Abstract
Thyroid hormones act on testis in multiple ways and exert their effect on different cell types, including Leydig and Sertoli cells, and germ cells. An excess or deficit of thyroid hormones results in alterations of testis function, including semen abnormalities. More frequently, hyperthyroidism has been associated with reduced semen volume and reduced sperm density, motility, and morphology, whereas hypothyroidism is associated with reduced sperm morphology. Therefore, thyroid function tests should be part of the diagnostic workup of the infertile man. This article is aimed at (1) elucidating how hyperthyroidism and hypothyroidism lead to a reduction in semen quality, briefly reviewing the current literature on murine models and humans, and (2) pinpointing the limitations of the studies carried out so far and identifying new perspectives for future research.
Keywords: male infertility, semen quality, thyroid hormones
Introduction
The relationship between thyroid and testis has been adequately studied only in the past decades. Particularly, a number of papers have focused on the effects of thyroid hormone on testicular development and function1 and on the relationship between altered thyroid status and infertility.2,3
3,5,3′-triiodothyronine (T3) and thyroxine (T4) regulate testis functioning by genomic and nongenomic effects.2 Genomic effects result from the binding of T3 to its cognate receptor (thyroid hormone receptor, TR) in the nucleus of Sertoli and Leydig cells, where upon binding to the thyroid hormone response elements the hormone–receptor complex activates gene transcription and protein synthesis1,4 (Figure 1). Two genes (TRα and TRβ) encode five isoforms that are obtained by alternate splicing (TRα1, TRα2, TRα3, TRβ1, and TRβ2). TRα2 and TRα3 are devoid of the hormone-binding domain and have been shown to compete with T3 for the binding of the thyroid hormone response element (TRE), suppressing transcription.4 Particularly, TRα1 is the predominant isoform in germ cells (from intermediate spermatogonia to pachytene spermatocyte) and in Sertoli cells, whose development is regulated also by TRβ1 and TRβ2.1 T3 acts on nongerm cells by regulating their proliferation and differentiation1,2 (Figure 1). Particularly, T3 has a double action on Leydig cell, in that in rats it acutely stimulates luteinizing hormone (LH)-mediated steroidogenesis, but chronically inhibits it.3,4 T3 stops Sertoli cell proliferation, determining their number at puberty,5 and alters the attachment between them and gonocytes by inhibiting the expression of the neural cell adhesion molecule5 (Figure 1).
Figure 1.
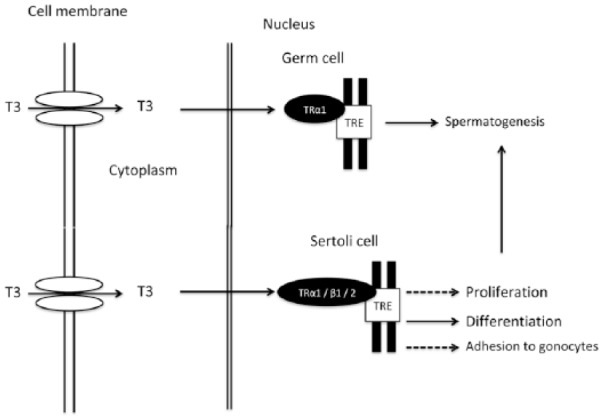
Summary of the effects of T3 on spermatogenesis.
Nongenomic effects of thyroid hormones result from their binding to nonnuclear receptors sited in the cytoplasmic membrane, cytoplasm, cytoskeleton, and mitochondria of the spermatozoon, en-hancing cyclic adenosine monophosphate (cAMP) synthesis and Ca2+ release and ultimately sperm motility.2,6 Recently, T4 was demonstrated to rapidly increase flagellar movements of spermatozoa (hypermotility) and consequently to increase the number of spermatozoa recovered by swim-up.6 All the samples studied (100%) achieved the 5 million threshold of motile sperm for the intrauterine insemination, compared to the 60% of samples treated with pentoxifylline, an inhibitor of cAMP phosphodiesterase.6 Other than T3 and T4, in the literature, other iodothyronines have been reported to act through nongenomic mechanisms by binding to cytoskeleton (3,5′,3′-triiodothyronine, rT3) or mitochondria (T2),7 but their effects on spermatozoa have never been investigated so far.
Finally, thyroid hormones regulate the redox status of testis that relies on a number of antioxidant systems.2 The most abundant mitochondrial protein in the sperm midpiece is glutathione peroxidase (GPx), which includes a family of selenium-containing proteins.8 Selenium is a micronutrient that is fundamental for thyroid homeostasis, as it is incorporated in iodothyronine deiodinases, another class of selenium-containing proteins, which catalyzes conversion of T4 to T3.9 In the testis, GPx has both antioxidant action (when sited in the cytoplasm) and antiapoptotic action (when sited in the mitochondrial capsule).9 Notably, supplementation of selenium-deficient men resulted in the improvement of sperm motility other than improvement in thyroid auoimmunity.8 Other antioxidant systems in the testis include superoxide dismutase, γ-glutamil transferase, catalase, glutathione-S-transferase, cytochrome C, melatonin, vitamin C, vitamin E, and zinc.2 Interestingly, the expression of γ-glutamil transferase and catalase is enhanced by thyroid hormones, while that of GPX and cytochrome C is regulated negatively.10,11
Given these data, it is not difficult to imagine that an altered thyroid function affects spermatogenesis and therefore semen quality.3,4 Most of the studies mentioned in this article have been carried out in mice/rats and subsequently in humans.
In this article, we will interchangeably use the terms “hyperthyroidism” or “thyrotoxicosis” to identify thyroid hormone excess.
Hyperthyroidism
Hyperthyroid rats show a delay in spermatogenesis with maturation arrest, no pachytene spermatocytes, a decrease in seminiferous tubule diameters, an impairment of the mitochondrial activity, and a reduction in lipid concentration.11 Hyperthyroid rodents have also an alteration of antixoidant systems as catalase is upregulated while GPx is downregulated.10 In another animal model, the ram, levothyroxine administration causes a reduction in sperm motility and testis weight.12
In humans, the excess of circulating thyroid hormones during thyrotoxicosis results in asthenozoospermia in more than half of the patients.13 Oligozoospermia and teratozoospermia are found in about 40% of thyrotoxic patients. These abnormalities frequently associate with a reduced semen volume (hypoposia).13 Thus, reduced sperm density, motility, and morphology together with an overall decrease in semen volume are the main semen alterations of thyrotoxic male patients.3
Hudson and Edwards14 reported, first, a lower progressive forward motility in 16 adult men with thyrotoxicosis due to Graves’ disease compared with 21 euthyroid controls. Subsequently, Abalovich et al.13 reported asthenozoospermia, oligozoospermia, and teratozoospermia in 85.7%, 42.9%, and 19%, respectively. Krassas et al.15 found that hyperthyroid patients have a lower sperm motility compared to euthyroid controls (mean ± SEM, 28 ± 8% vs 57 ± 7%, P < 0.01). Notably, semen parameters reverted to normality in the majority of cases upon treatment of hyperthyroidism.15 In a recent study on 163 men referred to an infertility clinic, the rate of subclinical or overt hyperthyroidism was 3.7% or nil.16 Hyperthyroid patients showed a greater difference in seminal vesicle volume before and after ejaculation compared to hypothyroid patients, which represented 7.4%. Seminal vesicle volume and emptying and fructose concentration correlated positively with serum FT3 levels.16 In contrast with the previous studies,3,13 Lotti et al.16 found also a positive correlation between FT3, FT4, and ejaculate volume.
Hypothyroidism
Sperm abnormalities associated with hypothyroidism are partly similar to those reported in hyperthyroidism.2,3 Rats whose thyroid have been blocked with antithyroid drugs show a decrease in seminal volume with an arrest of spermatogenesis and a decrease in the number and diameter of seminal tubules. A concomitant reduction in testis and accessory gland weight compared with euthyroid controls is observed.10 Progressive sperm motility, sperm transit time through the epididymis, and epididymal secretory activity are also affected.11 Furthermore, the number of testicular germ cells is decreased in rats with persistent hypothyroidism but not in those with transient hypothyroidism, while the number of live sperms is reduced both in rats with transient and persistent hypothyroidism.17 This reduction in sperm vitality may result from unbalance between the increased oxidative stress, given, for instance, by lipid peroxidation, and the reduced antioxidant systems, such as catalase and superoxide dismutase.10,17 Very recently, Sarkar and Singh18 have shown that oxidative stress reduces the expression of glucose transporter 3 (GLUT3) in Sertoli cells and glucose transporter 8 (GLUT8) in Leydig cells in propylthiouracil-dosed newborn mice, with a consequent decrease in testicular glucose levels and increased apoptosis of germ cells. Furthermore, oxidative stress downregulates the expression of connexin 43, a gap junctional protein in the seminiferous epithelium that regulates proliferation and apoptosis of germ cells.18 Since thyroid hormones promote Sertoli cell differentiation and inhibit their proliferation, lower concentrations of thyroid hormones after birth, such as in rats affected by congenital hypothyroidism, lead to the extension of the Sertoli cell proliferative period, delaying their differentiation and resulting in increase of testis weight and sperm production.19
In humans, the most frequent semen abnormality in patients with hypothyroidism is teratozoospermia. Indeed, teratozoospermia index, namely, the number of morphology alterations per spermatozoon, correlates negatively with serum T4 levels.20 Altered sperm motility, altered secretory activity of the accessory glands, and low ejaculate volume have been also reported.20 Similar to hyperthyroidism, semen alterations during hypothyroidism are reversible and mostly disappear upon achieving euthyroidism.20
Discussion
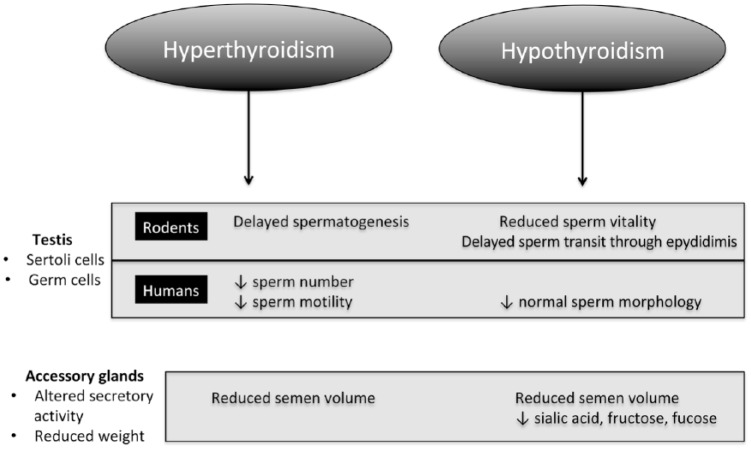
Overall, thyroid dysfunction leads to multiple alterations of semen quality that include reduced volume, sperm density, sperm motility, and sperm morphology. Particularly, concerning conventional parameters of the seminal fluid, hyperthyroidism causes hypospermia, oligozoospermia, asthenozoospermia, and teratozoospermia, whereas hypothyroidism is associated more frequently with teratozoospermia.2 The mechanism whereby thyroid hormone excess or deficiency affects semen quality is poorly understood in humans. It may result from a direct effect on sperm cell, as well as from an effect on nongerm cells (Figure 2). In thyrotoxic rats, sperm mitochondrial activity is reduced and antioxidant defense is altered. Spermatogenesis is also delayed.11 In addition, neonatal Sertoli cell proliferative period is shortened under hyperthyroid conditions.19 On the contrary, thyroid hormone deficiency reduces sperm vitality and delays sperm transit through the epididymis.11 Furthermore, both hyperthyroidism and hypothyroidism are associated with altered macroscopic characteristics of seminal fluid such as reduced volume because of reduced secretory activity of accessory glands (Figure 2).
Figure 2.
Effects of thyroid dysfunction on seminal characteristics in rodents and in humans.
To the best of our knowledge, only one recent study focused on biofunctional nonconventional sperm parameters in rats.11 In this study, the authors found that hypothyroidism causes a reduction in acrosome integrity and mitochondrial activity, while it increases plasma membrane integrity.11 Instead, hyperthyroidism decreases mitochondrial activity and increases plasma membrane integrity.11 Regarding humans, no study to date has addressed nonconventional sperm parameters during either the hyperthyroid or hypothyroid state.
Only one recent study has focused on fertility in men with subclinical thyroid dysfunction,16 but the small number of patients investigated prevents from drawing firm conclusions.
Limitations of the studies in literature are three. First, criteria used to diagnose semen abnormalities were frequently different from study to study. Second, in several studies, cohorts included men from infertile couples, namely, patients with low semen quality for reasons other than thyroid dysfunction. Third, studies performed so far have enrolled small cohorts of patients, hence lowering their statistical power.
In conclusion, although the screening of thyroid function is not recommended to date as part of the diagnostic workup of the infertile male, it might be reconsidered in light of the physiopathological background, provided that the evidence is further confirmed by multicentric studies on larger and homogeneous cohorts. Indeed, both hyperthyroidism and hypothyroidism are common in the general population, with a prevalence of 1% and at least 6%, respectively.
Further research is needed to investigate (1) whether hyperthyroidism or hypothyroidism affects nonconventional sperm parameters (2) whether subclinical thyroid dysfunction influences male fertility.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sandro La Vignera  https://orcid.org/0000-0002-7113-2372
https://orcid.org/0000-0002-7113-2372
Roberto Vita  https://orcid.org/0000-0002-5977-3062
https://orcid.org/0000-0002-5977-3062
References
- 1. Wagner MS, Wajner SM, Maia AL. (2008) The role of thyroid hormone in testicular development and function. Journal of Endocrinology 199(3): 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. La Vignera S, Vita R, Condorelli RA, et al. (2017) Impact of thyroid disease on testicular function. Endocrine 58: 397–407. [DOI] [PubMed] [Google Scholar]
- 3. Wajner SM, Wagner MS, Maia AL. (2009) Clinical implications of altered thyroid status in male testicular function. Arquivos Brasileiros de Endocrinologia e Metabologia 53(8): 976–982. [DOI] [PubMed] [Google Scholar]
- 4. Jannini EA, Ulisse S, D’Armiento M. (1995) Thyroid hormone and male gonadal function. Endocrine Reviews 16(4): 443–459. [DOI] [PubMed] [Google Scholar]
- 5. Holsberger DR, Cooke PS. (2005) Understanding the role of thyroid hormone in Sertoli cell development: A mechanistic hypothesis. Cell and Tissue Research 322(1): 133–140. [DOI] [PubMed] [Google Scholar]
- 6. Mendeluk GR, Rosales M. (2016) Thyroxin is useful to improve sperm motility. International Journal of Fertility & Sterility 10(2): 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis PJ, Goglia F, Leonard JL. (2016) Nongenomic actions of thyroid hormone. Nature Reviews Endocrinology 12(2): 111–121. [DOI] [PubMed] [Google Scholar]
- 8. Duntas LH, Benvenga S. (2015) Selenium: An element for life. Endocrine 48(3): 756–775. [DOI] [PubMed] [Google Scholar]
- 9. Ahsan U, Kamran Z, Raza I, et al. (2014) Role of selenium in male reproduction: A review. Animal Reproduction Science 146(1–2): 55–62. [DOI] [PubMed] [Google Scholar]
- 10. Choudhury S, Chainy GB, Mishro MM. (2003) Experimentally induced hypo- and hyper-thyroidism influence on the antioxidant defence system in adult rat testis. Andrologia 35(3): 131–140. [DOI] [PubMed] [Google Scholar]
- 11. Romano RM, Gomes SN, Cardoso NC, et al. (2017) New insights for male infertility revealed by alterations in spermatic function and differential testicular expression of thyroid-related genes. Endocrine 55(2): 607–617. [DOI] [PubMed] [Google Scholar]
- 12. Chandrasekhar Y, Holland MK, D’Occhio MJ, et al. (1985) Spermatogenesis, seminal characteristics and reproductive hormone levels in mature rams with induced hypothyroidism and hyperthyroidism. Journal of Endocrinology 105(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 13. Abalovich M, Levalle O, Hermes R, et al. (1999) Hypothalamic-pituitary-testicular axis and seminal para-meters in hyperthyroid males. Thyroid 9(9): 857–863. [DOI] [PubMed] [Google Scholar]
- 14. Hudson RW, Edwards AL. (1992) Testicular function in hyperthyroidism. Journal of Andrology 13(2): 117–124. [PubMed] [Google Scholar]
- 15. Krassas GE, Pontikides N, Deligianni V, et al. (2002) A prospective controlled study of the impact of hyperthyroidism on reproductive function in males. Journal of Clinical Endocrinology and Metabolism 87(8): 3667–3671. [DOI] [PubMed] [Google Scholar]
- 16. Lotti F, Maseroli E, Fralassi N, et al. (2016) Is thyroid hormones evaluation of clinical value in the work-up of males of infertile couples? Human Reproduction 31(3): 518–529. [DOI] [PubMed] [Google Scholar]
- 17. Sahoo DK, Roy A, Bhanja S, et al. (2008) Hypothyroidism impairs antioxidant defence system and testicular physiology during development and maturation. General and Comparative Endocrinology 156(1): 63–70. [DOI] [PubMed] [Google Scholar]
- 18. Sarkar D, Singh SK. (2017) Neonatal hypothyroidism affects testicular glucose homeostasis through increased oxidative stress in prepubertal mice: Effects on GLUT3, GLUT8 and Cx43. Andrology 5(4): 749–762. [DOI] [PubMed] [Google Scholar]
- 19. Rijntjes E, Gomes MLM, Zupanic N, et al. (2017) Transient hypothyroidism: Dual effect on adult-type Leydig cell and Sertoli cell development. Frontiers in Physiology 8: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krassas GE, Poppe K, Glinoer D. (2010) Thyroid function and human reproductive health. Endocrine Reviews 31(5): 702–755. [DOI] [PubMed] [Google Scholar]