Abstract
Background:
Femoroacetabular impingement (FAI) is a recognized cause of hip and groin pain and a significant factor in hip joint function during sport. Objective tests for understanding hip function are lacking in this population.
Purpose:
To determine whether biomechanical and electromyographic features of hip function during level-ground walking differ between a group diagnosed with FAI and those with no symptoms of FAI.
Study Design:
Controlled laboratory study.
Methods:
A total of 20 asymptomatic individuals and 20 individuals with FAI walked on a dual-belt instrumented treadmill at self-selected walking velocities. Sagittal and frontal plane joint motions, moments, and muscle activation for the gluteus medius, gluteus maximus, rectus femoris, and medial and lateral hamstrings were analyzed. Discrete measures were extracted from each biomechanical waveform, and principal component analysis was used to determine hip joint muscle activation and hip adduction moment patterns. Statistical significance was determined by use of Student t tests with Bonferroni adjustments for multiple comparisons (α = .05).
Results:
Individuals with FAI walked more slowly (P = .015) and had lower self-reported function (P < .001). No differences in muscle strength were found between the symptomatic and contralateral legs in the FAI group (P > .017), but those with FAI had lower strength in the knee extensors and flexors and the hip extensors, flexors, and adductors compared with the asymptomatic group (P < .017). Individuals with unilateral symptomatic FAI walked with similar biomechanical and hip muscle electromyographic results bilaterally. The only differences found were a greater amplitude of gluteus maximus activation in the FAI symptomatic leg compared with the asymptomatic group and greater medial hamstring activation than lateral hamstring activation in the FAI group in both limbs compared with the asymptomatic group.
Conclusion:
Individuals with FAI were generally deconditioned and reported significantly more functional limitations. No biomechanical differences existed between groups during level walking, yet hamstring and gluteus maximus activation differed when the symptomatic group was compared with the asymptomatic group.
Clinical Relevance:
The field lacks objective testing of hip joint function to understand implications of FAI for dynamic movements, particularly with applications to biomechanics and electromyography. Level walking was of limited value for understanding FAI hip function, and the development of a more challenging gait assessment is warranted.
Keywords: femoroacetabular impingement, gait, electromyography, strength, biomechanics, principal component analysis
Hip and groin pain is a significant concern for many young adults, particularly those involved in sport. Femoroacetabular impingement (FAI) is recognized as a common cause of these symptoms; the condition is often classified as pincer, cam, or a combination of both acetabular (pincer) and femoral (cam) abnormalities.35 Morphological abnormalities of the femoral head-neck junction, known as a cam, can lead to impingement during movement that limits range of motion of the hip joint, as abnormal contact forces occur between the proximal femur and the acetabular rim.3,12,13,35 While cam deformities can also be found in an asymptomatic population,15,33 continued impingement can lead to acetabular labral injury in the short term3,21 and possible cartilage degeneration in line with osteoarthritis (OA) in the long-term.1 Tannast et al32 found that the most severe hip damage corresponded with the zone that had the highest probability of acetabular impact, suggesting a direct relationship between joint biomechanics and hip joint abnormality.
To define a cam-type FAI deformity, the hip joint is typically assessed using metrics focused on joint impairment during static evaluations.7,8,15,20,21 These metrics are characteristically separated into clinical and radiographic methods. The most common radiographic method is to determine the alpha angle using a threshold of 50°.2,18 Clinical tests such as the impingement test (combined hip flexion-adduction–internal rotation) and FABER (combined hip flexion-abduction–external rotation) are also used.13,21,25 Tests that analyze hip joint impairments during routine activities of daily life such as walking or sport have not been routinely used.
Gait analysis has been used as a method to study joint mechanics in vivo because of its mechanical demands and its importance as a daily functional activity and as a precursor for return to sport. For lower extremity joints to remain functional, a balance must be established between stability and mobility. The passive osteoligamentous, muscular, and neurological subsystems are thought to be fundamental to this process at the knee31 and spine24; thus, there is reason to believe that the same theoretical framework could be applied to the hip joint.30 There is currently a lack of understanding of how the interrelationship between hip joint mechanics and muscle activity relates to or is affected by cam FAI.
Gait studies focusing on mechanical outcomes in a population with FAI have had mixed results. Individuals with FAI have been found to walk with less sagittal plane hip range of motion,10,17,19 while frontal and transverse motion limitations are more varied. Some studies have found no differences compared with asymptomatic individuals,10 yet others have reported less motion in the FAI group.5,17,19 Diamond et al10 found no significant differences in net external joint moments in all 3 planes of motion, a finding consistent with other studies.5,19 In contrast, reduced net external hip flexion and external rotation moments have been found in patients with FAI.17 Although biomechanical analysis is comprehensive, it still provides a limited understanding of overall joint function during walking. Generally, our understanding of muscle function during dynamic activities in FAI is deficient. Hip strength is generally reduced in individuals with FAI,11,22 suggesting the dynamics may also be altered during movement. Diamond et al11 found altered activation coordination of the deep hip rotators during early swing in a group with FAI compared with an asymptomatic group, thought to be a compensation during the movement of the femur toward the impingement position. Minimal literature exists that includes both biomechanical and muscle activations in a single study. Hip motions during gait fail to test the mechanical boundaries of the hip joint, relevant in FAI mechanics. Thus, outcomes may be more aligned with secondary compensations rather than the mechanics of cam structure, supporting the need to include a comprehensive assessment of joint mechanics and muscle activation patterning during dynamic activities.
The objective of this study was to determine whether hip motion, hip moments, and hip joint muscle activation characteristics during gait, in individuals with cam FAI, differ between the symptomatic and contralateral hip, and whether differences exist between patients with FAI and a healthy, aged-matched asymptomatic group. It was hypothesized that minimal joint biomechanical differences would exist between limbs of those with FAI and those from an asymptomatic group but that gluteal muscle activation patterns would be altered in the symptomatic hip, suggestive of altered joint function secondary to the symptomatic FAI injury.
Methods
Participants with unilateral symptomatic cam FAI were recruited after consultation with an orthopaedic surgeon. The inclusion criteria for the FAI group consisted of (1) age between 18 and 35 years, (2) unilateral symptomatic hip and/or groin pain, (3) alpha angle greater than 50°, (4) being deemed a candidate for surgical management, (5) no cardiovascular or neurological disease, (6) no musculoskeletal disease or injury other than cam FAI (ie, no acetabular dysplasia, no pincer lesions), (7) no lower limb surgery within the past year, and (8) ability to walk independently without the use of an ambulatory aid. The asymptomatic group was considered a sample of convenience, recruited through university and local community advertisements. All participants were required to have no neurological or cardiovascular disorder that would impair walking ability, to have no fracture or injury other than a sprain or strain, and to be able to walk independently. The study protocol was approved by the local institutional ethics review committee.
All participants were asked to complete the Hip Osteoarthritis Outcome Score (HOOS) and the international Hip Outcome Tool–33 (iHOT-33). Participants then changed into tight-fitting shorts and a T-shirt and removed their footwear; then their height and mass were recorded. All individuals completed at least 10 walking trials at a self-selected speed across the GaitRITE instrumented walkway (CIR Systems). Five trials were randomly recorded to determine average walking speed.
Participants were prepared for surface electromyography (EMG); skin was lightly shaved and cleaned with 70% alcohol wipes. Consistent with guidelines16 and standard procedures (Surface EMG for the Non-Invasive Assessment of Muscles, or SENIAM), Ag/AgCl surface electrodes (10 mm diameter, 30 mm interelectrode distance) (Red Dot; 3M Health Care) were placed bilaterally in a bipolar configuration over the gluteus maximus, gluteus medius, medial hamstring (MH), lateral hamstring (LH), and rectus femoris (Table 1). Surface EMG was recorded with two AMT-8 eight-channel Bortec systems at 2000 Hz using Qualisys Track Manager 2.10.
TABLE 1.
Position and Orientation of Surface Electrodes for Electromyography of Gluteus Maximus, Gluteus Medius, Rectus Femoris, and Medial and Lateral Hamstrings
| Muscle | Position | Orientation |
|---|---|---|
| Gluteus maximus | 50% of the distance between the second sacral vertebra and greater trochanter | Along the lead line from the second sacral vertebra and greater trochanter |
| Gluteus medius | 50% of the distance between iliac crest and greater trochanter | Along the lead line from the iliac crest and greater trochanter |
| Rectus femoris | 50% of the distance between anterior superior iliac spine and base of patella | Along the lead line from the anterior superior iliac spine and base of patella |
| Lateral hamstring | 50% of the distance between the ischial tuberosity and lateral tibial epicondyle | Along the lead line from the ischial tuberosity and lateral tibial epicondyle |
| Medial hamstring | 50% of the distance between the ischial tuberosity and medial tibial epicondyle | Along the lead line from the ischial tuberosity and medial tibial epicondyle |
Rigid plastic plates containing 4 retroreflective spheres were placed on the trunk, pelvis, lateral femur, lateral tibia, and foot by use of Velcro straps, and single markers placed on anatomic landmarks were secured with adhesive tape as per previously published procedures.26 Anterior superior iliac spines were determined by use of a calibrated wand and defined with respect to the pelvis cluster. The retroreflective spheres were tracked using eight Qualisys OQUS 500 motion analysis cameras at 100 Hz (Figure 1).
Figure 1.
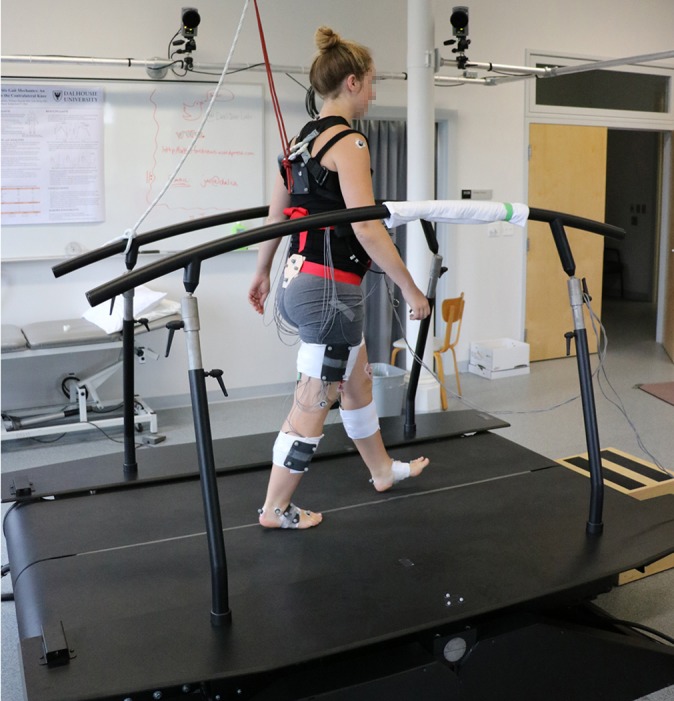
Image of a participant walking atop the dual-belt instrumented treadmill as part of this study. Markers shown are those tracked during walking, including a rigid plate on the pelvis, femur, shank, and foot and single markers placed on the shoulder, lateral epicondyle, lateral malleolus, calcaneus, and second metatarsal bilaterally. Electrodes are located as described in Table 1 and have been covered with tensor wrap.
Participants walked barefoot for at least 5 minutes on a dual-belt instrumented treadmill (R-Mill; Motekforce Link) for familiarization,36 which was set to the self-selected speed calculated from the GaitRITE walkway. A 20-second measurement was made after at least 5 minutes of walking.
After completion, a resting muscle activity trial was recorded with the participant lying supine. To elicit maximal voluntary isometric contractions (MVIC), Humac Norm Isokinetic Dynamometer (Computer Sports Medicine) procedures were used. Knee flexors and extensors were tested with the participant sitting, with the knee at 45° of knee flexion. Hip abduction and adduction were tested in the side-lying position with the hip placed in 15° of abduction. Hip flexion and extension were tested in the supine position with the hip in 45° of flexion. The resistance pad was placed at the distal femur for hip strength testing and at the distal tibia for knee strength testing. The limb and trunk were secured with Velcro straps. Following at least 1 practice and warm-up contraction, two 3-second maximal isometric contractions were completed. A 40-second rest period separated each contraction, and standardized verbal encouragement was given.
Data Processing
Custom programs, written in MatLab 2016b (The Mathworks), were used to complete data processing. Technical and local anatomic bone-embedded foot, shank, thigh, and pelvis coordinate systems were derived from virtual points and physical spheres. Marker motion and kinematic data were smoothed by use of a 6-Hz, low-pass, fourth-order Butterworth recursive filter. Ground-reaction force data were low-pass filtered by use of a 30-Hz, low-pass, fourth-order Butterworth recursive filter prior to processing. Hip joint angles were calculated using a 6 degrees of freedom model through Cardan/Euler rotations as previously described.26 Net external moments were calculated by use of an inverse dynamics model.34 Moments were projected into the joint coordinate system, amplitude normalized to body mass (N·m/kg), and low-pass filtered (10-Hz, fourth-order, recursive Butterworth) prior to analysis.26 All raw EMG signals were bandpass filtered (10- to 500-Hz, fourth-order, recursive Butterworth), corrected for resting bias, rectified, and filtered by use of a 6-Hz, low-pass, fourth-order, Butterworth recursive filter. EMG profiles were amplitude normalized to the highest 100-millisecond window from the MVIC trials.26 Motion and EMG waveforms were time normalized to the gait cycle (beginning and ending at heel strike), whereas the moment waveforms were time normalized to the stance phase (beginning at heel strike and ending at toe-off). Ensemble averages were calculated from the individual trials (at least 14 trials in 20 seconds) for each participant. Strength was determined using a 500-millisecond moving window algorithm that captured the maximum torque generated over the 3-second steady-state MVIC. The maximum value between the 2 trials was recorded as the maximal torque and amplitude normalized to body mass (N·m/kg).
Analysis
The symptomatic and contralateral leg in the FAI group and a random leg in the asymptomatic group were selected for analyses. HOOS and iHOT-33 scores were tabulated. Given the general feedback from patients with FAI and findings in the literature on walking ability, further investigation was conducted through subscale questions from the iHOT-33 and HOOS questionnaires. Specifically, 4 questions were extracted for further analysis: (1) “How difficult is it for you to walk long distances?” (2) “How much pain have you experienced in the past week while walking on a flat surface?” (3) “How much pain have you experienced in the past week while walking on a hard surface?” (4) “What is the degree of difficulty you have experienced in the past week due to your hip while walking on a flat surface?” For the first question (which was from iHOT-33), the individual’s response was scored on a visual analog scale, end marked by qualifiers of extremely difficult (0) and not difficult at all (100). For the remaining questions (from HOOS), the scores were based on a Likert scale (0, none; 1, mild; 2, moderate; 3, severe; 4, extreme).
Ranges of motion for stance phase-sagittal, frontal, and transverse plane (difference between maximum and minimum hip joint angles during the stance phase) were identified. Between-group and within-group (FAI) differences were calculated for peak flexion and extension net external sagittal plane moments, as well as hip adduction moment (HAM) peak and impulse. Principal component analysis was used to capture amplitude and temporal HAM features and EMG waveform features using custom MatLab 2016b programs. The use of this multivariate statistical technique for hip OA treadmill gait EMG29 and knee adduction moment analyses26 has been previously described in detail. Briefly, for each muscle group (hamstrings, rectus femoris, gluteus maximus, and gluteus medius), a matrix X (n waveforms × 101) was created and multiplied by the transpose of itself: Y = [X′] × [X]. For HAM assessment, a covariance matrix was created. The resultant matrices were then decomposed through eigenvector decomposition into eigenvectors (principal patterns [PPs]) and eigenvalues. PPs (PP1, PP2, PP3) that together explained greater than 90% of original waveform variability were retained for further analysis. PP scores were computed to provide a weighting coefficient for how each PP related to each waveform.
Student t tests were used to test for significant group differences in patient characteristics, walking velocity, and self-reported measures of function (HOOS and iHOT-33). Normality and equal variance of the response variables were determined from Kolmogorov-Smirnov and Levene tests, respectively. For gait biomechanical variables and PP scores, a series of t tests were used to test between-group (independent t test) and within–FAI group (paired t test) differences. Bonferroni adjustments were made for multiple comparisons, setting the significance level at α = .05/number of comparisons. Statistical procedures were completed by use of Minitab version 17.
Results
A total of 20 FAI and 20 asymptomatic individuals were included. Data on demographics, anthropometrics, iHOT-33, HOOS, and strength can be found in Table 2. Individuals with FAI walked more slowly (P = .015) and had lower iHOT-33 and HOOS outcomes (P < .001). No differences in muscle strength were found between the symptomatic and contralateral limbs in the FAI group (P > .017), but patients in the FAI group had lower strength in the knee extensors and flexors and the hip extensors, flexors, and adductors (P < .017) compared with the asymptomatic group (ie, the group with no symptoms of FAI in either limb). Hip abduction strength in the symptomatic versus contralateral limb of the FAI group was similar while the FAI contralateral limb had lower strength compared with the asymptomatic group (P = .014). Alpha angles averaged 71° in both hips of individuals with FAI (Table 2), and Tönnis scores were between 0 and 1.
TABLE 2.
Participant Demographics, Questionnaire Outcomes, Walking Speed, Muscle Strength, and Radiographic Resultsa
| Variable | Asymptomatic Group | FAI Group | |
|---|---|---|---|
| Sex, % female | 50 | 50 | |
| Age, y | 25 (3) | 28 (6) | |
| Mass, kg | 72.2 (11.7) | 74.6 (16.1) | |
| Body mass index, kg/m2 | 24.4 (3.3) | 25.6 (4.7) | |
| HOOS Symptoms | 92 (8) | 56 (19) | |
| HOOS Pain | 98 (3) | 62 (16) | |
| HOOS Activities of Daily Living | 100 (1) | 72 (18) | |
| HOOS Quality of Life | 98 (5) | 35 (14) | |
| HOOS Sport | 99 (2) | 50 (19) | |
| iHOT-33 | 97 (3) | 40 (14) | |
| Walking speed, m/s | 1.29 (0.12) | 1.19 (0.14) | |
| Strength, N·m | Asymptomatic Group | FAI Group, Symptomatic Limb | FAI Group, Contralateral Limb |
| Knee extension | 204.7 (63.7)b | 154.3 (43.7)c | 161.7 (53.3)c |
| Knee flexion | 120.7 (39.6)b | 89.8 (26.1)c | 90.3 (28.8)c |
| Hip extension | 147.0 (62.9)b | 87.1 (47.3)c | 95.4 (53.8)c |
| Hip flexion | 120.7 (39.6)b | 88.8 (29.7)c | 91.9 (23.7)c |
| Hip abduction | 98.2 (23.5)b | 84.2 (35.2)b,c | 83.7 (35.7)c |
| Hip adduction | 86.6 (39.3)b | 60.9 (27.9)c | 60.5 (28.1)c |
| Radiographic results | |||
| Alpha angle, deg | NA | 71.0 (7.5) | 71.6 (6.1) |
aValues are expressed as mean (SD) unless otherwise indicated. Boldface indicates that the result for the asymptomatic group was significantly different from that for the FAI group (P < .05). FAI, femoroacetabular impingement; HOOS, Hip Osteoarthritis Outcome Score; iHOT-33, international Hip Outcome Tool–33; NA, not applicable.
b,cFor the strength assessment among the 3 groups, like letters indicate no significant differences (P > .017); unlike letters indicate statistical significance (P < .017).
For the individual questions pertaining to walking, the mean iHOT-33 score regarding walking long distance was 99 (SD, 2) for the asymptomatic group and 43 (SD, 29) for the FAI group. In the FAI group, the median HOOS score for pain while walking on a flat surface was 1 (mild), for pain while walking on a hard surface was 2 (moderate), and for difficulty with walking on a flat surface was 1 (mild). The asymptomatic group scored 0 for all 3 HOOS questions.
Biomechanics
Table 3 shows the gait biomechanics outcomes. Hip sagittal plane angle and net external moment for the FAI group and asymptomatic group are shown in Figure 2, whereas HAM and PPs are shown in Figure 3. Within the FAI group, no biomechanical differences between symptomatic and contralateral hips were found (P > .017). No differences were found between the asymptomatic group and individuals with FAI, regardless of symptomatic or contralateral limb (P > .017).
TABLE 3.
Hip Biomechanical Outcomes Including Hip Adduction Moment Principal Component Analysis (PCA)a
| Variable | Asymptomatic Group | FAI Group, Symptomatic Limb | FAI Group, Contralateral Limb |
|---|---|---|---|
| Sagittal range of motion, deg | 36 (6) | 35 (4) | 34 (4) |
| Frontal range of motion, deg | 9 (2) | 10 (3) | 10 (2) |
| Transverse range of motion, deg | 10 (3) | 8 (2) | 8 (3) |
| Sagittal plane moment difference, N·m/kg | 1.7 (0.3) | 1.5 (0.2) | 1.5 (0.3) |
| Peak hip adduction moment, N·m/kg | 1.11 (0.18) | 1.11 (0.19) | 1.07 (0.19) |
| Hip adduction moment impulse, N·m/kg | 0.44 (0.09) | 0.48 (0.10) | 0.46 (0.11) |
| Hip adduction moment PCA | |||
| Principal pattern 1 | 7.7 (1.4) | 7.9 (1.5) | 7.6 (1.8) |
| Principal pattern 2 | 0.44 (0.57) | 0.02 (0.50) | –0.15 (0.56) |
| Principal pattern 3 | –1.33 (0.39) | –1.44 (0.46) | –1.32 (0.54) |
aValues are expressed as mean (SD). FAI, femoroacetabular impingement.
Figure 2.
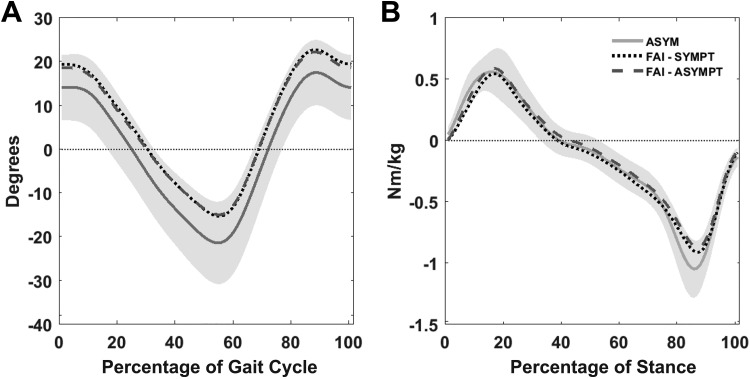
Ensemble averages of (A) sagittal plane hip motion, time normalized to gait cycle, and (B) sagittal plane net external moments, time normalized to stance, for the asymptomatic group (ASYM) and individuals with femoroacetabular impingement (FAI), including symptomatic (SYMPT) and contralateral (ASYMPT) limbs. Shaded areas represent 1 SD above and below the ASYM mean.
Figure 3.
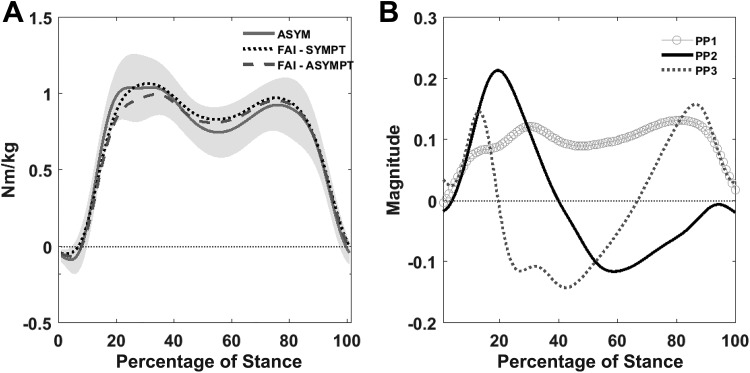
(A) Ensemble averages of frontal plane net external moments, time normalized to stance, for the asymptomatic group (ASYM) and individuals with femoroacetabular impingement (FAI), including symptomatic (SYMPT) and contralateral (ASYMPT) limbs. Shaded areas represent 1 SD above and below the ASYM mean. (B) Principal patterns (PP). The first pattern (PP1) captured the overall shape and magnitude, capturing 74% of the waveform variability. PP2 explained an additional 11%, capturing a difference between early and late stance, where high PP2 scores indicate a greater first peak and a lower second peak. Seven percent of the waveform variability was captured by PP3, a difference operator between early, mid-, and late stance, where high scores indicate a more pronounced dip between the first and second peaks during midstance.
The 3 PPs explained 92% of the net external hip adduction moment (Figure 3B). No differences were found between limbs in the FAI group or between groups (P > .017), with the exception of the difference in PP2 scores between the asymptomatic group and the contralateral limb of the FAI group (P = .002), where PP2 scores were greater in the asymptomatic group.
Electromyography
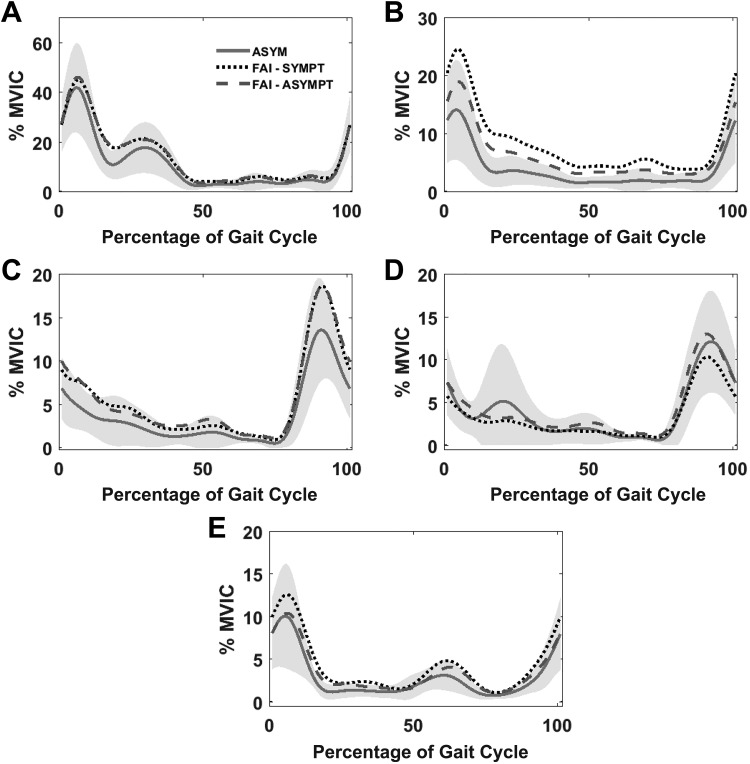
The gluteus medius electromyogram is illustrated in Figure 4A. PP1 explained 95% of the variability, capturing the overall waveform shape and magnitude, whereas 2% and 1% of the variability were explained by difference operator patterns of PP2 and PP3, respectively. No significant differences were found between the asymptomatic and FAI groups or between the contralateral and symptomatic limbs in the FAI group for any of the gluteus medius PP scores (P > .017).
Figure 4.
Ensemble averages of electromyograms for (A) gluteus medius, (B) gluteus maximus, (C) medial hamstring, (D) lateral hamstring, and (E) rectus femoris, time normalized to the gait cycle and amplitude normalized to percentage of maximal voluntary isometric contractions (% MVIC). Data given for the asymptomatic group (ASYM) and individuals with femoroacetabular impingement (FAI), including symptomatic (SYMPT) and contralateral (ASYMPT) limbs. Shaded areas represent 1 SD above and below the ASYM mean.
Figure 4B illustrates the gluteus maximus electromyogram. PP1 captured the overall magnitude and shape, explaining 93% of the waveform variability, whereas 4% and 2% were explained by PP2 and PP3 patterns capturing amplitude differences (difference operators) across the gait cycle. PP1 scores were greater in the FAI symptomatic limb compared with the asymptomatic group (P = .006), whereas no differences were found between the FAI contralateral limb and the asymptomatic group or within the FAI group itself (P > .017).
Medial and lateral hamstring electromyograms are shown in Figures 4C and 4D, respectively. PP1 captured the overall magnitude and shape, explaining 74% of the waveform variability. PP2 and PP3 captured muscle activation differences across the gait cycle, explaining 11% and 7%, respectively. In the FAI group, a significant PP1 score difference was found, with MH values greater than LH values for both limbs (P < .001), whereas this effect was not found in the asymptomatic group (P > .017). MH or LH did not differ between the asymptomatic group and either leg of the FAI group (P > .017). No other PP2 or PP3 differences were found between groups or between legs within the FAI group (P > .017).
Regarding the rectus femoris (Figure 4E), PP1 captured the overall magnitude shape, explaining 93% of the waveform variability. PP2 and PP3 captured amplitude differences (difference operators) across the gait cycle, explaining 3% and 2%, respectively. No significant differences were found between the contralateral and symptomatic legs in individuals with FAI, and no differences were found between the FAI group and asymptomatic group (P > .017) for any of the rectus femoris PP scores.
Discussion
In this study, we sought to understand the mechanical asymmetries during level-ground walking between the symptomatic and contralateral hips of individuals with cam FAI and compare these biomechanics and muscle activation patterns to an asymptomatic group of individuals with no known hip injury or disease. The study hypotheses were partially supported. To summarize, individuals with unilateral symptomatic cam FAI walked with similar biomechanics and hip muscle EMG bilaterally. The only differences found between the symptomatic limb in the FAI group and the asymptomatic group were a greater gluteus maximus activation amplitude (as captured by PP1), and MH > LH PP1 scores for the hamstrings.
This study has implications for biomechanical and EMG research in FAI but also provides information that could be integrated within clinical practice structures. The usefulness of gait analysis or gait retraining in the clinical evaluation and treatment of FAI was challenged, and results suggest that strength evaluation in the assessment of symptomatic FAI should be applied more broadly to both ipsilateral and contralateral limbs of individuals with unilateral symptoms to understand the implications of the condition of physical function.
In general, the FAI group was deconditioned in comparison with the asymptomatic group. Despite similar sex profiles of the groups, those with FAI reported HOOS and iHOT-33 scores that were comparable with, if not lower than, scores previously found for individuals with moderate hip OA,29 and those with FAI walked more slowly, at a speed comparable with healthy older adults.26 Regardless of statistical outcomes (see Table 2), individuals with FAI generated approximately 15% to 25% less torque during the strength testing in both limbs when compared with the asymptomatic group.
While symptom duration is not known for the FAI group, these overall findings suggest 1 of 2 predispositions to the following state: (1) the symptoms of FAI result in limitations that keep individuals from being active, resulting in physical deterioration over time, or (2) the FAI group is naturally deconditioned, which may predispose these individuals to symptomatic disease. While the former is more likely, given the incidence of FAI in the athletic population,6 it was surprising that no differences between legs in the cam FAI group were found in any of the variables measured, despite only 1 hip being symptomatic and requiring arthroscopic procedures. All individuals with FAI had bilateral disease on radiograph, evidenced by alpha angles greater than 50°, but the symptom presentation did not alter mechanical outcomes. Individuals with FAI did report some difficulty with walking-based outcomes on HOOS and iHOT-33 questions; however, the walking assessment conducted in this study revealed few walking-based biomechanical differences, with differences from the asymptomatic group occurring on EMG only.
Morphological abnormalities predispose individuals with cam FAI to abutment of the femoral neck and acetabular rim with positions of flexion, adduction, and internal femoral rotation.25 This combined position does not occur during the typical ranges of motion noted during gait, because flexion, the first component of the hip impingement test, does not often reach beyond 30° to 40°, as shown in Figure 2A and by other investigators.5,10 No biomechanical differences in motion or moments were shown between legs in the FAI group or between the FAI symptomatic leg and the asymptomatic group, corroborating previous work.5,10
These data suggest that symptoms due to cam deformity have limited impact on walking mechanics over the short term. Individuals with FAI scored 43 of 100 for the iHOT-33 question pertaining to long-distance walking, suggesting some difficulty with this dynamic task as duration increases. While immediate pain responses were not captured in this study, it is possible that repetitive hip joint stress, while not necessarily within the impingement position, will foster a biomechanical and biochemical environment for altered symptoms. Testing of functional tasks that put the hip joint further into the impingement positions is required, such as stair climbing, squatting, or hill walking; however, these current data also suggest that further work with longer duration walking (>6 minutes) is required to determine whether mechanics begin to break down with continued demands.
The comprehensive biomechanical assessment, including the use of principal component analysis for HAM evaluation, a technique used extensively in knee OA gait literature for knee adduction moment assessments,9,23 revealed limited alterations to biomechanical joint stresses during the walking task. In the majority of muscles analyzed, no amplitude or activation pattern differences were found, giving support to the conclusion that mechanical alterations to joint function during gait in individuals with FAI are minimal. Given that knee and hip flexion and extension strength was lower in the FAI group and that no amplitude differences (as a percentage of maximum) occurred during gait in these muscles, the findings suggest that the FAI group was lowering the demands of the walking task in comparison with the asymptomatic group. This strategy does not, however, have the effect of altering the general mechanical demands, as evidenced in the current data.
Despite many null findings, individuals with FAI did walk with greater gluteus maximus activation (PP1 scores) in the symptomatic hip compared with the asymptomatic group. Diamond et al10 found altered coordination of deep hip muscles during gait, particularly at the time of early swing, but those investigators stated that further work is required regarding additional superficial muscles that span the hip, such as the gluteus maximus. No other studies could be found that investigated the gluteus maximus in a population with FAI. Rutherford et al29 recently found certain activation patterns in the gluteus maximus that suggest prolonged activation in individuals with severe hip OA, with no differences found between an asymptomatic group and a moderate hip OA group. Amplitude differences, such as those of the current study, could not be interpreted because of the dissimilarities in normalization technique, thus limiting application to the study of FAI. The greater gluteus maximus amplitudes, while partially explained by the lower strength bilaterally in the FAI group, may be a strategy to limit the potential for hip flexion in the FAI group. While similar sagittal plane moments and motions were found, the EMG results suggest that alterations are occurring to preserve these biomechanical outcomes in this group.
Differential hamstring activation, with amplitudes of MH > LH, was found in the FAI group. Similar differential activation, where MH > LH, has been found previously in healthy older adults during treadmill walking26 and in those with moderate hip OA.29 The opposite, with LH > MH, was found for those with medial compartment knee OA, thought to be a mechanism by which active stability is required on the lateral knee joint27 during walking. It is difficult to determine the mechanism by which a greater MH would be required during walking, although previous work in gait modification may provide guidance. When individuals with knee OA actively adopt a toe-in gait through hip internal rotation, MH amplitudes increase to levels greater than LH.4 However, when a toe-out gait is adopted, no differences occur.28 It appears that individuals with cam FAI may be actively stabilizing their hip joints using muscles involved with internal rotation rather than external rotation in comparison with asymptomatic individuals. Electromyography provided information that control parameters in individuals with cam FAI differ slightly from those of asymptomatic individuals during level treadmill walking, despite hip mechanics being generally symmetrical and not different from those of asymptomatic individuals. Given the current focus on cam FAI, further work is required to determine whether these results apply to individuals with pincer or combined pincer-cam FAI.
Considerations are required for the interpretation of the current work. First, all individuals in the FAI group had bilateral cam FAI, despite unilateral symptoms, and we did not have radiographs for the asymptomatic group. Therefore, the results must be interpreted in this context; it is possible that asymptomatic individuals may have had asymptomatic cam deformities. Understanding why individuals with FAI had unilateral symptoms was outside the scope of this study. While soft tissue injuries, such as labral tears, are known to occur as a result of FAI14 and may have been present unilaterally, a radiograph would not be sensitive to their identification. Despite this possibility, hip mechanics and muscle activation patterns, with the exception of those for the gluteus maximus, were not affected during level walking.
Second, there is debate as to whether walking is sufficient to investigate hip function in FAI, particularly as walking is not an activity that is functionally limiting in comparison with other functional tasks that require greater hip ranges of motion. We do not have information pertaining to pain or self-reported function during the 6 minutes of walking in this study. Therefore, it is possible that these individuals had no pain during this assessment, despite having unilateral symptoms and functional deficits warranting orthopaedic consultation and arthroscopic intervention.
Third, we did not measure history of strength training, physical activity levels, or past physical therapy treatments in our asymptomatic or FAI participants. Therefore, the possibility exists for one of these groups to be more physically active than the other, which may have implications for interpretation of the findings. Last, this investigation involved muscles that could be assessed through surface EMG methods. We acknowledge that other hip muscles, such as iliopsoas, may be affected by FAI; however, further work regarding methodological considerations for surface-based methods is needed to understand the implications for measuring these deeper muscles during dynamic activities like gait. Future work not only should address tasks that place the hip joint in positions of greater range but should also further assess the standardized walking task by increasing the demands (eg, longer duration, walking on declines or inclines, perturbations).
Conclusion
Understanding the joint mechanics and muscle activation patterns in individuals with cam FAI may assist with predicting long-term implications of this dysfunction, which typically affects a young, athletic population. Knee and hip flexion and extension as well as hip adduction strength were reduced bilaterally in the FAI group compared with an asymptomatic group. Using gait as the functional activity, we found no between-group differences in 3-dimensional hip joint motions or HAM, nor did we note any between-leg differences within the FAI group. The FAI group recruited the gluteus maximus to a higher level than did the asymptomatic group; the FAI group also demonstrated higher levels of MH activity relative to the LH, whereas this differential activation did not occur in the asymptomatic group. While these findings add to our understanding of the implications of cam FAI, they also highlight the need for studies that include functional activities that put the hip further into a position of impingement, potentially elucidating more definitive differences between the two groups.
Acknowledgment
We acknowledge Matthew Baker for assistance with data collection; Meaghan MacDonald, Nicole Paquet, and Jalisa den Hartog for recruitment; Hillie Devries and Lindsey Buckingham (physical therapists) for reviewing the manuscript; and our participants for taking time to help us with our work.
Footnotes
One or more of the authors has declared the following conflict of interest or source of funding: This work was funded by the Faculty of Health, Dalhousie University.
Ethical approval for this study was provided by the Nova Scotia Health Authority Research Ethics Board (Romeo No. 1016790).
References
- 1. Agricola R, Heijboer MP, Bierma-Zeinstra SM, et al. Cam impingement causes osteoarthritis of the hip: a nationwide prospective cohort study (CHECK). Ann Rheum Dis. 2013;72(6):918–923. [DOI] [PubMed] [Google Scholar]
- 2. Barton C, Salineros MJ, Rakhra KS, Beaule PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res. 2011;469(2):464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87-B:1012–1018. [DOI] [PubMed] [Google Scholar]
- 4. Booij M, Richards R, Harlaar J, et al. Knee muscle activation patterns are altered in patients with moderate knee osteoarthritis during gait retraining designed to reduce the knee adduction moment. Gait Posture. 2016;49(suppl 2):71. [Google Scholar]
- 5. Brisson N, Lamontagne M, Kennedy MJ, Beaule PE. The effects of cam femoroacetabular impingement corrective surgery on lower-extremity gait biomechanics. Gait Posture. 2013;37(2):258–263. [DOI] [PubMed] [Google Scholar]
- 6. Byrd JW. Femoroacetabular impingement in athletes: current concepts. Am J Sports Med. 2014;42(3):737–751. [DOI] [PubMed] [Google Scholar]
- 7. Casartelli NC, Maffiuletti NA, Item-Glatthorn JF, et al. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthritis Cartilage. 2011;19(7):816–821. [DOI] [PubMed] [Google Scholar]
- 8. Davis AM, Bridge P, Miller J, Nelson-Wong E. Interrater and intrarater reliability of the active hip abduction test. J Orthop Sports Phys Ther. 2011;41(12):953–960. [DOI] [PubMed] [Google Scholar]
- 9. Deluzio KJ, Wyss UP, Zee B, et al. Principal component models of knee kinematics and kinetics: normal vs. pathological gait patterns. Hum Mov Sci. 1997;16:201–217. [Google Scholar]
- 10. Diamond LE, Wrigley TV, Bennell KL, et al. Hip joint biomechanics during gait in people with and without symptomatic femoroacetabular impingement. Gait Posture. 2016;43:198–203. [DOI] [PubMed] [Google Scholar]
- 11. Diamond LE, Wrigley TV, Hinman RS, et al. Isometric and isokinetic hip strength and agonist/antagonist ratios in symptomatic femoroacetabular impingement. J Sci Med Sport. 2016;19(9):696–701. [DOI] [PubMed] [Google Scholar]
- 12. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466(2):264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. [DOI] [PubMed] [Google Scholar]
- 14. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hack K, Di Primio G, Rakhra K, Beaule PE. Prevalence of cam-type femoroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am. 2010;92(14):2436–2444. [DOI] [PubMed] [Google Scholar]
- 16. Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. [DOI] [PubMed] [Google Scholar]
- 17. Hunt MA, Guenther JR, Gilbart MK. Kinematic and kinetic differences during walking in patients with and without symptomatic femoroacetabular impingement. Clin Biomech (Bristol, Avon). 2013;28(5):519–523. [DOI] [PubMed] [Google Scholar]
- 18. Kappe T, Kocak T, Bieger R, et al. Radiographic risk factors for labral lesions in femoroacetabular impingement. Clin Orthop Relat Res. 2011;469(11):3241–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kennedy MJ, Lamontagne M, Beaule PE. Femoroacetabular impingement alters hip and pelvic biomechanics during gait walking biomechanics of FAI. Gait Posture. 2009;30(1):41–44. [DOI] [PubMed] [Google Scholar]
- 20. Lerch S, Kasperczyk A, Warnecke J, et al. Evaluation of cam-type femoroacetabular impingement by ultrasound. Int Orthop. 2013;37(5):783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin RL, Enseki KR, Draovitch P, et al. Acetabular labral tears of the hip: examination and diagnostic challenges. J Orthop Sports Phys Ther. 2006;36(7):503–515. [DOI] [PubMed] [Google Scholar]
- 22. Mayne E, Memarzadeh A, Raut P, et al. Measuring hip muscle strength in patients with femoroacetabular impingement and other hip pathologies: a systematic review. Bone Joint Res. 2017;6(1):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Newell RS, Hubley-Kozey CL, Stanish WD, Deluzio KJ. Detecting differences between asymptomatic and osteoarthritic gait is influenced by changing the knee adduction moment model. Gait Posture. 2008;27(3):485–492. [DOI] [PubMed] [Google Scholar]
- 24. Panjabi MM. The stabilizing system of the spine, part 1: function, dysfunction, adaptation and enhancement. J Spinal Disord. 1992;5(4):383–389. [DOI] [PubMed] [Google Scholar]
- 25. Philippon MJ, Maxwell RB, Johnston TL, et al. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041–1047. [DOI] [PubMed] [Google Scholar]
- 26. Rutherford D, Baker M, Wong I, Stanish W. The effect of age and knee osteoarthritis on muscle activation patterns and knee joint biomechanics during dual belt treadmill gait. J Electromyogr Kinesiol. 2017;34:58–64. [DOI] [PubMed] [Google Scholar]
- 27. Rutherford DJ, Hubley-Kozey CL, Stanish WD. Changes in knee joint muscle activation patterns during walking associated with increased structural severity in knee osteoarthritis. J Electromyogr Kinesiol. 2013;23(3):704–711. [DOI] [PubMed] [Google Scholar]
- 28. Rutherford DJ, Hubley-Kozey CL, Stanish WD. The neuromuscular demands of altering foot progression angle during gait in asymptomatic individuals and those with knee osteoarthritis. Osteoarthritis Cartilage. 2010;18(5):654–661. [DOI] [PubMed] [Google Scholar]
- 29. Rutherford DJ, Moreside J, Wong I. Hip joint motion and gluteal muscle activation differences between healthy controls and those with varying degrees of hip osteoarthritis during walking. J Electromyogr Kinesiol. 2015;25(6):944–950. [DOI] [PubMed] [Google Scholar]
- 30. Sims K. The development of hip osteoarthritis: implications for conservative management. Man Ther. 1999;4(3):127–135. [DOI] [PubMed] [Google Scholar]
- 31. Solomonow M, Krogsgaard M. Sensorimotor control of knee stability: a review. Scand J Med Sci Sports. 2001;11:64–80. [DOI] [PubMed] [Google Scholar]
- 32. Tannast M, Goricki D, Beck M, et al. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res. 2008;466(2):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tresch F, Dietrich TJ, Pfirrmann CWA, Sutter R. Hip MRI: prevalence of articular cartilage defects and labral tears in asymptomatic volunteers: a comparison with a matched population of patients with femoroacetabular impingement. J Magn Reson Imaging. 2017;46(2):440–451. [DOI] [PubMed] [Google Scholar]
- 34. Vaughan CL, Davis BL, O’Conner JC. Dynamics of Human Gait. 2nd ed Cape Town, South Africa: Kiboho Publishers; 1999. [Google Scholar]
- 35. Wisniewski SJ, Grogg B. Femoroacetabular impingement: an overlooked cause of hip pain. Am J Phys Med Rehabil. 2006;85(6):546–549. [DOI] [PubMed] [Google Scholar]
- 36. Zeni JA, Jr, Higginson JS. Gait parameters and stride-to-stride variability during familiarization to walking on a split-belt treadmill. Clin Biomech (Bristol, Avon). 2010;25(4):383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]