) for 20%–50% positive cells, and 3 (strong) for >50% positive cells.[16]
STATISTICAL ANALYSIS
Analyses were conducted through SPSS software version 22.0 (SPSS, Inc., Chicago, IL, USA). Chi-Square test was used to examine the differences between the variables. Significant level was set at 0.05. Pearson's correlation was used to assess the co-localization of the markers.
RESULTS
A total of 63 samples (40 men; 63.5%, and 23 women; 36.5%) were used for immunohistochemical study. Age ranged from 20 to 70 years with a mean age of 53.3 years. There were statistically significant differences between tumor grade and the expression levels of VE-cadherin, CD44, and vimentin (P = 0.000). In addition, significant differences were found between tumor grade and MVD (P = 0.000) and between tumor grade and VM (P = 0.000). Besides, there was a positive correlation between tumor grade and VE-cadherin expression level Pearson r = 0.925, P < 0.000) between tumor grade and CD44 expression level (Pearson r = 0.595, P < 0.000), and between tumor grade and vimentin expression level (Pearson r = 0.678, P < 0.000). The details are summarized in Table 1.
Table 1.
The relationships between VE-cadherin, CD44 and vimentin expression levels and histopathological variables in different grades of oral squamous cell carcinoma
DISCUSSION
In this study, the expression level of VE-cadherin, CD44, and vimentin was examined in different histological grades of OSCC. According to the previous studies, VM formation is seen involving in tumor growth and cancer metastasis,[11] and in OSCC, it is correlated to poor prognosis.[9] In addition, the elevated expression level of VE-cadherin is associated with the cancer growth and progression.[16] Besides, VE-cadherin is expressed by CSCs and is associated with VM.[34,35] A study on aggressive melanoma indicated the high expression level of VE-cadherin by cancer cells. The authors suggested that VE-cadherin expression by tumor cells enhances vasculogenic-like network formation. Furthermore, it is suggested that tumor plasticity allows VM formation which is correlated to the VE-cadherin expression.[14] VM formation has a crucial role in the tumor progression and metastasis.[18] Overexpression of VE-cadherin in cancers such as melanoma and breast cancer is associated with poor prognosis.[34] In a published work, Fry et al. suggested VE-cadherin expression as a metastatic biomarker in breast cancer.[35] VM and EMT promote invasion and metastasis.[36,37,38] During VM formation, highly aggressive epithelial tumor cells can overexpress the mesenchymal phenotype through EMT.[39] VM has been shown to present in 21/84 (25%) of gastrointestinal stromal tumors, which were significantly associated with tumor grade and liver metastasis.[38] In a previous study on OSCC, tumor cell-lined vessels were found in 18/33 (54.5%) of cases.[13] Besides, VM formation was found in 40% of adenoid cystic carcinoma (ACC) tissues, mainly in the solid pattern.[19] Importantly, a published work on the triple-negative breast cancer demonstrated a significant expression level of VE-cadherin in CD133+ CSCs. The authors proposed that CD133+ CSCs might have the ability of acquisition of endothelial cell phenotype and VE-cadherin expression to enhance VM formation.[39] In our study, strong expression level of VE-cadherin was found in all high-grade samples. In addition, VM formation (lined by VE-cadherin-positive cancer cells) was detected in 17 (81%) of intermediate-grade and 18 (85.7%) of high-grade samples [Figures 1 and 2]. Furthermore, VM channels lined by VE-cadherin-positive cancer cells were mostly detected in histologically higher degree samples and the VE-cadherin expression level increased by the increasing of tumor grade increase. The present study also demonstrated VE-cadherin positivity at invasive front [Figure 3]. These results may provide sufficient document for the role of VE-cadherin expression level and VM formation in the tumor growth and the risk of metastasis through CSCs and EMT. Moreover, VE-cadherin expression level is suggested as a metastatic biomarker for OSCC.
Figure 1.
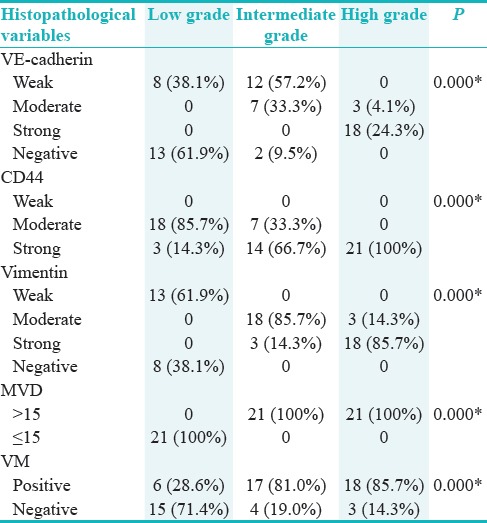
Histologic section of intermediate-grade tumor shows a strong vascular endothelial-cadherin positivity. Yellow arrows indicate vascular channels lined by vascular endothelial-cadherin-positive tumor cells (×400)
Figure 2.
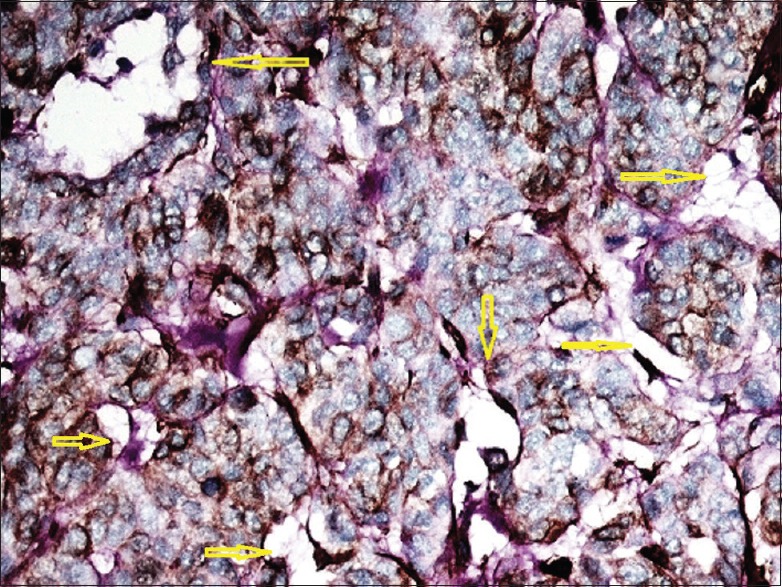
Paraffin section of high-grade tumor. The high-power magnification view shows a strong vascular endothelial-cadherin cell membrane staining of undifferentiated tumor cells. Green arrows indicate vascular channels lined by vascular endothelial-cadherin tumor cells
Figure 3.
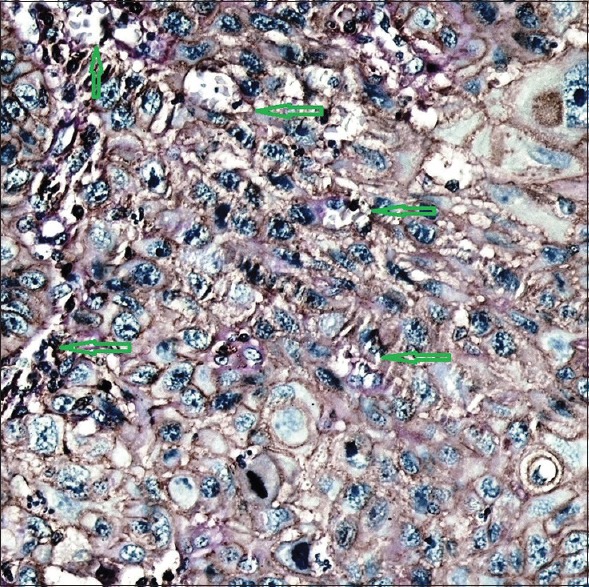
Formalin-fixed, paraffin-embedded tissue section indicating a strong vascular endothelial-cadherin positivity at invasive front (green arrows). Yellow arrow shows vascular endothelial-cadherin positivity in some detached tumor cells (×400)
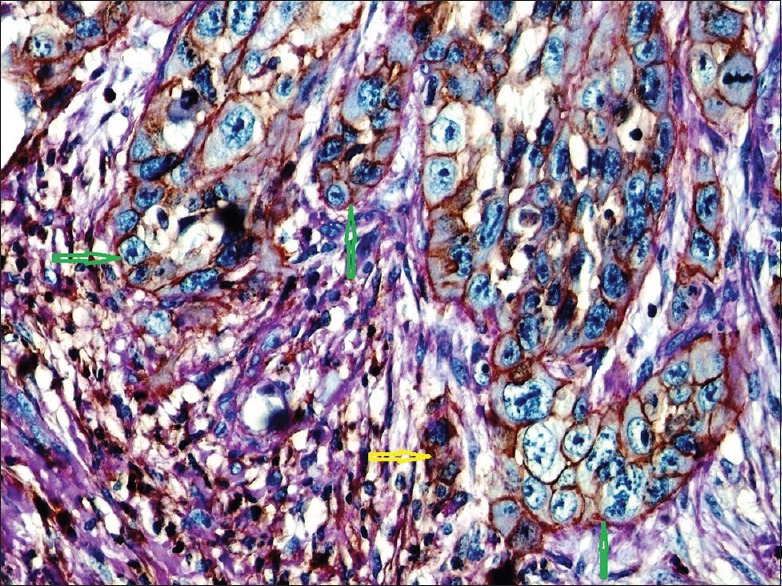
Regarding CD44 expression level in the present study, strong CD44 expression was found in all high-grade samples and 13 (61.9%) of intermediate-grade samples [Figures 4 and 5]. Besides, CD44-positive cancer cells were mainly found at invasive front and at the periphery of tumor islands [Figure 6]. Therefore, the present study provides enough evidence that the number of CSCs also increases by the increase of histological degree, and also, CD44 and VE-cadherin enhance tumor growth and metastasis by increasing the number of CSCs. CD44 expression is correlated with CSCs, and the aggressiveness of head and neck tumors[22] and CD44 plays a critical role in VE-cadherin expression.[40] For instance, a previously published paper indicated that CD44 plays an important role in controlling the proliferation and apoptosis of capillary endothelial cells through CD31 and VE-cadherin expression.[40] A published study demonstrated that CD44 variant mediates disassembly of endothelial VE-cadherin junction on metastatic melanoma cells. By doing this, CD44 mediates melanoma cell transendothelial migration.[41] CD44 is expressed in the basal cell layer of normal oral mucosa as well as CSCs.[21,42] A previous study on HNSCC samples found CD44 expression in all samples tested.[22] However, another published study on different histologic grades of OSCC indicated CD44 positivity in 80%–100% of well-differentiated cases, in 60%–85% of moderate-differentiated samples, and in 40%–62% of poor-differentiated cases. The reaction was mostly found at the periphery of tumor islands.[23]
Figure 4.
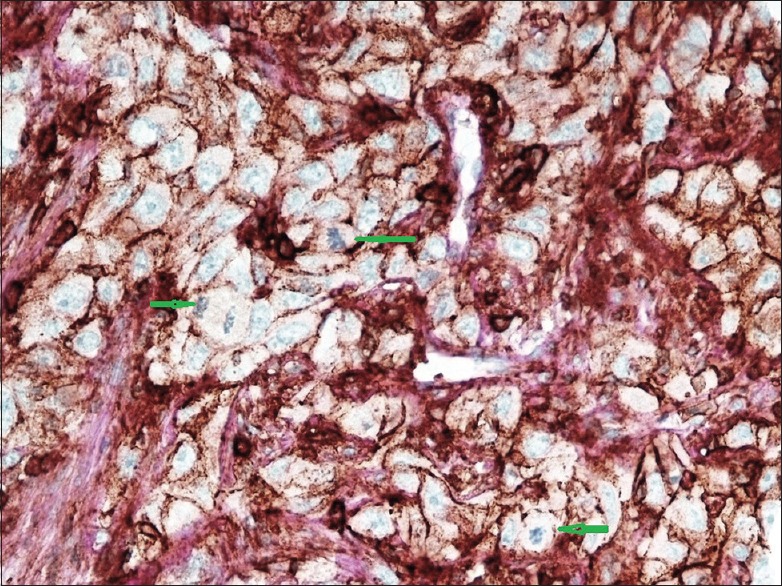
High-power section of high-grade tumor demonstrates a strong CD44 positivity in tumor cells. Green arrows indicate the mitotic figures
Figure 5.
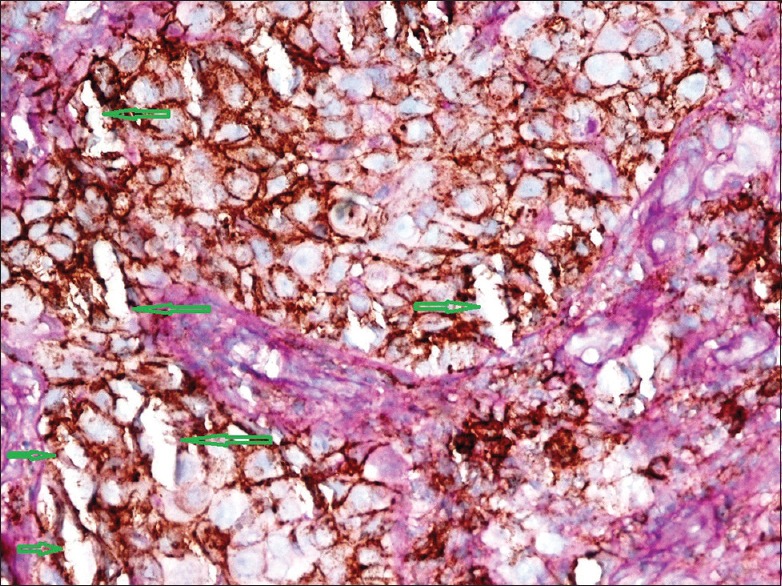
High-power section of intermediate-grade tumor demonstrates a strong CD44 positivity in tumor cells. Green arrows indicate vascular channels lined by CD44-positive tumor cells
Figure 6.
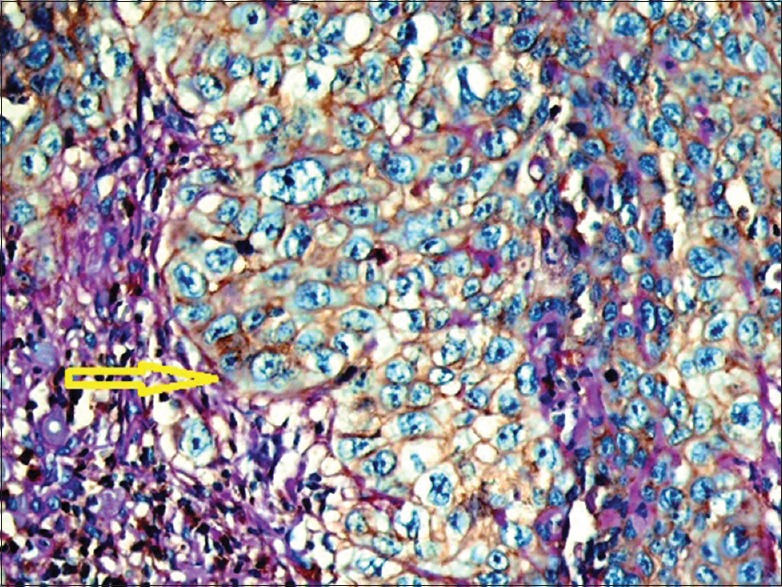
High power of intermediate-grade sample indicates CD44 positivity in all tumor cells. Yellow arrow indicates the tumor-stroma interface
According to the results obtained in the current investigation, elevated expression of vimentin was also indicated in tumor cells mainly at the periphery of tumor islands and invasive front in intermediate- and high-grade samples [Figures 7 and 8]. Moreover, vimentin positivity was found in some detached cancer cells, especially around the blood vessels, and in the stroma indicating the tumor cells which acquired EMT properties [Figure 9]. Previous studies on HNSCC samples, strong positivity of vimentin was found in the microenvironment. The authors proposed that cancer cell mobility enhances cancer progression and metastasis.[43,44] In other cancers such as colon, breast, and prostate cancers, vimentin expression level was indicative of aggressive cancer behavior and poor prognosis.[45,46] Recently published work has shown that elevated vimentin expression level is correlated with EMT process of cancers such as breast cancer[47] and is indicative of poor prognosis in OSCC.[44] Furthermore, vimentin expression is associated with esophageal squamous cell carcinoma lymph node metastasis.[28,29] EMT in an epithelial tumor may be an alternative mechanism of VM formation.[38] On the other hand, it is suggested that CSCs may be involved in vascular formation[13] and EMT to promote tumor cell invasion and metastasis.[14] The current study provides enough evidence that the higher expression levels of CD44 and vimentin at the periphery of tumor islands and invasive front mostly in higher grade samples may indicate that CSC properties are necessary to get EMT properties which, in turn, enhance the cancer metastasis. Taken together, in the current study, some tumor cells especially at the periphery of tumor islands and at invasive front got stained with VE-cadherin, CD44, and vimentin showing that they are CSCs which could get EMT phenotype. In addition, it can be suggested that these cells are involved in VM formation. Collectively, the current study can be another proof for the previous hypotheses regarding the role of CSC, EMT, and VM channels in cancer development and metastasis. Furthermore, the present study provides enough information about some other molecules and pathways in the cancer growth and metastasis in OSCC. More information about the involved molecules and pathways in tumor growth, especially in metastasis can help to design future investigations to provide new drugs to prevent metastasis as the most important reason for death in oral cancer patients.
Figure 7.
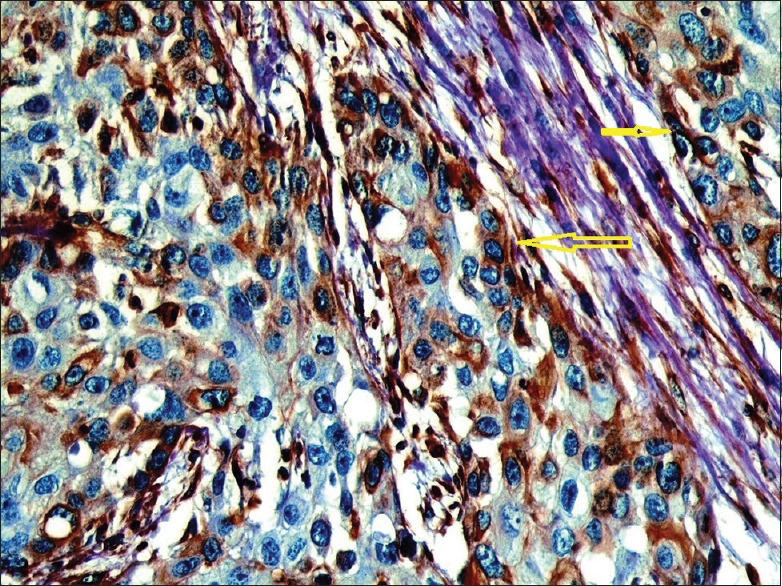
Paraffin section of intermediate grade tumor. The high-power magnification view shows a strong vimentin cytoplasmic staining of tumor cells mainly at the periphery of tumor islands (yellow arrows)
Figure 8.
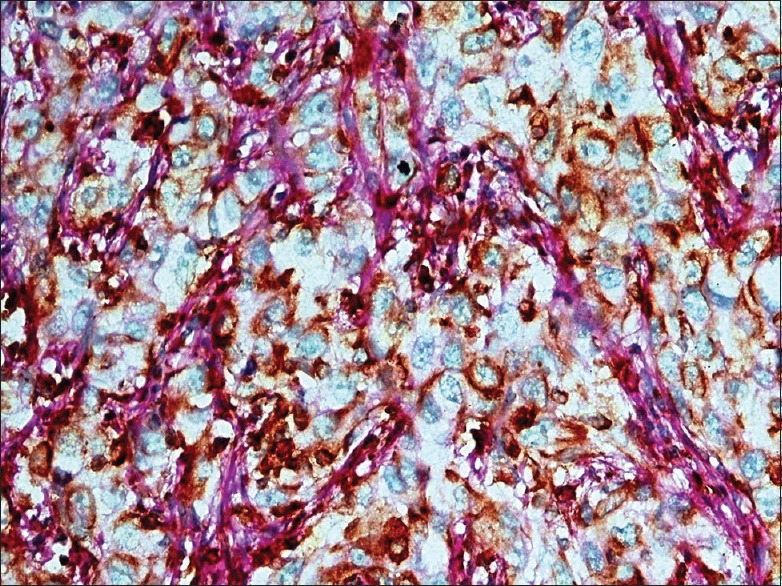
High-power photomicrograph from high-grade oral squamous cell carcinoma. Note the cytoplasmic positivity of vimentin in tumor cells
Figure 9.
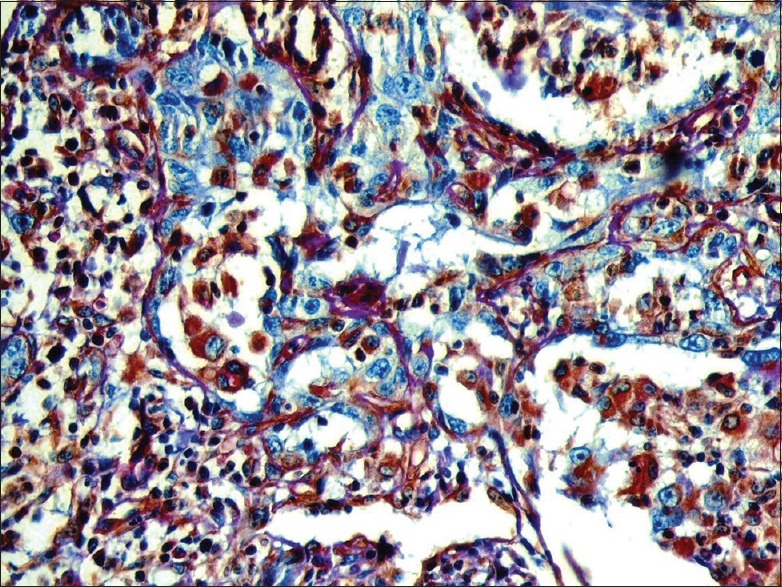
High-power magnification shows cytoplasmic vimentin positivity in detached tumor cells and stromal cells
MVD is another key player in tumor behavior. In the present study, MVD count was higher in intermediate- and high-grade samples compared to that of low-grade cases. Similar to the current study, a previous study on prostate cancer found a significant association between microvessel count (MVC) and tumor grade.[46] In ACC samples, MVD was correlated significantly with the clinical stage, vascular invasion, and metastasis.[48]
CONCLUSION
Our results may disclose a definite relationship between VE-cadherin, CD44 and vimentin expression levels, VM formation, EMT, CSCs, and MVC in OSCC samples. For this reason, it is suggested that VE-cadherin, CD44, and vimentin are related to angiogenesis and VM formation in OSCC, therefore, in tumor progression and metastasis. Recently, antitumor angiogenic therapies have been challenged. The presence of VM may explain the failure of antiangiogenic treatments.[18] Thus, the inhibition of VM formation has become a new strategy for anticancer therapy. Besides, mutations due to a genetic instability and environmental factors make the solid tumors such as OSCC heterogeneous.[49,50] Heterogeneity enhances tumor formation which may prove CSC hypothesis.[50] It is suggested that these cells give the tumor the ability to be resistant to chemoradiotherapy and metastasize.[51] Identifying the biomarkers of stem cells which acquire EMT characteristics may also improve the development of drugs targeting EMT CSCs.[52,53,54,55]
FINANCIAL SUPPORT AND SPONSORSHIP
This study was financially supported by Hamadan University of Medical Sciences.
CONFLICTS OF INTEREST
There are no conflicts of interest.
ACKNOWLEDGMENT
The authors would like to acknowledge the funding from Hamadan University of Medical Sciences.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA: Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Irani S, Bidari Zerehposh F, Sabeti Sh. Prevalence of Pathological Entities in Neck Masses: A Study of 1208 Consecutive Cases. Avicenna J Dent Res. 2016;8:1–5. [Google Scholar]
- 4.Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–49. doi: 10.1016/j.semcancer.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Irani S. Metastasis to the oral soft tissues: A review of 412 cases. J Int Soc Prev Community Dent. 2016;6:393–401. doi: 10.4103/2231-0762.192935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani S. Metastasis to the Jawbones: A review of 453 cases. J Int Soc Prev Community Dent. 2017;7:71–81. doi: 10.4103/jispcd.JISPCD_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irani S. Metastasis to head and neck area: a 16-year retrospective study. Am J Otolaryngol. 2011;32:24–7. doi: 10.1016/j.amjoto.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Irani S. Pre-Cancerous Lesions in the Oral and Maxillofacial Region: A Literature Review with Special Focus on Etiopathogenesis. Iran J Pathol. 2016;11:303–22. [PMC free article] [PubMed] [Google Scholar]
- 9.Irani S. Distant metastasis from oral cancer: A review and molecular biologic aspects. J Int Soc Prev Community Dent. 2016;6:265–71. doi: 10.4103/2231-0762.186805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 11.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res: CR. 2010;29:60. doi: 10.1186/1756-9966-29-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu SY, Chang LC, Pan LF, Hung YJ, Lee CH, Shieh YS. Clinicopathologic significance of tumor cell-lined vessel and microenvironment in oral squamous cell carcinoma. Oral Oncol. 2008;4:277–85. doi: 10.1016/j.oraloncology.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci U S A. 2001;98:8018–23. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irani S, Dehghan A. Expression of VE-Cadherin in Mucoepidermoid Carcinoma: Role in cancer development. J Int Soc Prev Community Dent. 2017;7:301–7. doi: 10.4103/jispcd.JISPCD_323_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, Kang YH, Lee JS, Byun JH, Kim UK, Jang SJ, et al. Positive expression of NANOG, mutant p53, and CD44 is directly associated with clinicopathological features and poor prognosis of oral squamous cell carcinoma. BMC oral health. 2015;15:153. doi: 10.1186/s12903-015-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao X, et al. Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol. 2013;108:414–9. doi: 10.1002/jso.23402. [DOI] [PubMed] [Google Scholar]
- 19.Wang SS, Gao XL, Liu X, Gao SY, Fan YL, Jiang YP, et al. CD133+ cancer stem-like cells promote migration and invasion of salivary adenoid cystic carcinoma by inducing vasculogenic mimicry formation. Oncotarget. 2016 doi: 10.18632/oncotarget.8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannelli G, Gallo O. Cancer stem cells hypothesis and stem cells in head and neck cancers. Cancer Treat Rev. 2012;38:515–39. doi: 10.1016/j.ctrv.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshua B, Kaplan MJ, Doweck I, Pai R, Weissman IL, Prince ME, et al. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck. 2012;34:42–9. doi: 10.1002/hed.21699. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca I, Pereira T, Rosa-Santos J, Soares J. Expression of CD44 isoforms in squamous cell carcinoma of the border of the tongue: A correlation with histological grade, pattern of stromal invasion, and cell differentiation. J Surg Oncol. 2001;76:115–20. doi: 10.1002/1096-9098(200102)76:2<115::aid-jso1021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Lindquist D, Ahrlund-Richter A, Tarjan M, Tot T, Dalianis T. Intense CD44 expression is a negative prognostic factor in tonsillar and base of tongue cancer. Anticancer research. 2012;32:153–61. [PubMed] [Google Scholar]
- 25.Mostaan LV, Khorsandi MT, Sharifian SM, Shandiz FH, Mirashrafi F, Sabzari H, et al. Correlation between E-cadherin and CD44 adhesion molecules expression and cervical lymph node metastasis in oral tongue SCC: Predictive significance or not. Pathol Res Pract. 2011;207:448–51. doi: 10.1016/j.prp.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigo JP, Dominguez F, Alvarez C, Herrero A, Suarez C. Expression of E-cadherin, CD44s, and CD44v6 in laryngeal and pharyngeal carcinomas. Am J Otolaryngol. 2003;24:384–9. doi: 10.1016/s0196-0709(03)00082-6. [DOI] [PubMed] [Google Scholar]
- 27.Luo T, Wang L, Wu P, Gong W, Chen W, Zhao H, et al. Downregulated vimentin and upregulated E-cadherin in T1 stage non-small-cell lung cancer: does it suggest a mesenchymal-epithelial transition? Neoplasma. 2017 doi: 10.4149/neo_2017_506. [DOI] [PubMed] [Google Scholar]
- 28.Irani S, Salajegheh A, Smith RA, Lam AK. A review of the profile of endothelin axis in cancer and its management. Crit Rev Oncol Hematol. 2014;89:314–21. doi: 10.1016/j.critrevonc.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Irani S, Salajegheh A, Gopalan V, Smith RA, Lam AK. Expression profile of endothelin 1 and its receptor endothelin receptor A in papillary thyroid carcinoma and their correlations with clinicopathologic characteristics. Ann Diagn Pathol. 2014;18:43–8. doi: 10.1016/j.anndiagpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Sun B, Zhang D, Zhao N, Zhao X. Epithelial-to-endothelial transition and cancer stem cells: two cornerstones of vasculogenic mimicry in malignant tumors. Oncotarget. 2017;8:30502–10. doi: 10.18632/oncotarget.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Yang Q, Yu S, Zhang X. Endocan: A new marker for cancer and a target for cancer therapy. Biomed Rep. 2015;3:279–83. doi: 10.3892/br.2015.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang GW, Tao YM, Ding X. Endocan expression correlated with poor survival in human hepatocellular carcinoma. Dig Dis Sci. 2009;54:389–94. doi: 10.1007/s10620-008-0346-3. [DOI] [PubMed] [Google Scholar]
- 33.Neville B DD, Allen C, Chi A. 4th ed. China: Elsevier; 2016. Oral and Maxillofacial Pathology. [Google Scholar]
- 34.Bartolome RA, Torres S, Isern de Val S, Escudero-Paniagua B, Calvino E, Teixido J, et al. VE-cadherin RGD motifs promote metastasis and constitute a potential therapeutic target in melanoma and breast cancers. Oncotarget. 2017;8:215–27. doi: 10.18632/oncotarget.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fry SA, Sinclair J, Timms JF, Leathem AJ, Dwek MV. A targeted glycoproteomic approach identifies cadherin-5 as a novel biomarker of metastatic breast cancer. Cancer Lett. 2013;328:335–44. doi: 10.1016/j.canlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Lin P, Sun B, Zhang S, Cai W, Han C, et al. Epithelial-mesenchymal transition regulated by EphA2 contributes to vasculogenic mimicry formation of head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:803914. doi: 10.1155/2014/803914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Qiao L, Liang N, Xie J, Zhang J, Deng G, et al. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. Journal of cellular and molecular medicine. 2016;20:1761–9. doi: 10.1111/jcmm.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun B Qie S, Zhang S, Sun T, Zhao X, Gao S. Role and mechanism of vasculogenic mimicry in gastrointestinal stromal tumors. Hum Pathol. 2008;39:444–51. doi: 10.1016/j.humpath.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–53. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 40.Tsuneki M, Madri JA. CD44 regulation of endothelial cell proliferation and apoptosis via modulation of CD31 and VE-cadherin expression. J Biol Chem. 2014;289:5357–70. doi: 10.1074/jbc.M113.529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, Fu C, Bai H, Song E, Dong C, Song Y. CD44 variant, but not standard CD44 isoforms, mediate disassembly of endothelial VE-cadherin junction on metastatic melanoma cells. FEBS letters. 2014;588:4573–82. doi: 10.1016/j.febslet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 42.Jones KB, Klein OD. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int J Oral Sci. 2013;5:121–9. doi: 10.1038/ijos.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutsch-Wicherek M. Am J Reprod Immunol. Vol. 63. New York, NY: 2010. RCAS1, MT, and vimentin as potential markers of tumor microenvironment remodeling; pp. 181–8. 1989. [DOI] [PubMed] [Google Scholar]
- 44.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers--E-cadherin, beta-catenin, APC and Vimentin--in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Cao Z, Shang B, Zhang G, Miele L, Sarkar FH, Wang Z, et al. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Biophys Acta. 2013;1836:273–86. doi: 10.1016/j.bbcan.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Dugonjic AS, Usaj SK, Eri Z, Latinovic LT. Significance of microvessel density in prostate cancer core biopsy. Vojnosanit Pregl. 2015;72:317–27. [PubMed] [Google Scholar]
- 47.Liu T, Zhang X, Shang M, Zhang Y, Xia B, Niu M, Liu Y, Pang D. Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast cancer. J Surg Oncol. 2013;107:188–94. doi: 10.1002/jso.23240. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Peng B, Chen X. Expressions of nuclear factor kappaB, inducible nitric oxide synthase, and vascular endothelial growth factor in adenoid cystic carcinoma of salivary glands: Correlations with the angiogenesis and clinical outcome. Clin Cancer Res. 2005;11:7334–43. doi: 10.1158/1078-0432.CCR-05-0241. [DOI] [PubMed] [Google Scholar]
- 49.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golub TR. Genome-wide views of cancer. N Engl J Med. 2001;344:601–2. doi: 10.1056/NEJM200102223440809. [DOI] [PubMed] [Google Scholar]
- 51.Allegra E, Trapasso S. Cancer stem cells in head and neck cancer. Onco Targets Ther. 2012;5:375–83. doi: 10.2147/OTT.S38694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–2. doi: 10.1016/j.stem.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–9. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albers AE, Chen C, Koberle B, Qian X, Klussmann JP, Wollenberg B, et al. Stem cells in squamous head and neck cancer. Crit Rev Oncol Hematol. 2012;81:224–40. doi: 10.1016/j.critrevonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Irani S, Jafari B. Expression of vimentin and CD44 in Mucoepidermoid Carcinoma: A role in tumor growth. Indian J Dent Res. 2018 doi: 10.4103/ijdr.IJDR_184_17. In press. [DOI] [PubMed] [Google Scholar]


