Abstract
Pasteurella multocida infection is most commonly associated with the immunocompromised, mostly in the form of soft-tissue infection, although other sites of infection are still possible and have been reported in the immunocompetent. We report a case of an immunocompetent male with a history of exposure to carrier organisms without portal of entry who developed P. multocida pneumonia with bacteremia. We undertook a focused review of literature of previously reported cases of P. multocida pneumonia in patients with chronic obstructive pulmonary disease. This literature review supports the use of penicillins as the first line of treatment over macrolides. Considering the high mortality rates with P. multocida bacteremia, it is important for clinicians to maintain a high level of suspicion for this organism in any patient with a history of carrier species exposure.
KEY WORDS: Chronic obstructive pulmonary disease, community-acquired pneumonia, Pasteurella multocida
INTRODUCTION
Pasteurella multocida is a Gram-negative coccobacillus zoonotic infectious organism. It is a facultative anaerobe that is nonmotile and nonflagellated. This pathogen is known to colonize the oropharynx of several healthy animals. In particular, cats and dogs have the highest carrier rates with 70%–90% of cats and 20%–50% of dogs being colonized.[1] Most human infections with P. multocida are secondary to animal bites causing soft tissue and skin infections, with second-most common cause arising from respiratory tract infections.[2] Of clinical significance, this organism harbors a polysaccharide capsule making its prevalence much higher among the immunocompromised. We present a case of an immunocompetent male with no skin or soft-tissue infection who developed healthcare-associated pneumonia (HCAP) and septic shock secondary to P. multocida.
CASE REPORT
The patient is a 78-year-old Caucasian male, former smoker (40 pack-year history of tobacco use), with medical history of chronic obstructive pulmonary disease (COPD) and prostate cancer status postradical prostatectomy and subsequent radiation therapy complicated by radiation colitis who presented to the emergency department with recent onset shortness of breath and altered mental status per family members. The patient reported that he had been experiencing a productive cough and subjective fevers for the past 2 days. The night before presentation, the patient’s wife noted that he was confused and was unable to get into bed. On these mentioned findings, the patient was rushed to the emergency department. On further questioning, the patient disclosed a recent hospitalization 2 months prior for a COPD exacerbation and suspected bowel obstruction.
On presentation, the patient was hypotensive with a blood pressure of 92/61, tachycardic with heart rate of 130, tachypneic with respiratory rate of 25, and hypoxic with oxygen saturation of 71%. The patient was afebrile with a temperature of 99.9°F (37.7°C). At this time, the patient was alert and oriented. On physical examination, breath sounds were diminished in all lung fields bilaterally along with scattered crackles throughout. While in the emergency department, the patient was started on noninvasive positive pressure ventilation with BiPAP. On initial laboratory studies, the patient had a leukocytosis (white blood cell 30.6 with 12% bands), lactic acidosis (lactic acid of 3.6 mmol/l), and acute kidney injury with creatinine of 1.5 mg/dl (baseline 0.8 mg/dl). Chest X-ray showed a left upper and lower lobe opacity concerning for pneumonia [Figure 1]. The patients calculated pneumonia severity index was 131, which correlates with a suggested Class V management strategy. Considering this classification and its associated mortality of 27%–31.1%, our patient was admitted to the intensive care unit for acute on chronic hypoxic hypercapnic respiratory failure secondary to left-sided pneumonia. Soon after admission, the patient’s condition deteriorated requiring intubation with mechanical ventilation. The patient was started on broad-spectrum antibiotics with vancomycin, piperacillin-tazobactam, and azithromycin for what was thought to be healthcare-associated pneumonia (HCAP).
Figure 1.
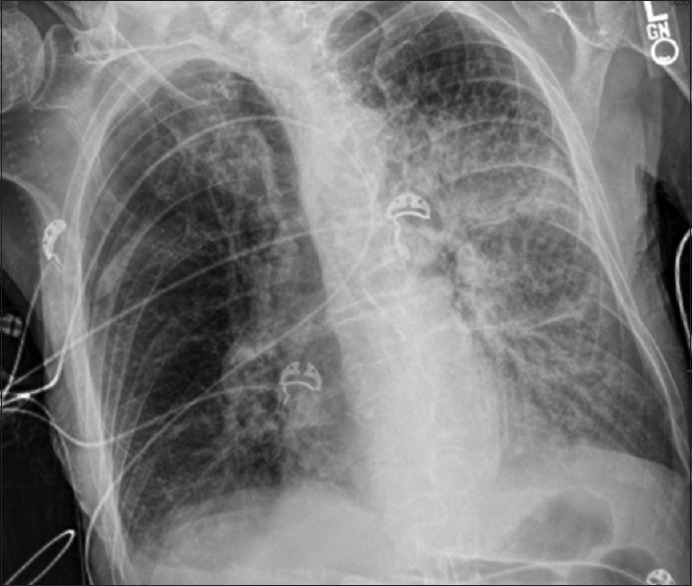
Chest roentgenogram demonstrating consolidation in the left upper lobe, lingula, and left lower lobe
On the 2nd day of hospitalization, initial blood cultures returned positive for P. multocida, confirmed by blood chocolate agar [Figure 2] and gram stain [Figure 3]. At this point, additional history obtained from the patient’s family revealed ownership over five cats and a deep involvement in their care and grooming. A thorough physical examination was performed; however, no scratch or bite marks were identified on the patient nor were any signs of skin infection or abscess found. Following the identification of P. multocida, the patient’s antibiotic regimen was switched to ampicillin-sulbactam. Several days after initiating targeted antibiotic therapy, the patients clinical status improved, leading to successful extubation and discharge on the 18th day of hospitalization. The patient was sent to an extended care facility with amoxicillin-clavulanic acid, with instructions to complete a total of 14 days of antibiotic therapy.
Figure 2.
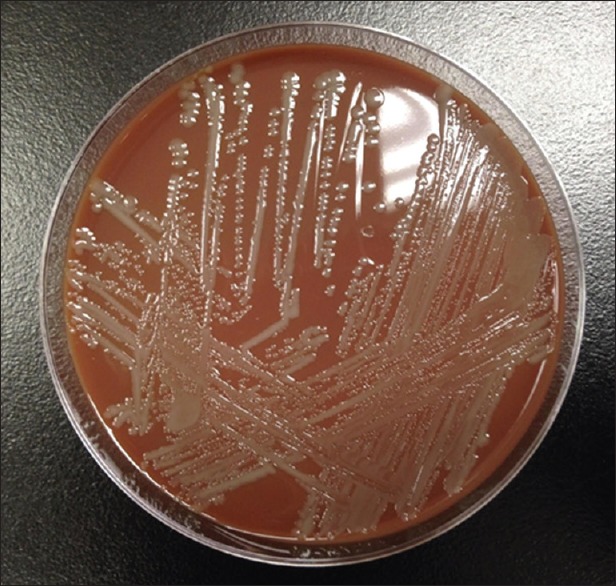
Gray nonhemolytic colonies of Pasteurella multocida on blood chocolate agar
Figure 3.
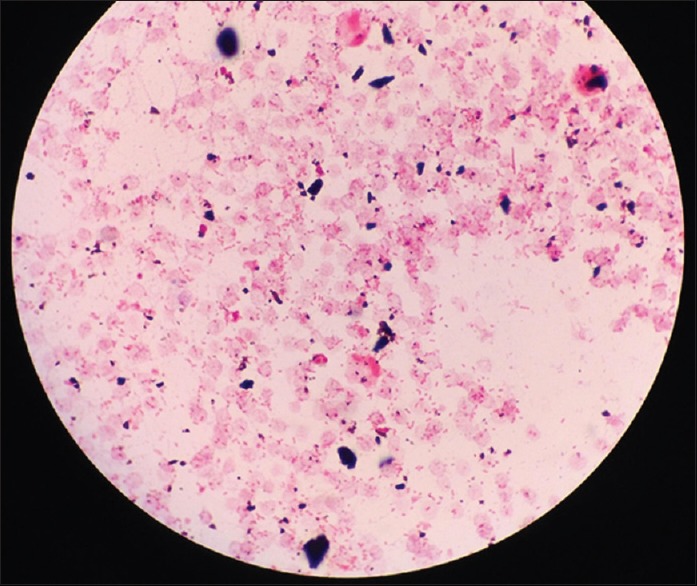
Gram stain of Pasteurella multocida shown as Gram-negative, nonmotile coccobacilli
DISCUSSION
P. multocida is common among domestic animals including cats, dogs, and pigs. Approximately 37% of the United States households own a dog and more than 30% a cat. This data are most consistent with the finding that most infections with P. multocida in humans are due to animal bites and scratches causing skin infections. In general, these animal bite infections account for 1% of all emergency department visits per year.[1] However, P. multocida is an opportunistic pathogen with predilection for causing bacteremia in the immunocompromised. Most often in this patient population, there is no history of animal bites, and infections are associated with poorer outcomes. A retrospective study by Giordano et al., at the Medical University of South Carolina found that 63% of patients with a P. multocida infection had one or more disease process which determined their immunocompromised status. They also noted that in these immunocompromised patients, there was no bite history documented (P = 0.006).[1] Factors which increase the risk for P. multocida infection include underlying lung diseases, cancer, dialysis, and cerebrovascular disease.[1,2]
In the case report, we present a case of P. multocida pneumonia in an immunocompetent patient. There is a pertinent history of recent hospitalization for COPD exacerbation and reported ownership of several cats. Of note, there was no obvious point of entry, bites or scratches, for this pathogenic organism. The patient was treated initially with ampicillin-sulbactam and discharged on amoxicillin-clavulanic acid, as is typical for resistance and sensitivity panels reported for this organism.[3] Although there are some reported cases of antibiotic resistance,[4] our patient’s symptoms and clinical improvement did not suggest this. Considering mortality rates between 7% and 31% with bacteremia,[5] it is imperative that clinicians maintain a high level of suspicion for P. multocida, even in an immunocompetent patient without evidence for portal of entry, when a history of exposure to carrier animals is present.
Systematic review of literature
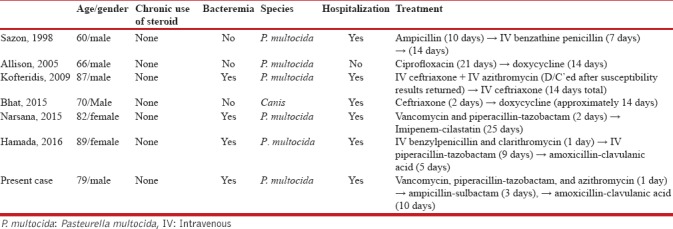
We undertook a focused review of literature of previously reported cases of Pasteurella pneumonia in patients with COPD. Six previously reported cases are described in Table 1.[2,6,7,8,9,10] This literature review supports the use of penicillins as the first line of treatment. In all cases, penicillins were used as the first line because of high susceptibility of Pasteurella to penicillins (97.3%) in respiratory tract infection.[11] However, macrolides were cautiously used after susceptibility testing as its reported susceptibility was as low as 89.4% among macrolides in respiratory tract infection.[11] Therapeutic strategies used in these cases emphasize the importance of susceptibility testing in selection of antimicrobials in Pasteurella pneumonia.
Table 1.
Cases of Pasteurella pneumonia in immunocompetent chronic obstructive pulmonary disease patients
CONCLUSION
Although P. multocida infection is most common in the immunocompromised, mostly in the form of soft-tissue infection, other sites of infection are still possible and have been reported in the immunocompetent. Considering the high mortality rates with P. multocida bacteremia, it is important for clinicians to maintain a high level of suspicion for these organisms in any patient with a history of carrier species exposure.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Giordano A, Dincman T, Clyburn BE, Steed LL, Rockey DC. Clinical features and outcomes of Pasteurella multocida infection. Medicine (Baltimore) 2015;94:e1285. doi: 10.1097/MD.0000000000001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkie IW, Harper M, Boyce JD, Adler B. Pasteurella multocida: Diseases and pathogenesis. Curr Top Microbiol Immunol. 2012;361:1–22. doi: 10.1007/82_2012_216. [DOI] [PubMed] [Google Scholar]
- 3.Wilson BA, Ho M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631–55. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michael GB, Freitag C, Wendlandt S, Eidam C, Feßler AT, Lopes GV, et al. Emerging issues in antimicrobial resistance of bacteria from food-producing animals. Future Microbiol. 2015;10:427–43. doi: 10.2217/fmb.14.93. [DOI] [PubMed] [Google Scholar]
- 5.Narsana N, Farhat F. Septic shock due to Pasteurella multocida bacteremia: A case report. J Med Case Rep. 2015;9:159. doi: 10.1186/s13256-015-0643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sazon DA, Hoo GW, Santiago S. Hemoptysis as the sole presentation of Pasteurella multocida infection. South Med J. 1998;91:484–6. doi: 10.1097/00007611-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Allison K, Clarridge JE., 3rd Long-term respiratory tract infection with canine-associated Pasteurella dagmatis and Neisseria canis in a patient with chronic bronchiectasis. J Clin Microbiol. 2005;43:4272–4. doi: 10.1128/JCM.43.8.4272-4274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kofteridis DP, Christofaki M, Mantadakis E, Maraki S, Drygiannakis I, Papadakis JA, et al. Bacteremic community-acquired pneumonia due to Pasteurella multocida. Int J Infect Dis. 2009;13:e81–3. doi: 10.1016/j.ijid.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Bhat S, Acharya PR, Biranthabail D, Rangnekar A, Shiragavi S. A case of lower respiratory tract infection with canine-associated Pasteurella canis in a patient with chronic obstructive pulmonary disease. J Clin Diagn Res. 2015;9:DD03–4. doi: 10.7860/JCDR/2015/13900.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada M, Elshimy N, Abusriwil H. Infective exacerbation of Pasteurella multocida. Case Rep Infect Dis. 2016;2016:2648349. doi: 10.1155/2016/2648349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lion C, Conroy MC, Carpentier AM, Lozniewski A. Antimicrobial susceptibilities of Pasteurella strains isolated from humans. Int J Antimicrob Agents. 2006;27:290–3. doi: 10.1016/j.ijantimicag.2006.02.004. [DOI] [PubMed] [Google Scholar]


