Abstract
Background:
Household and ambient air pollution are jointly responsible for about 7 million premature deaths annually. Women living in slums, with unhealthy environment, both indoors and outdoors, particularly those living close to industrial and/or vehicular pollution zones due to multiple sources of air pollution, are at the higher risk of having impaired lung function tests.
Objective:
The aim of this study was to estimate the prevalence of abnormal lung functions and to identify the environmental risk factors associated with them among adult women of 18–59 years.
Materials and Methods:
A total of 550 women aged 18–59 years were approached in a representative urban slum. Five hundred consented to participate and 299 had prebronchodilator spirometry satisfying ATS standards. House visits to assess environmental conditions were conducted to determine their association with forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC). Chi-square test was used to test the association of risk factors with lung functions. ANOVA was used to test the association of mean values of FEV1 and FVC with age.
Results:
Out of 299 participants with acceptable spirometric curves, 5% had reduced FEV1/FVC ratio than the normal and 26.8% and 17.4% had lower values than predicted for FVC and FEV1, respectively. Altered lung function was related to age, tobacco smoking, and history of respiratory disease.
Conclusions:
Both ambient and household air pollution have a deleterious pulmonary effect on long-term women residents of a representative urban slum in Delhi.
KEY WORDS: Air pollution, Delhi, environment, lung function, slum
INTRODUCTION
Household and ambient air pollution are jointly responsible for about 7 million premature deaths annually. These deaths attributed to 36% – ischemic heart disease, 33% – stroke, 17% – chronic obstructive pulmonary disease (COPD), 8% – acute lower respiratory infection, and 6% – lung cancer cases globally.[1] As per the 2011 census, 780 million individuals using solid fuels for cooking and heating live in India. Household air pollution is also a contributor to ambient air pollution-related deaths due to emissions in the ambient environment, responsible for 0.4 million deaths (12% of the total from ambient air pollution).[2] In 2012, roughly three-quarters of the global population was exposed to particulate matter in concentrations exceeding WHO Air Quality Guidelines. Through rigorous efforts, the Delhi government has been able to lower the air pollutant levels, and Delhi is in 12th position in May 2016 from the dubious distinction of being first in the list of cities with the worst ambient air quality worldwide.[3] As per Central Pollution Control Board air quality monitoring 2015 data, Delhi had 96% bad days, i.e., when one or the other parameter of air quality exceeded the norms.[4]
Another important risk factor for altered lung function is tobacco smoke. In poorly ventilated dwellings, indoor smoke can be 100 times higher than acceptable levels for fine particles. It is estimated that there are 1.1 billion tobacco smokers worldwide.[5] On the basis of the proportions of secondhand smoke exposure, as many as 40% of children, 35% of women, and 33% of men are regularly exposed to it.[6]
Earlier published studies to assess the altered lung functions and their risk factors have either been conducted in the hospital settings in males or children or in female population in the community settings in the rural areas with better ambient environmental factors, higher indoor pollution and lower outdoor pollution. Women living in slums, with unhealthy environment, both indoors and outdoors, particularly those living close to industrial and/or vehicular pollution zones due to multiple sources of air pollution, are at the higher risk of having impaired lung function tests.
We, therefore, conducted a study in an urban slum area of Delhi to estimate the prevalence of altered lung function tests and to identify the environmental risk factors associated with them among adult women of 18–59 years.
MATERIALS AND METHODS
The study was approved by the Ethical Committee of Lady Hardinge Medical College (LHMC) and data collection was done from January to December 2015. It was a cross-sectional study carried out at Vishwas Nagar slum in East Delhi with 528 houses,[7] approximately 5500 population and residing here for 15–25 years. It has been one of the most polluted zones in the city because of Inter State Bus Terminus at Anand Vihar, just half kilometer from the study site, with large vehicular density and traffic congestion. In addition, there are other sources of pollution from factories at Sahibabad, Ghazipur, and Jhilmil industrial areas and construction activities in nearby malls, metro railways, etc., all within 5 km radius from the study site.
Women who were residing in the study area for minimum of 1 year were eligible for the study while pregnant women or females with the history of heart disease or any other severe debilitating illness were excluded from the study. If a household had more than one eligible woman, only one was randomly selected. Of 528 households in the study site, 34 refused to give the consent while 6 households who were living on the rented upper floors gave the consent for the study. Out of 550 adult women aged 18–59 years in 528 houses, eventually, 500 females gave consent for participation in the study. Observations were made through home visits to know their living conditions such as type of house and ventilation, place of kitchen, type of fuel used for cooking and warming homes in winters, smoking (self and/or other family members within household), number of hours spent per day cooking within household, presence of exhaust, and smoke outlet within the kitchen, apart from interviewing their sociodemographic profile.
All the individuals were subjected to clinical history based on MRC questionnaire. Since lung volumes show diurnal variation, therefore, spirometry was performed as per ATS guidelines[8] in the community between 8 a.m. and 11 a.m. in the morning each time using a portable spirometer with turbine flow sensor technology “Spirolab II” (manufactured by SDI Diagnostics Pvt. Ltd.,) for this purpose. The investigator had undergone necessary training in the Department of Physiology at LHMC, New Delhi, for conducting the spirometry. Out of 500 participants, 101 refused to give consent for spirometry, and of 399 participants who consented for spirometry, 19 were not able to perform it. From rest 380 curves, only 299 curves were satisfactory and were categorized acceptable for analysis. A minimum of three acceptable maneuvers were performed in the all of these participants. In our analysis, we used the forced expiratory volume in 1 s (FEV1) and the forced vital capacity (FVC). Linear regression models were used to predict the lung function parameter FEV1 and FVC based on age and height. The prediction equations for creating reference values for these women were:[9]
FVC (L) = 20.07 − 0.010 × age − 0.261 × ht + 0.000972 × ht2
FEV1 (L) = −2.267 − 0.019 × age + 0.033 × ht
FEV1 and FVC values which were <5th percentiles define the lower limit of normal (LLN); therefore, any value below LLN was taken as abnormal.
LLN = Predicted value − (1.645 × S.E.E).
S.E.E – Standard error of estimate (constant for different pulmonary function parameters).
Biomass fuel exposure index was derived by multiplying duration of biomass fuel usage (years) and cooking duration/day.[10] Smokers were classified[11] as current smokers (who smoked regularly within 1 month before the interview); nonsmokers (who never smoked or occasionally smoked), and ex-smokers (who stopped smoking more than 1 month before the interview). One pack-year was defined as smoking 20 cigarettes per day for 1 year. In case of beedi smokers, the number of pack-years was further divided by 4 as the net weight of tobacco per beedi is about one-fourth as compared to weight in a cigarette.[12]
Chronic cough was defined as any cough lasting for 8 weeks or longer and chronic bronchitis when cough was present for most days in a month for 3 or more months in a year for 2 consecutive years (MRC, UK). Chronic phlegm production was defined as regular sputum production for 3 or more months in a year for 2 consecutive years. Dyspnea was defined as breathlessness when walking, which required the case to stop or slow down for breathing while walking on the level.[13]
Statistical analysis was done using SPSS version 12 (IBM SPSS Statistics). Chi-square test was done to test the association of risk factors with lung functions. ANOVA was used to test the association of mean values of FEV1 and FVC with age. Factors significant at P < 0.10 in the bivariate analysis were considered in multiple logistic regression. The results reported as OR (95% confidence interval). Thereby, multiple logistic regression analysis was done for variables – age group, duration of stay, tobacco smoking, and history of respiratory disease.
RESULTS
Ambient air pollution in the study area as indicated in Figures 1 and 2; past year records revealed levels of NO2, PM10, and PM2.5 higher than safe limits.[14] Surge of air pollutants was particularly noticed during winter months as climatic factors such as temperature, wind, and precipitation play important roles in determining patterns and concentrations of air pollution over multiple scales in time and space.
Figure 1.
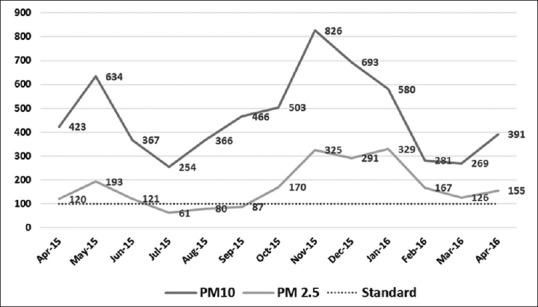
Line diagram showing monthly average of PM10 and PM2.5 levels of the study area (Central Pollution Control Board Anand Vihar station) for the past 1 year (prescribed standard for PM10 and PM 2.5–100.00 μg/m3)
Figure 2.
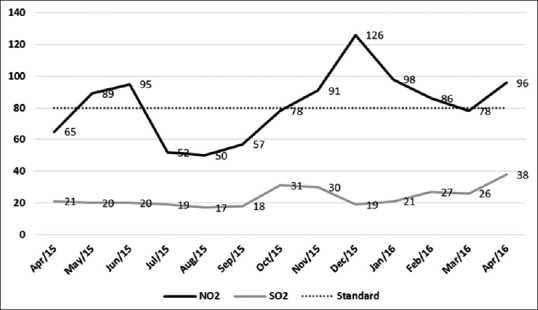
Line diagram showing monthly average of NO2 and SO2 levels of the study area (Central Pollution Control Board Anand Vihar station) for the past 1 year (prescribed standard for NO2 and SO2 – 80.00 μg/m3)
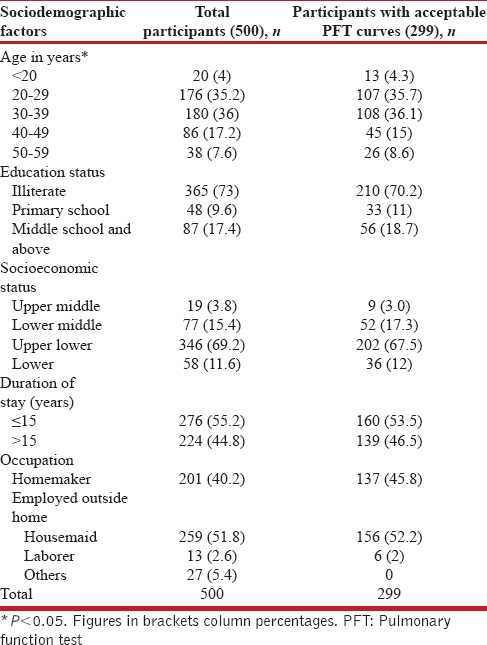
Most of the study participants (71%) were in the age group of 20–39 years and mean age was 35.4 ± 10.3 years. Majority of the participants were Hindu (92.4%) and were part of nuclear family (3/4th), almost 3/4th were illiterate, and 87% were currently married. About 80% of the families belonged to lower (12%) or upper lower socioeconomic (68%) status as per the modified Kuppuswamy scale. Nearly 60% of the participants have stayed in the study area for more than 10 years with a mean duration of stay 15.2 ± 9.5 years. Nearly 55% of the participants were employed outside their homes, mainly as housemaids, and majority (96%) had their jobs within 5 km of the residential area. Sociodemographic characteristics of the participants with acceptable pulmonary function test curves showed no significant difference than those of total number of participants initially screened for the study [Table 1].
Table 1.
Sociodemographic profile of the study participants
Tobacco smoking in the form of beedi was seen in 3.8% of the participants while 1.4% participants were ex-smokers. Majority (73.6%) of the smokers had smoked for <2.5 pack-years. About 31% of the participants were exposed to secondhand smoke, and in 13 instances, both the study participants and their spouses were currently smoking indoors, mostly (90.2%) beedis.
Dyspnea while walking at normal pace or at rest, chronic cough, chronic phlegm, and wheeze were observed in 14.2%, 5.6%, 2.4%, and 2.6%, respectively, among 500 participants interviewed, while out of 299 study participants with acceptable spirometric curves, 5% had reduced FEV1/FVC ratio than the normal and 26.8% and 17.4% had reduced values for FVC and FEV1, respectively. The mean values of FEV1 were 5.2% to 18.3% lower than the mean predicted FEV1 while the mean values of FVC were 17.4% to 26.7% lower than the predicted value of FVC. After applying ANOVA, it was observed that with increasing age, FEV1 and FVC decreased significantly [Figure 3].
Figure 3.
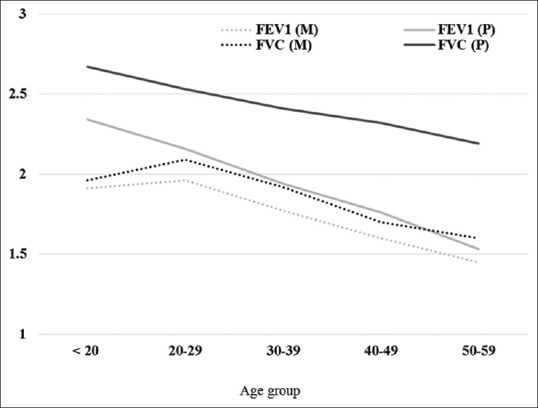
Distribution of study participants by age and mean values of lung volumes. M: Measured value, P: Predicted value
Multiple logistic regression model was significant with χ2 = 26.115, P = 0.006, 13% variance (R2) Nagelkerke; 82.6% cases were correctly classified with abnormal FEV1. Smokers and participants with a history of respiratory disease had 4.619 (1.075–19.851) and 3.479 (1.121–10.798) at P < 0.05 higher chance of abnormal FEV1. Participants who stayed more than 15 years in the study area had 15% more odds with P = 0.005 of having abnormal FEV1 values. Since none of the factors in the bivariate analysis showed significant P < 0.10 with FVC, therefore, it was not considered for multivariate analysis.
DISCUSSION
As per literature, coarse particles (PM10) consisting mainly of organic material, silicates and larger carbon aggregates, cause damage to larger airways and provoke higher inflammatory response than smaller particles[15] while deficits in the lung function are correlated with a set of pollutants that include nitrogen dioxide, acid vapor, and fine particulate matter (PM2.5) that reach the alveoli.[16]
Stankovic et al. (2007)[17] commented that women residing in highly polluted areas had a higher prevalence of all respiratory symptoms than women living in the lower polluted area. Most of these studies were conducted in females of age 35 and more. Lung functions start declining after 25 years of age that is accelerated on increased exposure to tobacco smoke, higher indoor and outdoor air pollution, uncontrolled asthma, and working in dusty occupations. Schikowski et al.[18] in their study among 55-year-old women showed that increasing exposure to PM10 was associated with reduction in both FEV1 and FVC by 5.1% and 3.7%, respectively. We observed a similar reduction for FEV1 but observed much higher reductions in FVC (23.7%).
Nearly 26% dwellings in the study area were of kucha type and most of them were ill ventilated (98.6%). Contrary to this, Singh and Jamal (2013)[19] observed one-third of kucha dwellings and only 26% being poorly ventilated. They have also observed that built materials such as mud, bamboo, thatched, tin sheets, and polythene (used in kucha houses) adsorb the smoke and other pollutants emitted from cooking fuels for longer period and thus increasing the exposure duration of the individuals. They also observed that very low-income group participants, 64% of those who were staying in kutcha house and mostly using biomass fuel; out of them, 85% had one of the respiratory diseases. Our study reported that out of all those symptomatic and staying in kutcha house, 58% of them were using biomass fuel for cooking.
In our study, only 13.6% of the households had a separate kitchen, 2.8% had kitchen in the outdoor setting, while the rest (83.6%) were cooking within the living room. Out of those women who were cooking indoors, 60% and 72% lacked smoke outlet and exhaust in the kitchen, respectively. On the other hand, Johnson et al. (2011)[20] found 50.7% households had outdoor kitchen while 49.3% had indoor kitchen. This study was conducted in rural areas in much cleaner background environment. Liquid petroleum gas (LPG) was recently introduced in the study area through different government run schemes, and therefore, majority (85%) of the study participants were using LPG for cooking and 10% were utilizing both LPG and biomass fuels such as wood and coal for cooking and rest 5% were totally dependent on wood for cooking fuel. Nearly one-fifth of the households used biomass fuels for warming homes during winters. These figures were quite different as compared to observations made under NFHS-3 by Agrawal[21] where cleaner fuels were used by 29.8% and biomass fuels by 70.2% households for cooking. Mean biomass exposure index was 52.15 ± 25.20 hour year by Bihari et al.[10] in a cross-sectional study in UP while mean biomass exposure index in our study was 24.98 ± 21.6 h years.
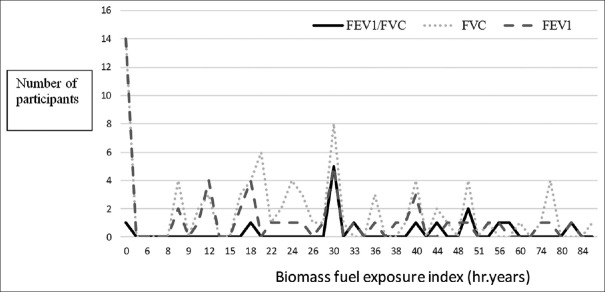
We observed a significant portion of participants had a restrictive pattern with lower FVCs than predicted [Figure 3]. These findings are similar to the BOLD study that observed a restrictive pattern not only in India but also other low- and middle-income countries that was mainly attributed to poor socioeconomic status which is an indirect marker for nutritional status.[22] We observed that most of our participants with abnormal FEV1 (49/52 [94.2%]), and abnormal FVC (74/80 [96.2%]) had biomass exposure index <60, respectively [Table 2]. We observed that there was almost even distribution of lung function impairments across the biomass exposure index [Figure 4]. Since the minimum threshold of biomass exposure index to have significantly increased risk of chronic bronchitis is above 60,[23] most of our participants had a biomass exposure index below 60; we hypothesize that ambient air quality has a significant additive effect to biomass fuel exposure.
Table 2.
Relationship of the altered lung functions with the environmental determinants (n=299)
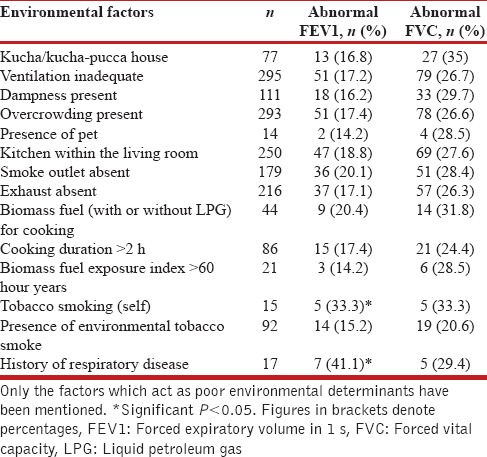
Figure 4.
Line diagram of dose–response relationship between biomass exposure index and altered lung function tests
The overall prevalence of tobacco smoking was 5%, which is almost twice the national average (2.9%) in females.[24] We observed a significant proportion of participants had impaired lung functions. Reduced FEV1/FVC ratio was seen in 20% of ever smokers and 4.2% among nonsmokers, with OR for abnormal FEV1 value as 4.619 (1.075–19.851), while it was 2.9 (1.1–7.6) for COPD among females.[25] Furthermore, they mentioned higher odds of developing COPD and abnormal FEV1 in those who were exposed to environmental tobacco smoke. Although we could not find any such association, higher prevalence in our study could be due to higher exposures to outdoor air pollutants owing to the location and other sources of air pollution in the study area.
Altered lung function tests were significantly associated with age, tobacco smoking, and history of respiratory disease and duration of stay more than 15 years. In addition to household air pollution, ambient air pollution was found a risk factor for altered lung function tests.
CONCLUSIONS AND RECOMMENDATIONS
Both ambient and household air pollution are affecting the health of residents staying in the study area for a long time. Further, research needs to be done to find the exact association of different air pollutants on their health. To mitigate the effects of ambient air pollution, regular flow of traffic needs to be ensured by avoiding traffic congestions and using laser-based traffic signals on roads. To decrease household air pollution, regular supply of LPG at subsidized rates will tend to reduce the use of biomass fuel. Housing conditions in the community need to be improved by taking simple affordable measures such as providing ventilation within the kitchen and use of chimneys for smoke outlet.
Limitations
Individual exposure to indoor air pollutants could not be measured due to the high cost.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Geneva: World Health Organization; 2014. [[Last accessed on 2015 Feb 12]]. Burden of Disease from the Joint Effects of Household and Ambient Air Pollution for 2012. Available from: http://www.who.int/phe/health_topics/outdoorair/databases/AP_jointeffect_BoD_results_March2014.pdf . [Google Scholar]
- 2.New Delhi: Ministry of Health and Family Welfare, Government of India; 2015. Report of the Steering Committee on Air Pollution and Health Related Issues, August 2015 Report no.: F. No. T.21022/41/2013-NCD. [Google Scholar]
- 3.Geneva: World Health Organization; 2016. [[Last accessed on 2016 Jul 30]]. Ambient Air Pollution Database. Available from: http://www.who.int/phe/health_topics/outdoorair/outdoorair_aqg/en/ [Google Scholar]
- 4.Kamyotra SJ, Sinha D. [[Last accessed on 2016 Sep 17]];CPCB Bulletin: Central Pollution Control Board. 1 Delhi; 26 July, 2016. Available from: http://www.cpcb.nic.in . [Google Scholar]
- 5.Geneva: World Health Organization; 2015. [[Last accessed on 2016 Feb 18]]. WHO Report on the Global Tobacco Epidemic 2015. Available from: http://www.who.int/tobacco/global_report/2015/en/ [Google Scholar]
- 6.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet. 2011;377:139–46. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 7.Delhi Urban Shelter Improvement Board. 2014. [[Last updated on 2014 Aug 17; Last accessed on 2014 Sep 22]]. Available from: http://www.delhishelter.nic.in/JJclustersDetails/htm .
- 8.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 9.Chhabra SK, Kumar R, Gupta U, Rahman M, Dash DJ. Prediction equations for spirometry in adults from Northern India. Indian J Chest Dis Allied Sci. 2014;56:221–9. [PubMed] [Google Scholar]
- 10.Bihari V, Iqbal SM, Srivastava LP, Kesavachandran C, Siddique MJ. Lung function impairment in women exposed to biomass fuels during cooking compared to cleaner fuels in Uttar Pradesh, India. J Environ Biol. 2013;34:971–4. [PubMed] [Google Scholar]
- 11.Schenker MB, Speizer FE, Samet JM, Gruhl J, Batterman S. Health effects of air pollution due to coal combustion in the chestnut ridge region of Pennsylvania: Results of cross-sectional analysis in adults. Arch Environ Health. 1983;38:325–30. doi: 10.1080/00039896.1983.10545815. [DOI] [PubMed] [Google Scholar]
- 12.Malik SK. Profile of chronic bronchitis in North India: The PGI experience (1972-1985) Lung India. 1986;4:89–100. [Google Scholar]
- 13.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. [[Last accessed on 2016 Feb 02]]. Available from: http://www.goldcopd.org .
- 14.Central Pollution Control Board. 2016. [[Last updated on 2016 Apr 28; Last accessed on 2016 Apr 28]]. Available from: http://www.cpcb.gov.in .
- 15.Happo M, Markkanen A, Markkanen P, Jalava P, Kuuspalo K, Leskinen A, et al. Seasonal variation in the toxicological properties of size-segregated indoor and outdoor air particulate matter. Toxicol In Vitro. 2013;27:1550–61. doi: 10.1016/j.tiv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Xu L, Cai Y. Monetary valuation of PM10-related health risks in Beijing china: The necessity for PM10 pollution indemnity. Int J Environ Res Public Health. 2015;12:9967–87. doi: 10.3390/ijerph120809967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankovic A, Nikic D, Nikolic M, Bogdanovic D, Stosic L, Milutinovic S, et al. Air pollution and respiratory symptoms in female population. J Health Var. 2007;46:47–54. [Google Scholar]
- 18.Schikowski T, Sugiri D, Ranft U, Gehring U, Heinrich J, Wichmann HE, et al. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6:152. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh AL, Jamal S. Risk assessment for indoor air pollution from Urban households in a sub-tropical climate. Bull Environ Sci Res. 2013;2:15–22. [Google Scholar]
- 20.Johnson P, Balakrishnan K, Ramaswamy P, Ghosh S, Sadhasivam M, Abirami O, et al. Prevalence of chronic obstructive pulmonary disease in rural women of Tamilnadu: Implications for refining disease burden assessments attributable to household biomass combustion. Glob Health Action. 2011;4:7226. doi: 10.3402/gha.v4i0.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal S. Effect of indoor air pollution from biomass and solid fuel combustion on prevalence of self-reported asthma among adult men and women in India: Findings from a nationwide large-scale cross-sectional survey. J Asthma. 2012;49:355–65. doi: 10.3109/02770903.2012.663030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): A population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 23.Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijaysimha R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J Med Res. 2013;137:87–94. [PMC free article] [PubMed] [Google Scholar]
- 24.Global Adult Tobacco Survey (GATS) Factsheet India: 2009-10. Ministry of Health and Family Welfare. [[Last accessed on 2016 Feb 10]]. Available from: http://www.who.int/tobacco/surveillance/en_tfi_India_gats_fact_sheet.pdf .
- 25.Mohammad Y, Shaaban R, Al-Zahab BA, Khaltaev N, Bousquet J, Dubaybo B, et al. Impact of active and passive smoking as risk factors for asthma and COPD in women presenting to primary care in Syria:First report by the WHO-GARD survey group. Int J Chron Obstruct Pulmon Dis. 2013;8:473–82. doi: 10.2147/COPD.S50551. [DOI] [PMC free article] [PubMed] [Google Scholar]