Abstract
Background:
Asymmetric dimethylarginine (ADMA) has emerged as a risk marker for many conditions related to pulmonary hypertension (PH); however, little is known about ADMA and symmetric dimethylarginine (SDMA) plasma concentrations in chronic obstructive pulmonary disease (COPD). Our interest centers on the role of ADMA in regulation of endothelial function in COPD and secondary PH. The aim of the present study was to evaluate the serum ADMA, SDMA, and L-arginine concentrations in COPD and its association with PH.
Methods:
Patients with diagnosis of COPD underwent pulmonary function tests, echocardiography, and laboratory investigations including ADMA, SDMA, and L-arginine.
Results:
Serum concentrations of ADMA, SDMA, and L-arginine tend to increase as COPD progresses. Patients with PH had higher concentrations of ADMA, SDMA, and L-arginine compared to cases with normal pulmonary arterial pressure (PAP); the difference was not statistically significant.
Conclusions:
Our results show that increased ADMA, SDMA, and L-arginine concentrations are associated with increased PAP measurements in patients with COPD, however, the relationship is not statistically significant.
KEY WORDS: Asymmetric dimethylarginine, chronic obstructive pulmonary disease, symmetric dimethylarginine
INTRODUCTION
Nitric oxide (NO) is a major endothelium-derived vasoactive substance playing a crucial role in maintaining vascular homeostasis and mediating regulation of local vasomotor tone. It is a potent endogenous vasodilator. Decreased levels of NO are associated with endothelial dysfunction. It is synthesized from L-arginine through the action of NO synthase (NOS), which is blocked by endogenous L-arginine analogs such as asymmetric dimethylarginine (ADMA). ADMA is an endogenous competitive inhibitor of NOS which has now fully emerged as a novel risk marker in a variety of conditions such as hypercholesterolemia, coronary artery disease,[1,2] peripheral arterial disease,[3,4,5] chronic heart failure,[6] pulmonary hypertension (PH),[7] stroke,[8] hypertrophic cardiomyopathy,[9] diabetes mellitus, and chronic renal failure.[10]
Another dimethylated L-arginine analog is the symmetric dimethylarginine (SDMA), but its role in the endothelial NO pathway is still unclear. SDMA and ADMA are able to interfere with the substrate availability of NOS. Some recent studies suggest that SDMA is also associated with cardiovascular events.[11,12] Studies have shown that PH in patients with congenital heart disease or undergoing hemodialysis is associated with elevated plasma levels of ADMA.[7,13] ADMA has emerged as a risk marker for many conditions related to PH; however, little is known about ADMA and SDMA plasma concentrations in chronic obstructive pulmonary disease (COPD). PH secondary to COPD is often thought to occur as a consequence of hypoxia. However, there is much debate on the etiology of COPD-related PH and the relevance of a vascular pathology in COPD. Our interest centers on the role of ADMA in regulation of endothelial function in COPD and secondary PH.
The aim of the present study was to evaluate the serum ADMA, SDMA, and L-arginine concentrations in COPD and its association with PH.
MATERIALS AND METHODS
Patients who applied to our Pulmonology Department from January 2013 to June 2013 with a diagnosis of COPD verified with clinical and spirometric data were prospectively enrolled in the study. All cases were in the stable stage. Patients with comorbid conditions likely to cause secondary PH such as chest wall disease, interstitial lung disease, previous pulmonary embolism, collagen vascular disease, congestive heart failure, valvular heart disease, chronic renal failure, and chronic liver disease and patients with acute exacerbation of COPD were not included in the study population.
The demographic data, clinical features including the presenting symptoms, past medical history, smoking habits, and medications were recorded. Physical examinations were performed. All patients were evaluated with echocardiography and blood tests.
In all patients, the presence of COPD had been confirmed by pulmonary function tests. Pulmonary function tests were performed by the same experienced technician. Each patient received exactly the same instructions. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/%FVC, forced expiratory flow 25%–75%, and peak expiratory flow were measured. All patients had FEV1/FVC <0.70. COPD was assessed for its severity according to FEV1 level as mild COPD if FEV1 ≥0.80 predicted, moderate COPD if 0.50≤ FEV1 ≤0.80 predicted, severe COPD if 0.30≤ FEV1 ≤0.50 predicted, and very severe COPD if FEV1 <0.30 predicted.
Echocardiographic examinations were performed by the same operator by GE Logiq 7 ultrasound device with 3 MHz probe. Pericardiac anatomy, valvular anatomy, left and right atria and ventricles, and cardiac function were evaluated by M-mode, 2-dimensional, pulsed-wave examinations; color flow Doppler imaging was used for the assessment of tricuspid regurgitation. PH was defined as a systolic pulmonary arterial pressure (PAP) >36 mmHg.
Blood was taken from all patients in the stable period of COPD, on the same day with the echocardiography in the morning after an overnight fast. Routine laboratory investigations included complete blood cell count, biochemical analyses, and C-reactive protein (CRP) and ADMA, SDMA and L-arginine.
Concentrations of ADMA, SDMA, and L-arginine were measured in serum samples using an enzyme-linked immunosorbent assay (Immundiagnostik AG, Bensheim, Germany) kit. Normal range for ADMA was 0.45 ± 0.19 μmol/L, normal range for SDMA was 0.47 ± 0.02 μmol/L, and normal range for L-arginine was 33.7–131.4 μmol/L. L-arginine/ADMA ratio is an index of NOS impairment, it was calculated for all cases. Normal values for CRP were values <3.45 mg/L.
Statistical analyses were performed with SPSS for Windows version 17.0. Continuous data are presented as a mean ± standard deviation. Correlations between parametric data sets were examined using Pearson’s correlation test. Differences between groups were analyzed using Mann–Whitney U-test. P < 0.05 was considered significant for all statistical tests. All reported P values are two sided.
RESULTS
Forty-two cases were recruited in the study. The study population consisted of 32 (76.2%) male and 10 (23.8%) female cases. The mean age was 65.98 (45–86). All the patients had been using bronchodilators for COPD, and none were under steroids. The patients were grouped according to COPD stages; 6 had mild disease, 19 had moderate disease, 12 had severe disease, and 5 had very severe disease. Mild and moderate cases were grouped as early stage, and severe and very severe cases were grouped as late stage COPD. PH was determined in 16 cases (38.1%). The study population had a mean smoking history of 25 pack-years (10–80).
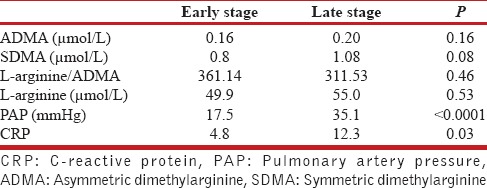
PAP measurements and CRP values of each stage of COPD patients are presented in Table 1. PAP measurements were significantly higher in late stage COPD (17.5 mmHg vs. 35.1 mmHg with P < 0.0001). When CRP concentrations were compared among these stages, patients with late-stage disease tended to have significantly higher CRP levels than early stage (12.3 and 4.8, respectively, P = 0.03).
Table 1.
Pulmonary arterial pressure measurements and C-reactive protein values in early and late stages of chronic obstructive pulmonary disease
Serum concentrations of ADMA, SDMA, and L-arginine in different stages of COPD are shown in Figures 1-3. Mean ADMA levels were 0.11 μmol/L in mild disease, 0.18 μmol/L in moderate disease, 0.19 μmol/L in severe disease, and 0.23 μmol/L in very severe disease. Mean SDMA levels were 0.59 μmol/L in mild disease, 0.88 μmol/L in moderate disease, 1.12 μmol/L in severe disease, and 0.99 μmol/L in very severe disease. L-arginine levels were 45.3 μmol/L in mild disease, 51.3 μmol/L in moderate disease, 53 μmol/L in severe disease, and 59.8 μmol/L in very severe disease. It can be seen that the concentrations of all three parameters tend to increase as the stage of the disease progresses. ADMA, SDMA, L-arginine, and L-arginine/ADMA values were compared between early and late stages of COPD, ADMA, SDMA, and L-arginine concentrations were higher in the latter group; however, no statistically significant difference could be found [Table 1]. Thirty cases had normal PAP and 12 cases had increased PAP; two of these were > 50 mmHg. Mean concentrations of ADMA, SDMA, L-arginine, and L-arginine/ADMA were compared between cases with and without PH. Patients with PH had higher concentrations of ADMA, SDMA, and L-arginine compared to cases with normal PAP; however, the difference between two groups was not statistically significant [Table 2]. ADMA levels were 0.21 μmol/L versus 0.16 μmol/L (P = 0.11), SDMA levels were 1.08 μmol/L versus 0.8 μmol/L (P = 0.08), and L-arginine levels were 55 μmol/L versus 49.9 μmol/L (P = 0.53).
Figure 1.
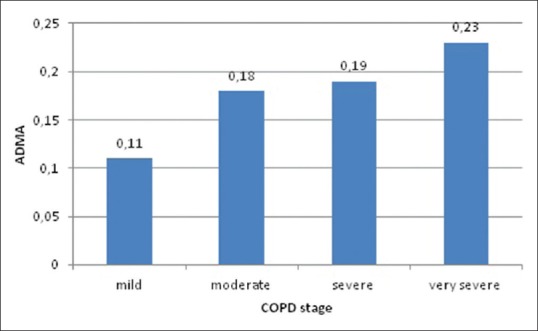
Asymmetric dimethylarginine levels in different chronic obstructive pulmonary disease stages
Figure 3.
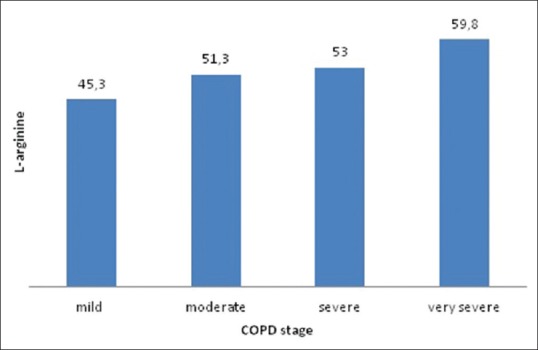
L-arginine levels in different chronic obstructive pulmonary disease stages
Table 2.
Asymmetric dimethylarginine, symmetric dimethylarginine, L-arginine, and L-arginine/asymmetric dimethylarginine in cases with and without pulmonary hypertension

Figure 2.
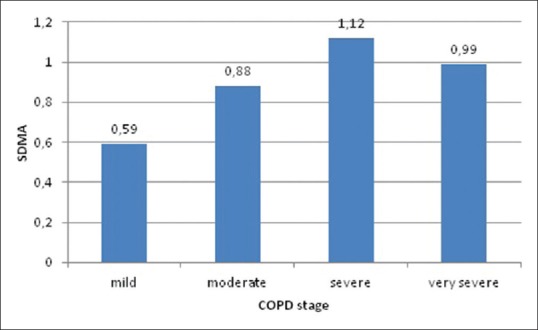
Symmetric dimethylarginine levels in different chronic obstructive pulmonary disease stages
Correlation analyses were made to demonstrate the relationship between CRP levels and ADMA, SDMA, and L-arginine concentrations. Correlation coefficient values were −0.09, 0.2, and 0.25 for ADMA, SDMA, and L-arginine, respectively. In this context, no significant correlation could be found among the mentioned parameters.
DISCUSSION
Our analysis demonstrates a positive correlation between serum levels of endogenous L-arginine analogs and severity of COPD. FEV1 tends to decrease as ADMA, SDMA, and L-arginine concentrations increase. However, the current study fails to show a statistically significant correlation among the mentioned parameters. We also report that increased ADMA, SDMA, and L-arginine concentrations are associated with increased PAP measurements, still the relationship is not statistically significant.
It has been reported that ADMA levels are increased in hypercholesterolemia, atherosclerosis, hypertension, chronic heart failure, diabetes mellitus, and chronic renal failure. ADMA is considered as an important cardiovascular risk marker and independent predictor of future cardiovascular events. Nevertheless, there are only a few studies on the relationship between ADMA concentrations and pulmonary diseases.[10]
The lung generates a significant amount of ADMA, potentially contributing to plasma ADMA levels that have been related to endothelial dysfunction. It has been hypothesized that ADMA levels are associated with inflammation, collagen deposition, nitrosative stress, and altered lung function. In accordance with this relationship, several studies have been conducted to assess ADMA levels in obstructive lung diseases.[14,15] Scott et al. evaluated L-arginine, ADMA, and SDMA levels in a murine model of allergic airways and in humans in adult lung specimens and sputum samples from pediatric asthmatic patients and found out that levels of ADMA were increased in plasma as well as lung specimens, sputum, and exhaled breath condensate.[15] In exhaled breath condensate of asthmatic patients, increased ADMA and SDMA concentrations were seen.[16]
NO is a key player and is closely linked to the vascular pathology in emphysema. It plays a crucial role in the maintenance of ventilation/perfusion matching as a response of hypoxia in lungs.[17] In an established experimental mouse model exposed to tobacco smoke, the inhibition of NOS protected against the development of PH and emphysema, and NOS downregulation was associated with a reduced number of pro-inflammatory cells such as granulocytes, macrophages, activated macrophages, and T cells and has potential to reverse emphysema.[18]
A report on the relationship between ADMA and COPD has recently been published. The authors have quantified the L-arginine metabolites, namely, L-arginine, L-ornithine, and L-citrulline. They also quantified ADMA levels in sputum samples of COPD patients as a measure of NOS activity and dysfunction and examined their relationship with spirometric values. The authors found that the sputum concentrations of L-arginine, L-citrulline, L-ornithine, ADMA, and SDMA were lower than reported in previous studies of CF and asthma sputum samples but higher than in healthy controls.[19]
ADMA levels were previously found to be increased in patients with PH of different etiologies. The exact pathophysiological role of ADMA in PH has not been clearly defined.
It was documented that ADMA concentrations were increased in PH in congenital heart disease. It was proposed that inhibition of NOS by increased levels of ADMA might contribute to PH in patients with congenital heart disease. To our knowledge, the role of ADMA in prediction of PH in COPD patients has not been elucidated.
It was previously reported that the levels of ADMA and SDMA were significantly correlated with each other.[12] However, in a large population-based cohort study, SDMA, but not ADMA, was found to be an independent predictor of all-cause and cardiovascular mortality.[11] In this study, the levels of the two markers were found be correlated with each other.
ADMA is a naturally occurring inhibitor of NOS. The degrading enzyme of ADMA is dimethylarginine dimethylaminohydrolase (DDAH). Almost 80% of ADMA is enzymatically hydrolyzed by DDAH. Being increased in patients with PH, ADMA is associated with unfavorable pulmonary hemodynamics and worse prognosis. These patients have a decreased expression and activity of DDAH. Degradation of ADMA by DDAH is considered the principal determinant for ADMA concentrations in patients with normal renal function.[17]
Recently, a prospective study in patients with peripheral artery disease has identified ADMA as an independent predictor of major adverse cardiovascular events. The authors reported that ADMA was not related to CRP levels.[4] Our present findings are compatible with these data and demonstrate no relationship between CRP and ADMA levels.
To our knowledge, this is the first study assessing the alterations of serum ADMA, SDMA, and L-arginine levels in COPD as well as their relationship with pulmonary arterial pressure.
An important limitation of our study is that PAP was noninvasively measured by transthoracic echocardiography without obtaining direct invasive measurements, while right heart catheterization is considered the gold standard for the diagnosis of pulmonary arterial hypertension. Nonetheless, transthoracic echocardiography is also an easily applicable, noninvasive, and accurate diagnostic tool for measuring PAP.
CONCLUSION
Our study demonstrates that endogenous competitive NOS inhibitors tend to increase as PAP increases in COPD. However, these results give no evidence to suggest that increased ADMA levels are significantly correlated with COPD severity and PH development. As the significant correlation between ADMA concentrations and COPD stages, as well as PH could not be demonstrated, firm conclusions on the relationship between ADMA levels and PH in COPD would be too ambitious. The role of NO pathway in the development of pulmonary vascular disease in COPD is doubt, and in this context, therapeutic regimens targeting this pathway are far from being successful. Further evaluations with larger study groups are necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Valkonen VP, Päivä H, Salonen JT, Lakka TA, Lehtimäki T, Laakso J, et al. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–8. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 2.Böger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality – An update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60:481–7. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Böger RH, Bode-Böger SM, Thiele W, Junker W, Alexander K, Frölich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95:2068–74. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 4.Mittermayer F, Krzyzanowska K, Exner M, Mlekusch W, Amighi J, Sabeti S, et al. Asymmetric dimethylarginine predicts major adverse cardiovascular events in patients with advanced peripheral artery disease. Arterioscler Thromb Vasc Biol. 2006;26:2536–40. doi: 10.1161/01.ATV.0000242801.38419.48. [DOI] [PubMed] [Google Scholar]
- 5.Böger RH, Endres HG, Schwedhelm E, Darius H, Atzler D, Lüneburg N, et al. Asymmetric dimethylarginine as an independent risk marker for mortality in ambulatory patients with peripheral arterial disease. J Intern Med. 2011;269:349–61. doi: 10.1111/j.1365-2796.2010.02322.x. [DOI] [PubMed] [Google Scholar]
- 6.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–30. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 7.Gorenflo M, Zheng C, Werle E, Fiehn W, Ulmer HE. Plasma levels of asymmetrical dimethyl-L-arginine in patients with congenital heart disease and pulmonary hypertension. J Cardiovasc Pharmacol. 2001;37:489–92. doi: 10.1097/00005344-200104000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Yoo JH, Lee SC. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis. 2001;158:425–30. doi: 10.1016/s0021-9150(01)00444-0. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep. 2007;59:715–20. [PubMed] [Google Scholar]
- 10.Sibal L, Agarwal SC, Home PD, Boger RH. The role of asymmetric dimethylarginine (ADMA) in endothelial dysfunction and cardiovascular disease. Curr Cardiol Rev. 2010;6:82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gore MO, Lüneburg N, Schwedhelm E, Ayers CR, Anderssohn M, Khera A, et al. Symmetrical dimethylarginine predicts mortality in the general population: Observations from the Dallas heart study. Arterioscler Thromb Vasc Biol. 2013;33:2682–8. doi: 10.1161/ATVBAHA.113.301219. [DOI] [PubMed] [Google Scholar]
- 12.Kiechl S, Lee T, Santer P, Thompson G, Tsimikas S, Egger G, et al. Asymmetric and symmetric dimethylarginines are of similar predictive value for cardiovascular risk in the general population. Atherosclerosis. 2009;205:261–5. doi: 10.1016/j.atherosclerosis.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Meng J, Li ZX, Jiang W, Xu C, Chun Li Y, Huang J, et al. Relationship of serum ADMA with pulmonary hypertension in patients on hemodialysis. Dial Transplant. 2010;39:242–6. [Google Scholar]
- 14.Ahmad T, Mabalirajan U, Ghosh B, Agrawal A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am J Respir Cell Mol Biol. 2010;42:3–8. doi: 10.1165/rcmb.2009-0137RC. [DOI] [PubMed] [Google Scholar]
- 15.Scott JA, North ML, Rafii M, Huang H, Pencharz P, Subbarao P, et al. Asymmetric dimethylarginine is increased in asthma. Am J Respir Crit Care Med. 2011;184:779–85. doi: 10.1164/rccm.201011-1810OC. [DOI] [PubMed] [Google Scholar]
- 16.Di Gangi IM, Pirillo P, Carraro S, Gucciardi A, Naturale M, Baraldi E, et al. Online trapping and enrichment ultra performance liquid chromatography-tandem mass spectrometry method for sensitive measurement of “arginine-asymmetric dimethylarginine cycle” biomarkers in human exhaled breath condensate. Anal Chim Acta. 2012;754:67–74. doi: 10.1016/j.aca.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Lüneburg N, Harbaum L, Hennigs JK. The endothelial ADMA/NO pathway in hypoxia-related chronic respiratory diseases. Biomed Res Int. 2014;2014:501612. doi: 10.1155/2014/501612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 19.Scott JA, Duongh M, Young AW, Subbarao P, Gauvreau GM, Grasemann H. Asymmetric dimethylarginine in chronic obstructive pulmonary disease (ADMA in COPD) Int J Mol Sci. 2014;15:6062–71. doi: 10.3390/ijms15046062. [DOI] [PMC free article] [PubMed] [Google Scholar]


