Abstract
Iron deficiency or iron deficiency anemia (IDA) are some of the most common systemic complications of inflammatory bowel diseases (IBD). Symptoms such as fatigue, reduced ability to concentrate and reduced exercise tolerance can mimic common symptoms of IBD and can therefore easily be overseen. Furthermore, clinicians tend to see mild to moderate anemia as an inevitable accompaniment of IBD that is sufficiently explained by the underlying disease and does not require further workup. But in contrast to these clinical routines, current guidelines recommend that any degree of anemia in patients with IBD should be further evaluated and treated. Multiple studies have shown that anemia is a main factor for decreased quality of life (QoL) in patients with IBD. Correction of anemia, however, can significantly improve the QoL of patients with IBD. It is therefore recommended that every patient with IBD is regularly screened for iron deficiency and anemia. If detected, appropriate workup and treatment should be initiated. Over the last years, a number of new diagnostic tools and treatment options have been developed. Multiple studies have demonstrated the safety of newer formulations of intravenous iron in patients with IBD and have compared oral and intravenous iron in various situations. Treatment recommendations have changed and new evidence-based guidelines were developed. However, to date these guidelines are still not widely implemented in clinical practice. The aim of this review is to draw attention to the need for treatment for every level of anemia in patients with IBD and to provide some practical guidance for screening, diagnostics, treatment and follow up of IDA in patients with IBD following current international guidelines.
Keywords: Crohn’s disease, inflammatory bowel disease, iron deficiency, iron deficiency anemia, ulcerative colitis
Clinical relevance
Epidemiology
Anemia is the most common systemic complication in patients with inflammatory bowel disease (IBD). A recent meta-analysis by Gisbert and colleagues found a prevalence of approximately 16% in outpatients with IBD and 68% in inpatients with IBD.1 The main cause of anemia in patients with IBD is iron deficiency and the mean prevalence of iron deficiency was 45% in all patients with IBD combined.1 In another meta-analysis by Kulnigg and colleagues the prevalence of iron deficiency in patients with Crohn’s disease (CD) ranged from 36% to 90% depending on the cohort studied and the definition of iron deficiency used, with a higher proportion of iron deficiency in inpatients compared with outpatients as well as in older studies compared with newer studies.2
Pathogenesis
The human body contains approximately 3–5 g of iron. The majority of iron occurs in the hemoglobin (Hb) of red blood cells (2–4 g), followed by storage iron in the form of ferritin or hemosiderin (1–1.5 g), myoglobin (100–300 mg) and iron bound to enzymes (less than 100 mg). Plasma iron and iron bound to transferrin together sum up to approximately 3 mg. In healthy adults, daily iron loss is about 1–2 mg/day in the form of desquamation of epithelial cells from the intestinal mucosa, the bile tract, the urinary tract and the skin, as well as in the form of menstrual blood loss.3 The same amount of iron is normally absorbed from food sources. The maximal iron absorption occurs in the duodenum and to a lesser extent in the proximal ileum.3
The most important cause of iron deficiency in patients with IBD is increased iron loss due to ongoing gastrointestinal (GI) blood loss from the inflamed mucosa. In addition, iron absorption can be reduced due to inflamed intestinal mucosa, especially if the duodenum is affected.4 Other causes include patients who have undergone upper GI surgery. Dietary restrictions in patients with IBD may also play a prominent role.4 When iron storages are exhausted, anemia occurs.
Although iron deficiency can often be identified as the most important cause of anemia, the pathogenesis of anemia in patients with IBD is complex and often multifactorial. The second most common cause is anemia of chronic disease (ACD) and in a relevant proportion of patients, both etiologies are present.1 In fact, IBD is a classic example for concurrent iron deficiency anemia (IDA) and ACD and anemia due to both entities is a clear marker of active disease in patients with IBD.5,6
However, ACD is not an IBD-specific clinical problem. It can be found in patients with a variety of chronic infections or inflammation as well as other chronic conditions that are associated with immune activation such as malignancies or other autoimmune diseases.7
In ACD inflammatory cytokines are upregulated and can induce changes in the iron homeostasis through multiple pathways. Thereby, the acute phase protein hepcidin plays a central role. It is generated in the liver and upregulated by inflammatory cytokines such as interleukin 6 and lipopolysaccharides. If upregulated, hepcidin blocks the basolateral iron exporter ferroportin 1 on duodenal enterocytes, macrophages and hepatocytes. This results in a reduced intestinal absorption of iron and in a shift of iron from the circulation to the storage sites. These effects combined can in turn create functional iron deficiency.7 In addition, other proinflammatory cytokines, most notably tumor necrosis factor α (TNFα), interleukin 1 and interferon α, β and γ, can have an inhibitory effect on multiple steps of erythropoiesis: The proliferation and differentiation of erythroid progenitor cells are directly impaired by inflammatory cytokines, erythropoietin response is often inadequate for the level of anemia and the responsiveness of the erythroid progenitor cells to erythropoietin is also decreased when inflammatory cytokines are elevated. In addition, free radicals and proinflammatory cytokines can potentially cause direct damage to erythrocytes resulting in an increased degree of erythrophagocytosis and a reduced erythrocyte half-life.7
Other, less common risks of anemia in patients with IBD include vitamin B12 or folate deficiency, especially in patients with malabsorption of the upper GI tract due to small bowel manifestations of IBD or extensive small bowel resections.1 Anemia can also be induced by medications used for IBD (e.g. sulfasalazine, thiopurines).1 As in otherwise healthy individuals, other causes of anemia such as inborn hemoglobinopathias, myelodysplastic syndrome or hemolysis can be present and should be ruled out, depending on the individual patient background. An overview of possible causes of anemia is provided in Table 2 (in the section ‘Diagnosis’).
Table 2.
Causes of anemia and influence on red blood cell morphology and reticulocyte count.
| Morphology | Reticulocyte count | Examples of causes of anemia |
|---|---|---|
| Macrocytic anemia (MCV > 100 fl) | normal/low | Vitamin B12 or folate deficiency |
| Drug induced (azathioprin, sulfasalazin, methotrexate) | ||
| Myelodysplatic syndrome | ||
| elevated | Hemolysis | |
| Myelodysplastic syndrome with hemolysis | ||
| Normocytic anemia (MCV between 80 and 100 fl) | normal/low | Early iron deficiency anemia |
| Anemia of chronic disease | ||
| Aplastic anemia | ||
| Renal anemia | ||
| Acute hemorrhage | ||
| elevated | Hemolysis | |
| Myelodysplastic syndrome with hemolysis | ||
| Microcytic anemia (MCV < 80 fl) | normal/low | Iron deficiency anemia |
| Anemia of chronic disease (mostly normocytic) | ||
| Hereditary anemia | ||
| elevated | Hemoglobinopathies (e.g. thalassemia) |
MCV, mean corpuscular volume.
Clinical presentation
Typical symptoms of IDA include fatigue, decreased physical performance, dizziness, headaches, dyspnea on exertion and pallor of the skin, nails and conjunctiva. In severe cases, tachycardia, a functional systolic heart murmur, syncope, dyspnea at rest or angina pectoris symptoms can occur.8
In addition, iron deficiency with or without anemia can create several nonhematologic symptoms. Iron is essential for the energy metabolism of all human cells and almost every organ system. Resulting symptoms include decreased exercise tolerability,9 impaired cognitive performance,9 increased risk of infection,9 alterations of thyroid hormones, catecholamines and neurotransmitters,9 dryness of the skin, breakage of the hair8 and restless leg syndrome. 8
Overall symptoms of iron deficiency are often vague and nonspecific. In chronic iron deficiency and IDA, the symptoms may be less pronounced than otherwise expected from low Hb levels. This can be due to adaption of the body to low Hb values or due to adaption to the resulting functional deficits. In many cases, the extent of the symptoms of anemia can only be identified in retrospect after treatment. In patients with IBD, this is further complicated by the fact that symptoms from IDA and symptoms from the underlying disease can be difficult to distinguish.
The impact of IDA on the quality of life (QoL) can be significant. In fact, chronic fatigue caused by anemia can worry patients with IBD as much as abdominal pain or diarrhea.1 It was demonstrated that there is a demand from patients with IBD for additional psychotherapy that depends on social characteristics such as QoL.10 Multiple studies over recent years have shown that IDA correlates with a significantly reduced overall QoL and that successful treatment of iron deficiency can improve QoL unrelated to IBD disease activity.1,11,12
Screening
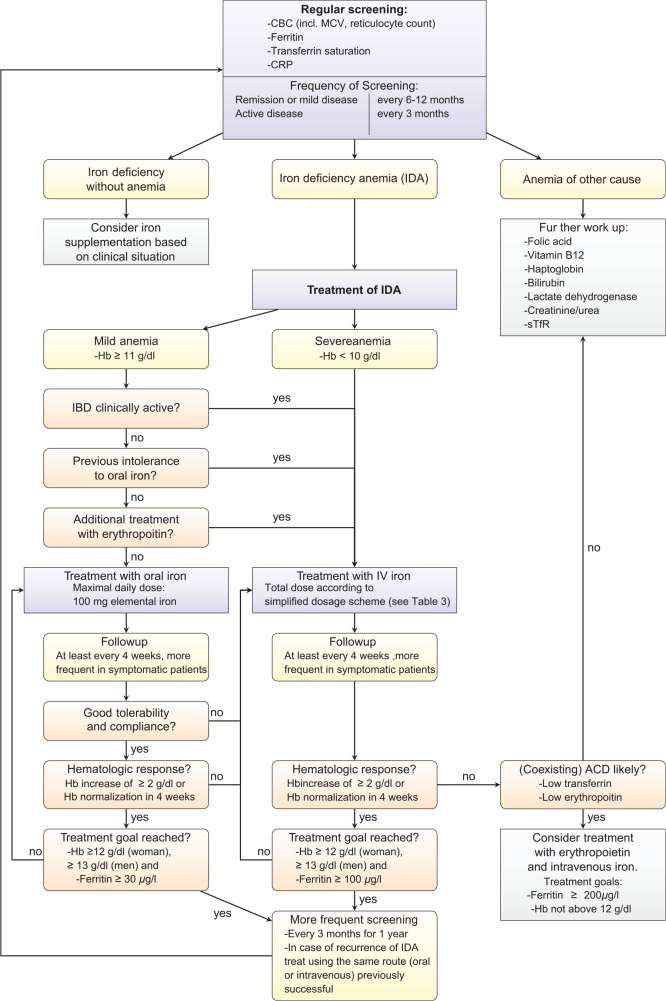
As outlined above, patients with IBD have a high risk of anemia, and if present, anemia is a main factor contributing to morbidity. It is therefore important that every patient with IBD is screened for anemia. The European Crohn’s and Colitis Organization (ECCO) recommend a laboratory screening using complete blood count (CBC), serum ferritin and C-reactive protein (CRP) for every patient with IBD in their 2015 guidelines.6 Screenings should be repeated every 6–12 months for patients in remission or with mild disease and at least every 3 months for patients with active disease in the outpatient setting. Vitamin B12 and folic acid should also be tested at least once a year if a patient has risk factors (e.g. extensive small bowel disease or resection, ileoanal pouch) or if macrocytosis is present.6,13 Figure 1 in section ‘Summary’ visualizes the recommended screening algorithm.
Figure 1.
Algorithm for screening and treatment of iron deficiency anemia of patients with inflammatory bowel disease (IBD) as described in this paper and based on previous publications by Dignass and colleagues and Gasche and colleagues.6,25
ACD, anemia of chronic disease; CBC, complete blood count; CRP, C-reactive protein; IDA, iron deficiency anemia; Hb, hemoglobin; MCV, mean corpuscular volume; sTfR, soluble transferrin receptor.
Diagnosis
The diagnosis of IDA in patients with IBD is complicated by the fact that ACD with or without functional iron deficiency can overlap in one patient. Chronic inflammatory state can impact the values of iron storage proteins as these are also acute phase proteins. For this end, as described below, some of the standard values have to be adjusted in this group of patients.
Hb values and definition of anemia
The World Health Organization (WHO) defines cutoff values below which anemia is present in a population at sea level (see Table 1). It should be kept in mind that these normal values vary depending on factors such as age, sex, smoking habits, altitude and genetic influences. However, it is a common misconception that cuff of values for anemia are different in patients with IBD. Instead, the same definition of anemia applies for anemia in patients with IBD and in healthy controls.6
Table 1.
The World Health Organization definition of anemia: hemoglobin levels below which anemia is present at sea level.9
| Age or sex group | Hemoglobin (g/dl) |
|---|---|
| Children 6–59 months | 11 |
| Children 5–11 years | 11.5 |
| Children 12–14 years | 12 |
| Nonpregnant women (above 15 years of age) | 12 |
| Pregnant women | 11 |
| Men (above 15 years of age) | 13 |
Adapted from World Health Organization. “Nutritional anaemias: tools for effective prevention and control.”, Page 7, Copyright (2017).
Complete blood cell count
A CBC for diagnostic evaluation of anemia should include the following cell indices: red blood cell count (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), reticulocytes count/production index, red cell distribution width (RDW), thrombocyte count and leucocyte count. In order to differentiate between various forms of anemia, the first step is to look at the MCV and MCH to identify macrocytic/normocytic/microcytic anemia.13 The next step is to evaluate the reticulocyte count and to differentiate between appropriate and inappropriate erythropoiesis. The morphology of red blood cells and the reticulocyte count can help to identify the various causes of anemia, as shown in Table 2.
In clinical practice, it has to be considered that multiple causes of anemia can be present in one patient. For example, coexisting IDA and ACD can be microcytic or normocytic. In IDA with concurrent drug-induced anemia (e.g. in the case of treatment with azathioprine), the lab test can be microcytic or even show a macrocytic anemia. This shows that the presence of macrocytic anemia alone should not be mistaken as a reason to exclude iron deficiency. MCH and MCHC are usually decreased in iron deficiency anemia, but are usually normal in ACD.7
The RDW shows how much the erythrocytes differentiate in their size. It is commonly increased in IDA and within normal range in thalassemia. However, in patients with IBD, it has to be considered that nutritional deficits and disease activity can also increase the RDW.14
Reticulocyte count is reduced (or ‘normal’, although it should physiologically be upregulated in anemia) in iron deficiency as a sign of inappropriate erythropoiesis. If the reticulocyte count is increased, hemolysis is an important differential diagnosis (lactate dehydrogenase (LDH), bilirubin and haptoglobine are parameters that help to further investigate the presence of hemolysis). The reticulocyte production index (RPI) puts the reticulocyte count in relation to the severity of anemia (RPI = reticulocyte count in % × hematocrit of the patient/ideal hematocrit (=0.45) × reticulocyte maturation time).3 As a result of the shorter half-life, reticulocyte indices can reflect changes in the iron homeostasis within 3–10 days, much faster than erythrocyte indices.
Iron storage indices
Serum ferritin is the most common parameter to evaluate iron storage. Ferritin is an intracellular cytosolic protein that stores and releases iron in an organized fashion, but is also secreted in the serum. Normal values are 15–100 μg/liter for women and 30–200 μg/liter for men. Thereby, a serum ferritin level of 100 μg/liter correlates with a total iron storage of 1000 mg. In patients with IBD the usage of ferritin is complicated by the fact that it is an acute phase protein and can increase in the setting of inflammation.3 However, if serum ferritin is below the lower cutoff, iron deficiency can be diagnosed, but if ferritin is normal, iron deficiency cannot be excluded in patients with IBD. The 2015 ECCO guidelines therefore recommend a serum ferritin 30 μg/liter as a cutoff in patients with clinical, endoscopical and biochemical remission. In patients with active inflammation a serum ferritin 100 μg/liter may still be consistent with iron deficiency.6
Transferrin is the main protein for iron transport in the plasma. Normal values depend on the laboratory but are approximately 200–400 mg/dl. In a healthy adult, the transferrin saturation (TfS) with iron is approximately one third (16–45%). It should be mentioned that serum iron and TfS are subject to quite significant circadian effects.3 In the case of iron deficiency, transferrin is upregulated and TfS decreases. A TfS less than 20% is consistent with iron deficiency with a sensitivity of 90% and a specificity of only 40–50%.3 Transferrin is downregulated in the case of iron overload, malignant diseases or chronic infection. Therefore, interpretation is difficult in active IBD. The 2015 ECCO guidelines define ACD as ferritin greater than 100 μg/liter and TfS less than 20%. If ferritin is between 30 and 100 μg/liter, and TfS is less than 20%, a combination of IDA and ACD is likely.6
Soluble transferrin receptor (sTfR) is a relative new marker for iron metabolism. It is a truncated soluble form of the transferrin receptor that correlates with the total mass of cellular transferrin receptors on erythroid precursor cells of the bone marrow. Its concentration correlates with increased iron need in the bone marrow either in the case of (functional or absolute) iron deficiency or in situations with increased erythropoiesis (e.g. hemolysis). It is reduced in conditions with hypoproliferative erythropoiesis (aplastic anemia, renal anemia). An advantage compared with the above discussed markers is that the concentration is entirely independent from inflammation or hepatic function.3 sTfR and an index of sTfR/ferritin have been proposed as indices to differentiate between IDA and ACD.15 Nevertheless, data are conflicting about its sensitivity and specificity compared with conventional markers like ferritin.3 But a relatively new study from 2011, investigating 100 patients with IBD, suggested that the sTfR/ferritin index was the most sensitive marker for IDA in IBD with the benefit of being independent of disease activity.16 Disadvantages are the high costs and the lack of a uniform reference range up to now.3
Clinical routine
As described above, many diagnostic options exist to identify iron deficiency and IDA. However, depending on the clinical situation, a small test panel is usually sufficient. The 2015 ECCO guidelines recommend a CBC (including MCV and reticulocyte count), ferritin, TfS and CRP.6 More extensive workup depends on the clinical situation and availability. It may include vitamin B12, folic acid, haptoglobin, bilirubin, LDH, creatinine and urea, sTfR and other parameters.6
Diagnosis of iron deficiency without anemia
Iron deficiency with relevant clinical symptoms can be present even before anemia develops as a long-term consequence. In such cases, early changes in the CBC, for example an increased RDW or an MCH close to the lower limit of normal, can represent early manifestations of iron deficiency.17 As described above, the reticulocyte count can be decreased even before erythrocyte indices are affected. Iron storage indices can also be interpreted in a similar way as in the diagnosis of IDA: in patients with IBD and no clinical, endoscopic or biochemical sign of active disease, a ferritin level below 15–30 μg/liter is diagnostic for iron deficiency.17 In the case of active disease, a ferritin level up to 100 μg/liter can still be consistent with iron deficiency. A TfS below 20% reflects a reduced availability of iron for erythropoiesis, either due to total iron deficiency or due to functional iron deficiency (e.g. in chronic inflammation or following treatment with erythropoietin). It was suggested that a TfS below 20% with a ferritin below 100 μg/liter defines total iron deficiency. A TfS below 20% and a ferritin above 100 μg/liter were defined as functional iron deficiency.17 In otherwise unclear cases, sTfR can also be useful.
Treatment
Anemia is one of the manifestations of IBD that often remains undertreated. It has to be considered that IDA is already the endpoint of iron deficiency. As described above, multiple studies have shown a strong correlation between Hb levels and quality of life in patients with IBD.1,11,12 Therefore, IDA should be treated in every patient with IBD, for every level of anemia. The goal of treatment should be complete normalization of anemia and iron stores.6
In patients with IBD and iron deficiency without anemia, there is no clear evidence of benefits of iron supplementation. Studies in patients with congestive heart failure or in patients with chronic fatigue have demonstrated an increase in QoL when iron deficiency was treated, but there are no data available for patients with IBD. Therefore, the decision to treat iron deficiency without anemia should be based on the symptoms of the individual patient.6
A number of comparative studies between oral and intravenous iron supplementation have been conducted and have confirmed that GI side effects are significantly less frequent when intravenous iron is used.18–20 More recently, two meta-analyses could confirm these results.21,22 Furthermore, intravenous iron could replenish iron storage and improve anemia faster and more efficiently than oral iron in clinical studies18,23 as well as in a recent meta-analysis.22 However, the clinical significance of faster Hb level increase remains unclear as differences in QoL were not different between the intravenous and the oral treatment arm. Disease activity was also not significantly different.22
The choice of front-line therapy varies between continents. While intravenous iron is considered the standard treatment in patients with IBD (with active disease) in Europe, oral iron remains the first-line treatment in the US.24 Some authors propose intravenous administration as the preferred route for all patients with IBD (e.g. Gasche and colleagues25), while other authors point out that both routes can be efficient and safe to use, especially when considering the facts that GI side effects to oral iron may be dose dependent26 and that newer oral formulations might minimize these effects.27
Taking together all the evidence today and in line with current international guidelines,6 oral iron is sufficient in some situations but intravenous iron should be preferred in other specific clinical situations depending, for example, on side effects to oral iron, disease activity and level of anemia. The recommended algorithm for the treatment of IDA in patients with IBD is summarized in Figure 1 in section ‘Summary’.
Oral iron
Oral iron is cost effective and widely available. It is the standard first-line treatment in otherwise healthy patients with IDA. In patients with IBD, oral iron is recommended for patients with mild anemia (Hb ⩾ 11 g/dl) whose disease is clinically inactive and who have not been previously intolerant to oral iron.6
Multiple studies have shown that oral iron is effective and safe in patients with IBD. For example, a study from 2009 in 78 patients with mild anemia (Hb > 10 g/dl) and inactive disease showed a hematologic response (complete normalization of Hb) in 89%. Intolerance was seen in only 5.1% of the patients, who were then subsequently switched to intravenous iron. IBD activity did not increase in any patient and QoL increased in line with normalization of Hb.12
However, there are some drawbacks for oral iron supplementation in patients with IBD. Most importantly, GI side effects are frequently associated with oral iron application. Nonabsorbed iron is exposed to the inflamed intestinal mucosa and can increase inflammation and disease activity. Multiple animal studies have shown that iron enhances intestinal inflammation.2 In humans, a recent meta-analysis on 669 patients with IBD and IDA could demonstrate that GI side effects were more common with oral iron supplementation compared with intravenous application.21 Intolerance to oral iron in turn is leading to discontinuation of treatment in as many as 21% of the patients treated.2
In clinical practice, oral iron supplementation is further complicated by the fact that mucosal inflammation can cause ongoing malabsorption of iron.4 This effect is even stronger if ACD is also present due to the increased concentration of hepcidin that further downregulates the intestinal iron absorption.7
Another consideration is the microbiome of the gut. Noticeable shifts in gut bacterial diversity have been described in patients with IBD and iron deficiency following oral iron supplementation (however, other changes were also observed following intravenous treatment) and the gut microbiome in turn might have an effect on disease activity in IBD.28
A study from 2010 in anemic African children could further demonstrate these changes in the gut microbiota. In the group that was treated with oral iron for 6 months, fecal enterobacteria increased and fecal bifidobacteria decreased compared with the placebo group. Fecal calprotectin levels that are associated with intestinal inflammation were also increased in the iron group.29 This can be explained by the fact that some bacteria are more dependent on iron than others.
Another recent study in a mouse model could show that an iron sulfate free diet in systemic iron deficient mice prevents the development of chronic ileitis in a mouse model for Crohn’s disease. It was suggested that luminal iron may trigger epithelial stress and apoptosis as well as changes in the microbiotic homeostasis.30
To address these potential negative effects of oral iron supplementation in clinical practice, some new alternatives of oral iron treatment have been proposed. A recent work suggests that standard doses of oral iron may be too extensive. Daily iron absorption can vary from 0.5 mg in a healthy adult to 20 mg in iron deficiency and excess of oral iron intake can increase oxidative stress and inflammation in the colon and can cause disturbances of the microbiome. Low-dose oral iron with no more than 100 mg of total elemental iron has been shown to be equally effective with a preferable tolerability in new studies26 and is therefore recommended in the current ECCO guidelines.6 Of note, a 2015 meta-analysis did not show a dose-dependent effect of GI side effects of oral iron.21
Another new approach in oral iron treatment is the use of ferric (Fe 3+) iron in a complex with maltol. Traditional oral iron supplements contain ferrous (Fe 2+) iron in a complex with sulfate, gluconate or fumarate. It was suggested that ferric (Fe3+) iron can be better absorbed, that smaller doses are sufficient and that the risk of GI side effects is therefore decreased. In a recent phase III trial, 128 patients with quiescent, mild or moderate IBD and mild to moderate IDA were randomly assigned to receive ferric maltol or placebo. All participants had previously documented failure to respond to oral iron treatment. It was confirmed that ferric maltol can effectively increase Hb levels and that GI side effects or treatment compliance were not different compared with placebo. Although data are still limited, these results are promising and suggest that oral ferric maltol can become an alternative for patients with IBD previously intolerant to oral iron.27
Intravenous iron
The 2015 ECCO guidelines recommend intravenous iron as a first-line treatment in patients with active IBD, in patients with severe anemia (Hb < 10 g/dl), in patients with previous intolerance to oral iron and in patients with additional need for treatment with erythropoietin.6
As described above, intravenous iron was shown to increase Hb levels faster and this is of particular importance in patients with severe anemia. In patients with decreased intestinal absorption of iron due to active mucosal inflammation, the intestinal route can be bypassed by using intravenous iron supplementation. The same applies for patients with concurrent ACD with upregulated hepcidin and downregulated iron absorption. Although intravenous iron is recommended as a first choice agent in the above-mentioned scenarios, it is widely underused in clinical practice.31 This can be due to lower availability, increased costs and inconvenience of intravenous application, but there are also some preconceptions that remain common among medical practitioners:
One common concern is that intravenous iron can exacerbate inflammation in general. As described above, iron is a nutrient for some kinds of bacteria. It is also a pro-oxidant and as such it can theoretically aggravate inflammation. Studies in animal models have illustrated that iron can exacerbate experimental sepsis. Human studies in contrast have shown a transient increase in markers for oxidative stress but no negative clinical outcomes in terms of infectious complications following intravenous iron treatment. With current literature an exact conclusion is not possible, but the risk for increased infection, if there is any, is likely very small.32
It is also widely believed that intravenous iron is associated with a high risk of anaphylactoid reactions. While this was true for earlier formulations using high molecular weight (HMW) iron dextran, it is not true for the newer formulas that are the treatment standard today.
In summary, it can be said that intravenous iron with the formulations used today is safe to use in patients with IBD with a very low risk of adverse reactions and a generally favorable tolerability.32
Dosage of intravenous iron
Traditionally, the iron deficit (in mg) of a patient with IDA was calculated using the Ganzoni formula: [body weight in kg × (target Hb – actual Hb in g/dl) × 0.24 + 500].6
In 2011, the FERRIcor trial compared a new, simplified fixed dose regimen for carboxymaltose with individually calculated doses of iron sucrose following the Ganzoni formula in 489 patients with IBD and IDA. Subjects received up to three doses of 500 or 1000 mg of ferric carboxymaltose, or up to 11 infusions of 200 mg iron sucrose. Significantly more patients in the group of the new fixed dose regimen had a Hb response (Hb increase > 2 mg/dl) or Hb normalization by week 12. The compliance to study protocol was larger in this group; the safety profile was favorable in both groups.33 It was suggested that the Ganzoni formulation underestimates the iron requirements for patients with IBD because of ongoing GI bleeds and malabsorption. The 2015 ECCO guidelines therefore recommend the new simplified dosage scheme for treatment with intravenous iron in general (not only for ferric carboxymaltose for which it was validated).
Patients with severe anemia with an Hb greater than 7 g/dl were not included in the FERRIcor trial. In these cases, the ECCO guidelines recommend an additional 500 mg of intravenous iron.
In patients with symptomatic iron deficiency without anemia, a minimum of 500–1000 mg of iron is recommended in the current ECCO guidelines. This is based on a study that showed an improved QoL following treatment with 1000 mg of intravenous iron in otherwise healthy women with symptomatic iron deficiency.34 Another study in patients with IBD could demonstrate a decreased rate of recurrence of anemia when iron deficiency without anemia was treated with intravenous iron.35
Table 3 summarizes the simplified dosage scheme for intravenous iron recommended by the ECCO 2015 guidelines.
Table 3.
Simplified scheme for intravenous iron replacement therapy in patients with inflammatory bowel disease.
| Hemoglobin level | <70 kg | >70 kg | Source |
|---|---|---|---|
| Symptomatic iron deficiency without anemia > 12 g/dl (women) > 13 g/dl (men) |
500–1000 mg | 500–1000 mg | ECCO 20156based on Favrat et al.33 and Evstatiev et al.34 |
| 10–12 g/dl (women)10–13 g/dl (men) | 1000 mg | 1500 mg | ECCO 20156based on the FERRIcor trial32 |
| 7–10 g/dl | 1500 mg | 2000 mg | ECCO 20156based on the FERRIcor trial32 |
| < 7 g/dl | 2000 mg | 2500 mg | ECCO 20156 |
ECCO, European Crohn’s and Colitis Organization.
Selection of intravenous iron formulation
A number of different formulations for intravenous iron are available and have been shown to be effective in iron deficiency in patients with IBD.
Historically, the only available formulation of intravenous iron was HMW iron dextran (‘DexFerrum’, Vifor (International) Inc., Switzerland). Back then, intravenous iron was largely seen as dangerous and as a last resort for specific situations due to a risk of infrequent but severe anaphylactoid reactions to HMW iron dextran.36 Today, HMW iron dextran should be avoided due to its unfavorable safety profile.32
The risk of severe side effects is much lower in low molecular weight (LMW) iron dextran (‘CosmoFer’, Pharmacosmos, Holbaek, Denmark, ‘INFeD’, Allergan, Dublin, Ireland). A study that evaluated all reported side effects to the US Food and Drugs Administration (FDA) from 1998 to 2000 demonstrated that the risk of life-threatening and non-life-threatening side effects was 2- to 12-fold lower in patients receiving LMW iron dextran compared with HMW iron dextran.37 Another study in 50 patients with IBD demonstrated that LMW iron dextran can safely and effectively be used as a total dose infusion of the whole calculated iron deficit in one session using the Ganzoni formula.38 Disadvantages of LMW iron include a small risk of immunoglobulin E mediated anaphylactoid reactions. This risk is much smaller than in HMW iron dextran, but still larger than in new iron salt formulations.39
Iron sucrose (‘Venofer’, Vifor (International) Inc., Switzerland) is the formulation most extensively studied in patients with IBD.18–20 It has been shown to be safe and well tolerated even in patients previously intolerant to other intravenous iron products. It is a semi-stabile iron preparation with a half-life of 5–6 h so that iron is released relatively quickly. Iron sucrose is typically given as a slow injection of 100–200 mg iron two to three times a week or as a slow infusion of a maximum of 500 mg of iron once a week. This can be disadvantageous in severe anemia because multiple sessions are necessary to supplement the estimated iron deficit.40
Ferric gluconate (‘Ferrlecit’, Sanofi, Paris, France) is a liable iron formulation meaning that iron is also released relatively quickly. Most of the studies on ferric gluconate have been performed in patients with chronic kidney failure and it was shown to be safe and effective in patients with anemia receiving hemodialysis. A typical dosage would be eight infusions of 125 mg during eight consecutive sessions of dialysis.41
Ferric carboxymaltose (‘Ferinject’, Vifor (International) Inc., Switzerland, ‘Injectafer’, Vifor (International) Inc., Switzerland) is another iron formulation that was approved by the FDA in 2013. Up to 1000 mg of iron can be safely administered within 15 min. Safety and efficiency have been demonstrated in over 3500 patients with IDA and chronic kidney disease, pregnancy/postpartum or congestive heart failure.3 In patients with IBD, a large randomized controlled trial showed noninferiority in Hb increase compared with oral ferrous sulfate with a faster Hb increase.23 Another large randomized controlled trial compared ferric carboxymaltose following a new simplified fixed dose regimen with traditional treatment using up to 11 injections of iron sucrose following the Ganzoni calculation. By week 12, a larger proportion of the patients in the ferric carboxymaltose group had a Hb response.33
Iron isomaltoside (‘Monofer’, Pharmacosmos, Holbaek, Denmark) is another relatively new iron salt and was approved in Europe in 2009. It is safe to use as a total dose transfusion due to its stable formulation with little liable iron and its very small immunogenic potential.42 In patients with IBD, the PROCEED study compared iron isomaltoside in doses as calculated by the Ganzoni formula with 200 mg of oral iron sulfate daily. By week 8, noninferiority regarding average Hb increase could not be demonstrated. However, it has to be considered that patients with known intolerance to oral iron were excluded from the trials resulting in possible selection bias. Furthermore, it could be shown that the effects of iron isomaltoside were dose dependent (>1000 mg were more effective) and it was suggested by the authors that the Ganzoni formula may have underestimated the true iron demand in patients with IBD.43
The last paragraphs demonstrate that multiple different intravenous formulations exist and it can be expected that further formulations will be developed in the coming years. Clinical studies of the various formulations follow different protocols and there are no large competitive trials between the various formulations in terms of efficiency or safety profile.6 As described above, in patients with IBD the substances most studied are iron sucrose and iron carboxymaltose whereas other formulations have predominantly been tested in other patient groups (e.g. patients with chronic kidney disease). It can be assumed that the different formulations of intravenous iron are similarly efficient. In clinical practice, the selection of an intravenous agent therefore often depends on patient preferences, costs and local availability.
Blood transfusions
Blood transfusions should be the last resort in the treatment of IDA in patients with IBD due to high risks of side effects. Blood transfusions can immediately improve Hb levels in severe anemia but do not sufficiently correct the underlying iron deficiency. If iron deficiency also plays a role in the genesis of anemia, blood transfusions should always be followed by intravenous iron supplements with or without erythropoietin.6 Early treatment of IDA with iron supplementation can decrease the frequency of blood transfusions needed.41
Infliximab (+intravenous iron)
Successful treatment of the underlying IBD can improve mucosal inflammation and GI blood loss and thereby improve anemia (for treatment guidelines, see Dignass and colleagues, Wehkamp and colleagues and Gomollón and colleagues44–46). In addition to this effect, treatment with the anti-TNF agent infliximab is thought to have a direct beneficial effect on the pathogenesis of anemia in patients with IBD. As described above, IDA and ACD frequently coexist in patients with IBD and TNFα and other inflammatory cytokines are involved in the development of ACD. An in vitro study demonstrated that blockage of TNFα with infliximab could increase the amount of erythroid precursor cells.47 In a study of 87 patients with IBD receiving infliximab and intravenous iron sucrose, 49.4% could reach the target Hb level of 12 g/dl. Following treatment with infliximab, erythropoetin (EPO) levels and sTFR levels initially increased, which is consistent with functional iron deficiency. After subsequent therapy with iron sucrose, EPO and sTFR levels decreased significantly.48 These results demonstrate why the combination of intravenous iron and infliximab might be particularly beneficial.
Erythropoetin
When anemia in patients with IBD does not respond adequately to optimal treatment of the underlying IBD and intravenous supplementation of iron, (concurrent) ACD should be thought of. If ACD is likely, additional treatment with EPO should be considered.6
As described above, ACD is frequent in patients with IBD and frequently coexists with IDA. However, treatment with EPO remains a second-line treatment for patients with severe or symptomatic anemia refractory to intravenous iron alone due to high costs and potential side effects.2,6,49 A number of clinical trials could demonstrate successful treatment of IBD related anemia previously refractory to iron therapy using additional EPO.50–52 Thereby, laboratory parameters predictive for a poor response to iron supplementation alone are low serum EPO levels for the degree of anemia as well as low levels of transferrin and sTfR.53
To date, there are no large long-term studies available on EPO treatment in patients with IBD.6,49 Data are limited in aspects such as the preferred exact dosage, agent used, duration of therapy or the role of EPO in less severe anemia or prevention of recurrent anemia.
Multiple large studies about safety of EPO were performed in patients with chronic kidney disease. It was demonstrated that a target Hb of 13–14 g/dl as well as a rapid increase of Hb (>1 g/dl in 2 weeks) are harmful: the risk of thromboembolic events, stroke, cardiovascular events and death was higher than in a control group.54 Given the lack of data for patients with IBD, it is advisable to use the same caution measures as in patients with chronic kidney disease. In line with that, the 2015 ECCO guidelines recommend using a target Hb not above 12 g/dl to minimize side effects.6
During treatment with EPO it is has to be considered that increased erythropoiesis following treatment with EPO causes an increased demand for iron in the bone marrow.3,6,55 It is therefore recommended in the 2015 ECCO guidelines that complementary intravenous iron should always be given with a target ferritin level of over 200 μg/liter to prevent functional iron deficiency.6
In patients with IBD, the increased risk of venous thromboembolism requires particular consideration because patients with IBD already carry a higher risk for thromboembolic events, especially in active disease and in ulcerative colitis.3 Other potential side effects of treatment with EPO include hypertension (5–24% of the patients), edema, fever, dizziness and the very rare but serious development pure red cell aplasia secondary to anti-EPO antibodies.56 Another issue of concern is that several malignant cell lines express EPO receptors with contradictory reports of the effect of EPO on such cell lines.49 However, it was also suggested, that EPO may have beneficial effects on the underlying IBD. For example, a study on experimental colitis in a murine model demonstrated that EPO can exhibit protective, anti-inflammatory effects through various pathways.57
Follow up
The goal of iron supplementation should always be complete normalization of Hb and iron storage. It should be kept in mind that even after normalization of Hb levels, iron storages are still not filled up because anemia is only the end point of iron deficiency. Iron treatments should be followed up clinically and using laboratory tests at least within 4 weeks in asymptomatic patients and more frequently in symptomatic patients.25 An important surveillance factor is the wellbeing of the patient. Clinically, QoL can improve within a few days after initiation of iron therapy and previous symptoms of anemia decrease.
An adequate erythropoietic response has been defined as an increase of Hb of at least 2 g/dl or normalization within 4 weeks.6,25,55 As described above, reticulocyte count and reticulocyte production index are faster parameters of successful treatment and will be increased within 1–2 weeks following oral or intravenous iron with or without EPO.
As described above, iron storage can be assessed using ferritin and transferrin saturation. During oral or intravenous iron supplementation, transferrin saturation can be falsely elevated.25 Ferritin levels also overestimate the iron available for erythropoiesis in the bone marrow during intravenous iron treatment.58,59 Therefore, assessment of iron storage should only be performed a minimum of 4 weeks after the last intravenous iron application. After termination of iron therapy, the parameters of iron storage should be above the lower limits of normal.25,55 A recent study has pointed out that the time until reoccurrence of iron deficiency in patients with IBD can be delayed with higher post-treatment ferritin values above 100 μg and even more so above 400 μg/liter.58 However, it was suggested that a ferritin level of 800 μg/liter and a transferrin saturation of 50% should be used as upper limits for intravenous iron to prevent iron overload.6,55
Patients on oral iron should be monitored clinically about tolerability and side effects. In oral iron therapy, ferritin can be used for monitoring during treatment and is expected to increase within 1–2 weeks and Hb levels should also be monitored.
If a patient is on oral iron and the erythropoetic response is not adequate, or if oral iron is not tolerated, the patient should be switched to intravenous iron. If treatment with intravenous iron is not successful, other causes of anemia should be reevaluated. If (concomitant) ACD is possible, additional use of erythropoietin-stimulating factors should be considered.6 Optimal treatment of the underlying IBD is also an important factor in the treatment of anemia at all levels.
Maintenance therapy
After correction of the Hb level, iron deficiency and anemia reoccur frequently and surprisingly quickly in patients with IBD as the risk factors might continue. Thereby, a fast recurrence correlates with disease activity even in clinically asymptomatic patients with low inflammatory parameters.6 A recent follow-up study after intravenous iron replacement therapy in 88 patients revealed a median of only 10 months for reoccurrence of anemia and 19 months for reoccurrence of iron deficiency in patients with IBD and it was suggested that maintenance treatment may be needed.58
The FERGImain study investigated intravenous maintenance therapy in patients with IBD who were previously successfully treated with intravenous iron carboxymaltose. Ferritin was controlled every 2 months and if it dropped below 100 μg, 500 mg of intravenous iron carboxymaltose (n = 105) or placebo (n = 99) were given for 8 months. In the treatment arm of the study, anemia reoccurred in 27% of patients compared with 39% in the placebo arm of the study.35 This was the first study to evaluate ferritin-guided maintenance therapy with intravenous iron.
In line with these studies, the current guidelines recommend that every patient with IBD is monitored for recurrence of IDA every 3 months for at least 1 year following correction of iron and at least every 6–12 months thereafter.6 Laboratory parameters should include Hb, ferritin, transferrin saturation and CRP. As soon as ferritin drops below 100 μg/liter or Hb falls below normal values, iron should be substituted using the same route as in the initial treatment.6
Current situation
In 2013, a study across nine European countries was performed to evaluate the routine practice in the management of IBD-associated anemia. A total of 344 gastroenterologists from nine European countries were surveyed; 56% of the patients had at least moderate anemia (Hb < 10 g/dl), 15% had severe anemia (Hb < 6 g/dl). It was reported that 92% of the patients were treated with iron, but only 28% received intravenous iron (67% oral iron) despite international guidelines that recommend intravenous iron as first-line treatment in moderate to severe IDA in patients with IBD. This demonstrates that intravenous iron remains underused in Europe. There is a need to increase awareness and implementation of the international guidelines. The high prevalence of iron deficiency also indicates that monitoring is not sufficient.31
Another paper in 2012 evaluated 631 online patient surveys from patients with IBD and anemia. It was reported that 33% of these patients received no treatment for anemia. Of those treated, only 27% received intravenous iron. Depending on the formulation of oral iron, 68–77% of the patients were dissatisfied with oral iron, mostly because of poor tolerability. In contrast, 72% of the patients treated with intravenous iron were satisfied with their treatment.60
Summary
IDA is the most common systemic complication of patients with IBD and contributes significantly to the morbidity of the disease. The overall QoL in patients with IBD correlates strongly with Hb levels independently from underlying disease activity.
Iron deficiency and IDA in patients with IBD is still largely underdiagnosed and undertreated. Every patient with IBD should be screened regularly for IDA. The current ECCO guidelines recommend screenings every 6–12 months for quiescent IBD and at least every 3 months in active disease for outpatients. They recommend a screening panel including Hb, CBC, CRP and ferritin.
For the diagnosis of IDA, the same Hb cutoff values as in otherwise healthy adults apply (Hb < 12 g/dl for women and <13 g/dl for men). The analysis of iron storage in patients with IBD is complicated by the fact that ferritin is an acute phase protein and can be increased in the setting of chronic inflammation, whereas transferrin is a negative acute phase protein and can be decreased. The current ECCO guidelines suggest adjusted cutoff values with ferritin less than 30 μg/liter in patients with mild disease or in remission and a cutoff of ferritin less than 100 μg/liter in patients with active disease. A new alternative parameter for iron availability to erythropoiesis is sTfR. It can be useful in unclear cases because it does not correlate with inflammation.
Anemia of chronic disease is likely if ferritin is over 100 μg/liter with a TfS less than 20%. If ferritin is between 30 and 100 μg/liter, and TfS is less than 20%, a combination of IDA and ACD is likely.
The goal of treatment of IDA should always be complete normalization of anemia and iron stores. Oral iron is widely available and cost efficient, but has been associated with an increased risk of GI side effects. It is an adequate first-line treatment in patients with mild anemia (Hb > 11 g/dl) whose disease is clinically inactive and who have not been previously intolerant to oral iron. Intravenous iron has been shown to increase Hb faster than oral iron and the new formulations have a favorable safety profile. It is recommended as a first-line treatment in patients with active disease, in patients with severe anemia, in patients with additional need for EPO-stimulating agents and in those with previous intolerance to oral iron.
Early formulations of intravenous iron (HMW iron dextran and to a lesser extent LMW iron dextran) have been associated with a risk of severe anaphylactoid reactions. This is not true for a number of new iron formulations that generally have a favorable safety profile. Iron sucrose and iron carboxymaltose are the formulations most extensively studied in patients with IBD but there are no large comparative trials between different formulations available in terms of safety or efficiency.
If anemia does not respond sufficiently to intravenous iron and (concurrent) ACD is possible, treatment with EPO and intravenous iron is recommended. Thereby, the target Hb should not be above 12 g/dl to minimize potential side effects (e.g. thromboembolism).
Following initiation of treatment, asymptomatic patients should be followed up at least within 4 weeks and symptomatic patients more frequently. When oral iron is used, tolerability should be assessed. A hematologic response is defined as an Hb increase over 2 g/dl or Hb normalization within 4 weeks.
Recurrence of IDA in patients with IBD occurs frequently and surprisingly quick. Fast recurrence of IDA does correlate with IBD disease activity. It is therefore recommended that every patient is screened every 3 months for 1 year following correction and every 6–12 months thereafter. Figure 1 summarizes the screening and treatment algorithm for IDA in patients with IBD described in this paper.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: JW received a honorarium for a scientific lecture from Vifor Pharma.
Contributor Information
Dorothea Niepel, Department of Internal Medicine I (Gastroenterology, Hepatology, Infectious Diseases), University Hospital Tübingen, Tübingen, Germany.
Thomas Klag, Department of Internal Medicine I (Gastroenterology, Hepatology, Infectious Diseases), University Hospital Tübingen, Tübingen, Germany.
Nisar P. Malek, Department of Internal Medicine I (Gastroenterology, Hepatology, Infectious Diseases), University Hospital Tübingen, Tübingen, Germany
Jan Wehkamp, Department of Internal Medicine I, University Hospital Tübingen, Otfried-Müller-Str. 10, 72076 Tübingen, Germany.
References
- 1. Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol 2008; 103: 1299. [DOI] [PubMed] [Google Scholar]
- 2. Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther 2006; 24: 1507–1523. [DOI] [PubMed] [Google Scholar]
- 3. Stein J, Dignass AU. Management of iron deficiency anemia in inflammatory bowel disease–a practical approach. Ann Gastroenterol 2013; 26: 104. [PMC free article] [PubMed] [Google Scholar]
- 4. Gasche C, Lomer M, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut 2004; 53: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cronin CC, Shanahan F. Anemia in patients with chronic inflammatory bowel disease. Am J Gastroenterol 2001; 96: 2296. [DOI] [PubMed] [Google Scholar]
- 6. Dignass AU, Gasche C, Bettenworth D, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015; 9: 211–222. [DOI] [PubMed] [Google Scholar]
- 7. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011–1023. [DOI] [PubMed] [Google Scholar]
- 8. Lopez A, Cacoub P, Macdougall IC, et al. Iron deficiency anaemia. Lancet 2016; 387: 907–916. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. “Nutritional anaemias: tools for effective prevention and control.”, (2017). Available at http://apps.who.int/iris/bitstream/handle/10665/259425/9789241513067-eng.pdf?sequence=1 (accessed 4 October 2018).
- 10. Klag T, Mazurak N, Fantasia L, et al. High demand for psychotherapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2017; 23: 1796–1802. [DOI] [PubMed] [Google Scholar]
- 11. Wells CW, Lewis S, Barton JR, et al. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis 2006; 12: 123–130. [DOI] [PubMed] [Google Scholar]
- 12. Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis 2009; 15: 1485–1491. [DOI] [PubMed] [Google Scholar]
- 13. Klag T, Stange E, Wehkamp J. Labordiagnostik bei chronisch-entzündlichen darmerkrankungen, einschließlich blut-und stuhldiagnostik. Der Gastroenterologe 2014; 9: 117–126. [Google Scholar]
- 14. Cakal B, Akoz AG, Ustundag Y, et al. Red cell distribution width for assessment of activity of inflammatory bowel disease. Dig Dis Sci 2009; 54: 842–847. [DOI] [PubMed] [Google Scholar]
- 15. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clinica Chimica Acta 2003; 329: 9–22. [DOI] [PubMed] [Google Scholar]
- 16. Oustamanolakis P, Koutroubakis IE. Soluble transferring receptor-ferritin index is the most efficient marker for the diagnosis of iron deficiency anemia in patients with IBD. Inflamm Bowel Dis 2011; 17: E158–E159. [DOI] [PubMed] [Google Scholar]
- 17. Munoz M, Garcίa-Erce JA, Remacha ÁF. Disorders of iron metabolism. part II: iron deficiency and iron overload. J Clin Pathol 2011; 64: 287–296. [DOI] [PubMed] [Google Scholar]
- 18. Lindgren S, Wikman O, Befrits R, et al. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol 2009; 44: 838–845. [DOI] [PubMed] [Google Scholar]
- 19. Erichsen K, Ulvik RJ, Nysaeter G, et al. Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scand J Gastroenterol 2005; 40: 1058–1065. [DOI] [PubMed] [Google Scholar]
- 20. Schrӧder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease–a randomized, controlled, open-label, multicenter study. Am J Gastroenterol 2005; 100: 2503. [DOI] [PubMed] [Google Scholar]
- 21. Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side effects in adults: a systematic review and meta-analysis. PLoS One 2015; 10: e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee TW, Kolber MR, Fedorak RN, et al. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis? J Crohns Colitis 2012; 6: 267–275. [DOI] [PubMed] [Google Scholar]
- 23. Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT®) randomized controlled trial. Am J Gastroenterol 2008; 103: 1182. [DOI] [PubMed] [Google Scholar]
- 24. Jimenez K, Gasche C, Auerbach M. On both sides of the ocean. Blood Transfus 2016; 14: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gasche C, Berstad A, Befrits R, et al. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis 2007; 13: 1545–1553. [DOI] [PubMed] [Google Scholar]
- 26. Rizvi S, Schoen RE. Supplementation with oral vs. intravenous iron for anemia with IBD or gastrointestinal bleeding: is oral iron getting a bad rap? Am J Gastroenterol 2011; 106: 1872. [DOI] [PubMed] [Google Scholar]
- 27. Gasche C, Ahmad T, Tulassay Z, et al. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: results from a phase-3 clinical trial program. Inflamm Bowel Dis 2015; 21: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017; 66: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zimmermann MB, Chassard C, Rohner F, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr 2010; 92: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 30. Werner T, Wagner SJ, Martínez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 2011; 60: 325–333. [DOI] [PubMed] [Google Scholar]
- 31. Stein J, Bager P, Befrits R, et al. Anaemia management in patients with inflammatory bowel disease: routine practice across nine European countries. Eur J Gastroenterol Hepatol 2013; 25: 1456–1463. [DOI] [PubMed] [Google Scholar]
- 32. Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. ASH Educ Program Book 2010; 2010: 338–347. [DOI] [PubMed] [Google Scholar]
- 33. Evstatiev R, Marteau P, Iqbal T, et al. Fergicor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011; 141: 846–853. [DOI] [PubMed] [Google Scholar]
- 34. Favrat B, Balck K, Breymann C, et al. Evaluation of a single dose of ferric carboxymaltose in fatigued, iron-deficient women–prefer a randomized, placebo-controlled study. PLoS One 2014; 9: e94217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Evstatiev R, Alexeeva O, Bokemeyer B, et al. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013; 11: 269–277. [DOI] [PubMed] [Google Scholar]
- 36. Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. Lancet 2007; 369: 1502–1504. [DOI] [PubMed] [Google Scholar]
- 37. Chertow GM, Mason PD, Vaage-Nilsen O, et al. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant 2004; 19: 1571–1575. [DOI] [PubMed] [Google Scholar]
- 38. Koutroubakis IE, Oustamanolakis P, Karakoidas C, et al. Safety and efficacy of total-dose infusion of low molecular weight iron dextran for iron deficiency anemia in patients with inflammatory bowel disease. Dig Dis Sci 2010; 55: 2327–2331. [DOI] [PubMed] [Google Scholar]
- 39. Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 2005; 21: 378–382. [DOI] [PubMed] [Google Scholar]
- 40. Muñoz M, Gómez-Ramírez S, García-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol 2009; 15: 4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol 2004; 76: 74–78. [DOI] [PubMed] [Google Scholar]
- 42. Kalra PA, Bhandari S. Efficacy and safety of iron isomaltoside (Monofer®) in the management of patients with iron deficiency anemia. Int J Nephrol Renovasc Dis 2016; 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reinisch W, Staun M, Tandon RK, et al. A randomized, openlabel, non-inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (proceed). Am J Gastroenterol 2013; 108: 1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis 2012; 6: 991–1030. [DOI] [PubMed] [Google Scholar]
- 45. Wehkamp J, Gӧtz M, Herrlinger K, et al. Chronisch entzündliche darmerkrankungen. Dtsch Arztebl Int 2016; 113: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2016; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 47. Bergamaschi G, Di Sabatino A, Albertini R, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-α treatment. Haematol 2010; 95: 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katsanos K, Cavalier E, Ferrante M, et al. Intravenous iron therapy restores functional iron deficiency induced by infliximab? J Crohns Colitis 2007; 1: 97–105. [DOI] [PubMed] [Google Scholar]
- 49. Tsiolakidou G, Koutroubakis IE. Stimulating erythropoiesis in inflammatory bowel disease associated anemia. World J Gastroenterol 2007; 13: 4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schreiber S, Howaldt S, Schnoor M, et al. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N Engl J Med 1996; 334: 619–624. [DOI] [PubMed] [Google Scholar]
- 51. Gasche C, Dejaco C, Waldhoer T, et al. Intravenous iron and erythropoietin for anemia associated with Crohn disease: a randomized, controlled trial. Ann Intern Med 1997; 126: 782–787. [DOI] [PubMed] [Google Scholar]
- 52. Koutroubakis IE, Karmiris K, Makreas S, et al. Effectiveness of darbepoetin-alfa in combination with intravenous iron sucrose in patients with inflammatory bowel disease and refractory anaemia: a pilot study. Eur J Gastroenterol Hepatol 2006; 18: 421–425. [DOI] [PubMed] [Google Scholar]
- 53. Gasche C, Waldhoer T, Feichtenschlager T, et al. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol 2001; 96: 2382. [DOI] [PubMed] [Google Scholar]
- 54. Unger EF, Thompson AM, Blank MJ, et al. Erythropoiesis-stimulating agents—time for a reevaluation. N Engl J Med 2010; 362: 189–192. [DOI] [PubMed] [Google Scholar]
- 55. Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol 2010; 7: 599–610. [DOI] [PubMed] [Google Scholar]
- 56. López RM, Aladrén BS, García FG. Use of agents stimulating erythropoiesis in digestive diseases. World J Gastroenterol 2009; 15: 4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cuzzocrea S, Mazzon E, Di Paola R, et al. Erythropoietin reduces the development of experimental inflammatory bowel disease. J Pharmacol Exp Ther 2004; 311: 1272–1280. [DOI] [PubMed] [Google Scholar]
- 58. Kulnigg S, Teischinger L, Dejaco C, et al. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol 2009; 104: 1460. [DOI] [PubMed] [Google Scholar]
- 59. Ali M, Fayemi AO, Frascino J, et al. Failure of serum ferritin levels to predict bone-marrow iron content after intravenous iron-dextran therapy. Lancet 1982; 319: 652–655. [DOI] [PubMed] [Google Scholar]
- 60. Danese S, Hoffman C, Vel S, et al. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn’s and Ulcerative Colitis Association’s online survey. Eur J Gastroenterol Hepatol 2014; 26: 1385–1391. [DOI] [PubMed] [Google Scholar]