Abstract
Background:
Liver disease is an increasing cause of death worldwide but palliative care is largely absent for these patients.
Aim:
We conducted a feasibility trial of a complex intervention delivered by a supportive care liver nurse specialist to improve care coordination, anticipatory care planning and quality of life for people with advanced liver disease and their carers.
Design:
Patients received a 6-month intervention (alongside usual care) from a specially trained liver nurse specialist. The nurse supported patients/carers to live as well as possible with the condition and acted as a resource to facilitate care by community professionals. A mixed-method evaluation was conducted. Case note analysis and questionnaires examined resource use, care planning processes and quality-of-life outcomes over time. Interviews with patients, carers and professionals explored acceptability, effectiveness, feasibility and the intervention.
Setting/participants:
Patients with advanced liver disease who had an unplanned hospital admission with decompensated cirrhosis were recruited from an inpatient liver unit. The intervention was delivered to patients once they had returned home.
Results:
We recruited 47 patients, 27 family carers and 13 case-linked professionals. The intervention was acceptable to all participants. They welcomed access to additional expert advice, support and continuity of care. The intervention greatly increased the number of electronic summary care plans shared by primary care and hospitals. The Palliative care Outcome Scale and EuroQol-5D-5L questionnaire were suitable outcome measurement tools.
Conclusion:
This nurse-led intervention proved acceptable and feasible. We have refined the recruitment processes and outcome measures for a future randomised controlled trial.
Keywords: Liver failure, palliative care, supportive care, care planning, nurse specialist, feasibility trial, generalist palliative care
What is already known about the topic?
Palliative care is available increasingly for people with advanced heart, lung and kidney disease but tends to be absent or very late for people with liver disease.
Liver disease is a rising cause of death worldwide, affecting younger people and often ending with a hospital death.
Effective identification, ongoing support and care planning can improve quality of life for people with advanced liver disease.
What this paper adds?
A liver nurse specialist intervention proved acceptable and feasible.
Patients, families and professionals valued and benefitted from support and case management from a liver nurse specialist with some generic palliative care training.
The trial successfully identified recruitment processes, refined an acceptable intervention and tested the feasibility of collecting candidate outcome measures.
Implications for practice, theory or policy
This feasibility trial provided valuable information to inform the design of a future randomised controlled trial of a nurse-led intervention to improve the care of people with advanced liver disease.
Introduction
Liver disease is an increasing cause of death worldwide, with 90% of deaths under 70 years.1,2 Its trajectory is erratic and unpredictable, with increasingly frequent, unplanned hospital admissions.3 Patients experience many physical and psychosocial challenges including fatigue, anxiety and depression.4 Their everyday life is disrupted and uncertainty prevails.5 Comorbidities are common and 70% of deaths occur in hospital.2
Palliative care is available increasingly for people with heart, lung and kidney failure.6 However, supportive and palliative care needs in people with liver failure often go unrecognised and unaddressed.7,8 This is despite many national policies and the World Health Organization (WHO) calling for palliative care to be delivered according to need.9–11 Compared with other advanced progressive illnesses such as cancer, heart failure, respiratory disease or neurological conditions, involvement of nurse specialists is rare. In liver units, they usually support people being assessed for liver transplantation or work in hepatitis C services.12 Supportive care in the community, integrated with hospital hepatology/gastroenterology services, is increasingly seen as important.13 Effective identification, ongoing support and anticipatory care planning should enable people with advanced liver disease to maximise their quality of life and reduce the burden of unplanned hospital admissions that are so common in their final year of life.
Randomised controlled trials (RCTs) in liver failure focus predominantly on managing physical complications.14 We found no trials of palliative care for people with advanced liver disease; however, a quality improvement project of early palliative care for patients awaiting liver transplantation suggested that it could deliver potential improvements in symptom burden and mood.15 Evidence-based models in cancer have successfully introduced palliative care earlier and integrated with care by other specialists.16,17 Management of advanced liver disease can be complex and requires specific expertise that may not be available from palliative care clinicians or primary care teams.18 Integration of palliative care principles into the existing role of hepatology teams is potentially more acceptable, deliverable and accessible. We developed an intervention to deliver this model of care and assessed it within a feasibility trial.
We evaluated the feasibility of a nurse-led, supportive care intervention for patients with advanced liver disease for whom liver transplantation was contraindicated. We aimed to answer three questions:
Was the supportive care service acceptable to patients, their informal carers and health professionals?
How could this intervention be improved to ensure that it is robust, feasible, deliverable and potentially cost effective as part of routine health and social care services?
Is a randomised trial feasible in terms of recruitment, retention and qualitative and quantitative data collection to measure primary and secondary outcomes?
Method
We conducted a feasibility trial with a mixed-method evaluation.19,20 The study was designed, conducted and evaluated in collaboration with a patient and public involvement (PPI) group with extensive experience of advising on research in end-of-life care.
Recruitment and sample characteristics
We recruited patients aged over 18 with decompensated cirrhosis and their families from a tertiary gastroenterology/hepatology hospital ward in Scotland (April 2015–February 2016). Patients were eligible if they had two or more hospital admissions with advanced liver disease related to complications of ascites, gastrointestinal haemorrhage, encephalopathy, hepatorenal failure, sepsis or progressive hepatocellular carcinoma. We excluded patients with an expected survival of under 1 month, other conditions likely to cause death within 12 months, persistent cognitive impairment (assessed with the 4AT screening tool21) or those who lived over 50 miles from the recruitment site to make qualitative interviews at home feasible. We chose not to include patients on the transplant waiting list as they were already receiving care coordination from designated liver specialists. Patients nominated their main informal carer who was also invited to participate. Their general practitioner (GP) was recruited by letter.
The intervention
Two liver nurses with over 10 years of liver nursing experience shared the role and delivered the intervention for 21 h per week. In addition to completing a Good Clinical Practice in research course, they received specific training in palliative care consisting of an advanced communication topic workshop on talking about deteriorating health and opportunities to shadow palliative care staff working in hospital, hospices and the community. They also accompanied a heart failure nurse specialist on home visits. After an initial face-to-face assessment and care coordination meeting with the patients and their informal carers, the liver nurses provided supportive care mainly by telephone for up to 6 months (alongside the patients’ usual care). The supportive care liver nurse specialist had three defined roles:
Act as a case manager and coordinator liaising between patients/carers, health and social care professionals or services, and voluntary agencies;
Support patients and informal carers to live as well as possible by offering information about the illness and its management including financial and social support, providing psychological support and empowering patients/carers to seek help;
Support care delivered by professionals in the community through providing information and advice on the best practice (including integration of disease-focused treatments with supportive and palliative care) and facilitating anticipatory care planning through the use of the Scottish electronic summary care plan (Box 1). This is completed in primary care and accessed by emergency services and hospital staff. Details of the components and the delivery process of the intervention are shown in Figure 1.
Box 1. The Scottish Key Information Summary.
Introduced in 2013, the Key Information Summary (KIS) is a shared electronic medical record between healthcare professionals in Scotland. Using a template within the GP clinical system, a KIS is written for patients with the most complex care needs and notes key points of their anticipatory care plan, for example, medications, carer and next of kin details, the person’s wishes regarding place of death and resuscitation. The form may be updated as the patient’s condition progresses. It can be accessed by Out of Hours and some other services, for example, Accident & Emergency, Acute Receiving Unit and the Scottish Ambulance Service. Although other services can read a KIS, presently only General Practices can add information to the document. For further details, see http://www.scimp.scot.nhs.uk/key-information-summary/
Figure 1.
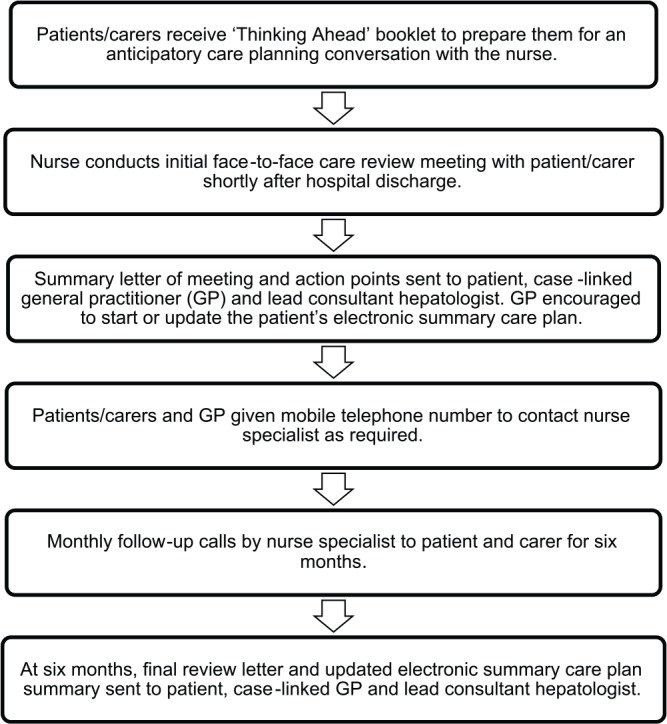
Components and delivery process of the intervention.
Evaluation of the intervention
Five datasets were integrated to provide a comprehensive, mixed-method evaluation. Anonymity of patient/carer/professional data was assured by assigning each patient a number at recruitment, which was used on all documentation and retained securely. Health and care resource use was monitored for the duration of the intervention period; number of planned and patient/carer-initiated contacts with the nurse, number and duration of unplanned and elective hospital admissions, reason(s) for admission, hepatology outpatient visits, primary care team contacts, use of informal and formal support agencies, place and cause of death; time from recruitment to death. All correspondence and the clinical records of interactions with the nurses were documented, anonymised and analysed. Questionnaires for patients and family carers were presented in attractive, readable booklets and approved by our PPI group. The questionnaires were chosen on the basis of extensive use for previous research in palliative care populations, ease of completion to reduce participant burden, and to provide a spectrum of complementary measures that could be considered for suitability in a large trial. They were administered by the nurse at baseline after recruitment to the study and sent by post at 3 and 6 months. Patients completed the Palliative care Outcome Scale (POS), which is a brief measure of palliative care service outcomes;22 the EuroQol-5D-5L, which assesses quality of life and can inform economic evaluations;23 the Hospital Anxiety and Depression Score (HADS), to screen for anxiety and depression which are common psychological symptoms in people with advanced illness;24 and the McGill Quality of Life questionnaire Part B, which invites participants to identify up to three troublesome physical symptoms or problems and rate their severity.25 Family carers completed the POS Carer Score and the Caregiver Quality of Life questionnaire to assess the impact of the intervention on the carer experiences.26 Qualitative interviews with a purposive sample of patients representing a range of aetiologies, demographics and care arrangements, their family carers and case-linked health and care professionals were conducted, using topic guides, by a female non-clinical researcher (B.K.). Her previous PhD had involved conducting in-depth, serial interviews with people with advanced liver disease.27 The interviews explored the acceptability, feasibility and perceived impact of the intervention. Patients and carers were interviewed (either alone or together if they preferred) for 20–60 minutes in the person’s home shortly after their initial face-to-face meeting with the nurse, 3 and 6 months later. Professionals were interviewed once, face-to-face or by telephone, at the end of the intervention.
Data analysis
The audio-recorded qualitative data and field notes were coded and analysed thematically according to the research questions and any emerging themes using NVivo qualitative data management software version 10 (QSR International (UK) Limited, London, UK). Questionnaire data were analysed using SPSS for Windows version 19 (IBM Corp., Armonk, NY). Descriptive statistics were used for the sample characteristics and quality-of-life outcomes. Continuous measures were summarised using the mean and standard deviation where these were normally distributed data and, if not, using the median and the interquartile range. Categorical variables were summarised by the frequency in each category. Outcomes were compared between time points using repeated-measures one-way analysis of variance (ANOVA) for normally distributed data and the Friedman test otherwise. Documentation generated by the nurses for each participant was reviewed systematically for type and content of interaction and resultant actions. Emerging findings were regularly discussed with the wider research team and the PPI group.
Results
Feasibility of the intervention and evaluation
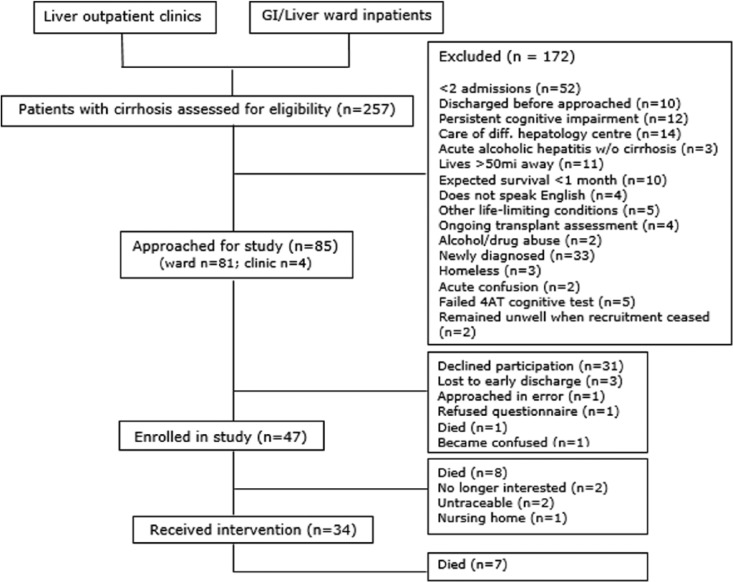
We recruited 47 patients over 46 weeks from gastroenterology/hepatology inpatient wards and outpatient clinics, of whom 13 died or were lost to follow-up before starting the intervention; hence, 34 (72%) went on to receive the intervention (Figure 2). Patient characteristics are shown in Table 1. During the initial 12 weeks, 82 of the 108 inpatients screened did not have multiple admissions as per the original inclusion criterion, so we revised this to ‘one or more’ unplanned hospital admissions to facilitate subsequent recruitment. The clinicians decided that this intervention could improve care for patients following any hospital admission with decompensated, advanced liver disease and so recommended this change. A family carer was nominated by 27 patients and all agreed to participate.
Figure 2.
Flowchart of patient recruitment.
Table 1.
Patient characteristics at time of recruitment.
| No. of patients recruited | 47 |
| Male/female | 31/16 |
| Age | Mean 60.5 years (range 31–87 years) |
| Liver disease aetiology | 31 alcohol-related liver disease (ALD) 5 non-alcoholic fatty liver disease 10 combination of causes 1 unknown |
| Carstairs deprivation score28 | Mean 3.5; median 4 (1 = most affluent, 7 = most deprived) |
| Time since diagnosis of cirrhosis | Mean 2.7 years (range 0–12 years) |
| Hepatology clinical scoresa | MELD: mean 16; median 16 (range 6–29) Child–Pugh: mean 10; median 9 (range 6–12) |
| Cirrhosis-related complications requiring previous admissions | 41 ascites 23 encephalopathy 20 bleeding varices |
| No. of ward admissions showing previous usage | In previous 5 years: mean 3.8; median 3 (range 1–17) In previous 12 months: mean 2.6; median 2 (range 0–9) |
| No. of inpatient days in the previous 12 months | Mean 22.8 days; median 16 (range 0–78 days) |
MELD: Model for End-Stage Liver Disease.
Different scoring systems exist to predict the prognosis of patients with chronic liver disease and to determine the need for liver transplantation. MELD score is calculated from the patient’s serum bilirubin, serum creatinine and the international normalised ratio (INR) for prothrombin time. A score of 10–19 indicates a 6% 3-month mortality. Child–Pugh considers bilirubin, serum albumin, prothrombin time and prolongation, ascites and hepatic encephalopathy. A score of 10–15 indicates a 45% chance of 1-year survival.
Of the 34 intervention patients, 27 (79%) were alive at study completion. The seven patients who died during the intervention were in the study for a median of 124.5 days. Six patients died in hospital from acute complications of advanced cirrhosis. None were admitted for terminal care and all admissions were considered clinically appropriate by the ward team. In total, 14 (52%) carers left the study due to patient death (n = 10), patient withdrawal (n = 1), relationship breakup (n = 1) and loss of interest (n = 2). We successfully recruited our target population of people with advanced liver disease at high risk of death but not imminently dying and their family carers. Resource use data including planned and unplanned hospital admission days and outpatient visits proved feasible to collect from hospital records. Of the 34 case-linked GPs, 18 provided patient contact data.
Questionnaire pack return rates were high at the initial time point, indicating that the instruments and delivery methods used were acceptable. Due to death, attrition and non-returns, only 14 patients returned their questionnaire packs at all three time points (30% of those recruited), with lower completion rates for some questionnaires. The rate of return for carer questionnaires was also high initially, but fell over time (Table 2).
Table 2.
Questionnaire returns over time.
| Patient questionnaires | Baseline (n = 42; %) | 3 months (n = 31; %) | 6 months (n = 27; %) |
|---|---|---|---|
| POS | 100 | 77 | 48 |
| HADS | 98 | 81 | 52 |
| EQ-5D | 100 | 81 | 52 |
| EQ-VAS | 90 | 81 | 48 |
| McGill | 95 | 81 | 48 |
| Continuity of care | 88 | 81 | 52 |
| Carer questionnaires | Baseline (n = 27; %) | 3 months (n = 17; %) | 6 months (n = 13; %) |
| Carer POS | 89 | 71 | 46 |
| Carer QOL | 85 | 71 | 38 |
POS: Palliative care Outcome Scale; HADS: Hospital Anxiety and Depression Score; EQ-5D: EruQol-5D; EQ-VAS: EuroQol-Visual Analogue Scale; QOL: quality of life.
n = number of questionnaires distributed; a returned questionnaire is a questionnaire returned with at least one item completed.
This feasibility study was not designed or powered to detect changes. However, exploratory data analysis revealed statistically significant improvements in key palliative care outcomes (POS) and quality of life (EQ-5D) over time (Table 3). The HADS showed little change in patient depression and anxiety, but its value may have been limited because fatigue and poor sleep are increasingly frequent symptoms as the liver function worsens. Likewise, the rating scales of the McGill tool seemed to be difficult for some participants to complete adequately. Six carers completed both the Carer POS and the Carer Quality of Life Index Scale at all three time points, but it was difficult to evaluate these data further due to our small sample size and participant attrition. The service use data did not show any reduction in hospital bed-nights and outpatient attendances after the intervention started, although some patients did give accounts of instances where this had happened in their interviews.
Table 3.
Outcomes’ scores over time.
| Outcomeb | Baseline | 3 months | 6 months | Change |
|---|---|---|---|---|
| POS (n = 11) | ||||
| Mean | 19.45 | 14.18 | 14.73 | p = 0.001* |
| SD | 6.80 | 9.11 | 9.41 | |
| EQ-5D index (n = 13)a | ||||
| Median | 0.50 | 0.69 | 0.67 | p = 0.037* |
| IQR | 0.33 | 0.22 | 0.32 | |
| EQ-VAS (n = 11) | ||||
| Mean | 41.82 | 51.36 | 62.27 | p = 0.004* |
| SD | 16.47 | 18.97 | 13.67 | |
| HADS–Anxiety (n = 14) | ||||
| Mean | 10.07 | 8.79 | 9.14 | p = 0.43 |
| SD | 5.20 | 5.96 | 4.27 | |
| HADS–Depression (n = 14) | ||||
| Mean | 9.57 | 8.86 | 8.93 | p = 0.74 |
| SD | 2.95 | 4.31 | 4.07 | |
POS: Palliative care Outcome Scale; SD: standard deviation; IQR: interquartile range; HADS: Hospital Anxiety and Depression Score.
Analysed using the Friedman test; repeated-measures one-way ANOVA was used for all other outcomes.
Based on those who completed a questionnaire at all three time points only.
Highlights statistically significant improvements.
For the qualitative interviews, all those we sampled agreed to an initial interview (13 patients, 10 family carers and 13 professionals), yielding 42 interviews (20 of these jointly with patient and carer). Only two patient–carer pairs opted out of follow-up interviews, confirming the acceptability and feasibility of multi-perspective, longitudinal, qualitative interviewing in this patient group.
Acceptability of the individual components of the intervention to patients, families and professionals
The initial face-to-face meeting was widely considered helpful due to the time pressures typical of medical appointments and the nurses’ informal approach. Visiting the patient’s home for this meeting was valued as it allowed the nurses to see people’s living arrangements and gauge the person’s support needs at home:
We felt we were getting more answers, whereas in the hospital you feel as if it is, ‘We’re too busy, we’ve got other patients, you’re not the only patient, we’ve got this patient to see’. […] When [study nurse] was here we got a chance to speak. It was good, we did get a lot of feedback; it was really helpful. (Patient and carer 30)
I think it was nice they came to the house after [patient] came home because then they know how you’re living, you know? If you were sitting in rented accommodation that was terrible they know the home life is sad or poor or whatever and they can support you. It’s good that they know your home surroundings as well and who you have at home, if you have nobody, you know. (Patient and carer 34)
The dedicated telephone line received a high number of calls (70 calls by patients, 44 calls by family carers and 28 by professionals). Participants considered the frequency of the planned monthly calls by the nurse to be adequate. Some felt that they could manage with fewer or no calls as long as they had access to a designated telephone number to contact the nurses if needed. The monthly calls were considered particularly valuable to socially isolated patients:
I think psychologically knowing that [the study nurses] were there if I needed them and the fact they were going to call me in a month anyway, that’s probably helpful in itself. (Patient 07)
Professionals particularly valued the quick resolution of clinical questions and problems, help with complex symptom management, support in decision-making about whether admission was required and the nurse’s ability to fast-track admission when needed:
To me she was like the missing link that could explain everything to everybody and certainly was a great support to [patient] and his family. (Social Worker 41)
[Study nurse]’s name appears in the notes on six different dates, referring to discussions had with her to do with symptom management or arranging admission. (GP 23)
Modifications to the intervention suggested by participants
The qualitative interviews generated several suggestions for improving the intervention in a future trial. Many patients and family carers would have appreciated the nurse support earlier in their illness journey and more frequent face-to-face meetings:
Interviewer: So would you have found having the sort of facility that the study nurses provided, having that earlier on, would that have been helpful or did it come at the right time?
Carer 46: No. I think even earlier on, because there was lots of questions, you don’t get told everything.
I think maybe in person would be a better option rather than a phone call, because […] if you were seeing that person face-to-face on a regular basis and you get to know that person then you can sort of, you tend to open up a wee bit more. (Carer 30)
Professional suggestions related to alternative ways of engaging with the nurse and additional responsibilities for the role and explicitly suggesting a shared-care model with primary care:
If they maybe had some clinical responsibilities then knowing these patients, going and seeing them, they should be able to say, ‘Right, you need to go into hospital’, and sort all that out and in a sense bypass us. So that might be an area where it might be able to be developed. (GP 42)
We’ve got the renal advice service and we just ping off an email […] and usually that is replied to within 24 hours, so we can get an answer about what to do with complicated patients, without necessarily being on hold to a registrar who doesn’t know them so well […]You don’t want delays to happen if patients really need to be in hospital, but email would be one option, some kind of direct advice line would be great. (GP 23)
Something summarising the anticipated prognosis […] that’s very helpful to allow us to have an initial pegging process to know where to pitch them in our order of priority. So people who are expected to do very well we would be less concerned about, but people who are going to have very frequent regular reviews, they will be brought to our attention much more readily if that is set out in a succinct summary from the liver clinic. That would be very helpful to know, how worried to be about them. (GP 36)
Impact of the intervention
The advice, support and continuity of care provided by the supportive care liver nurse intervention improved participants’ ability to cope:
[Study nurse] was very helpful, she put my mind at rest, she answered questions that I hadn’t thought about, and she gave me more confidence to be able to deal with what I’m doing. (Carer 31)
Some patients and family carers reduced their contacts with their GP, instead using the study nurse as first point of call for queries and concerns, with welcome consequences for busy community professionals:
It’s taking some workload off us, because [patient]’s not been in contact nearly as frequently as previously. (GP 26)
The study nurses successfully case-managed several patients who had otherwise disengaged from routine services. Their involvement also resulted in more timely, planned and person-centred hospital admissions:
[Study nurse] made [consultant] aware, the GI team aware, so we didn’t have to hang about A&E for a long time. They knew and came down almost immediately. Which is fine now, that’s kind of what happens, he’s normally seen pretty quickly. (Carer 08)
The nurses’ initial review meetings with patients and carers showed that patients generally had a poor understanding of their illness and its likely course and this hampered anticipatory care planning. However, the nurses’ correspondence prompted 21 GPs to create a new anticipatory care plan, while 8 updated a patient’s existing plan. The intervention thus increased the proportion of updated electronic summary care plans created in primary care and accessible to other staff in the community and hospitals from 29% before the intervention to 85% after it.
Discussion
Summary
The nurse-led intervention proved acceptable and feasible. Patients, families and professionals valued and benefitted from the support of a skilled nurse specialist and case manager. The nurse’s ability to help patients, carers and professionals to cope with the uncertainties inherent in managing advanced liver disease was appreciated.27 Improvements to people’s quality of life and care were demonstrated through the interviews and improvements in the quality-of-life questionnaire scores. Improvements in care coordination were evident from the study nurses’ engagement with professionals across a range of settings, their key role in facilitating more efficient hospital admissions and the informational and relational continuity of care the role afforded to patients, carers and professionals. Care planning became proactive and routine instead of opportunistic, with a marked improvement in the number and timely completion and updating of electronic summary care plans. These are of proven value for facilitating anticipatory care planning across settings in Scotland.28 Evidence from several participants indicated that the specialist nurse intervention has the potential to reduce unplanned hospital admissions. Other possible service use and economic benefits of the intervention are its potential to reduce outpatient clinic non-attendance, decrease primary care appointments and visits, and facilitate earlier discharge.
Comparison with the current literature
Most recent trials to integrate palliative care earlier in the illness trajectory of people with advanced diseases in general have involved the addition of specialist palliative care in parallel with continuing disease management.29–31 However, the WHO recommends a ‘palliative care approach’ that is delivered by the clinician or team already involved in the patient’s ongoing care.11 A recent systematic review has reported how this can be conducted by clinical nurse specialists, but found no such studies in liver disease.32 Baumann et al.15 have demonstrated that integrating early palliative care into the pre-transplant assessment process can be beneficial. This feasibility trial successfully developed and evaluated an innovative intervention that can be embedded in an existing gastroenterology/hepatology hospital service and then extended into the community through education and support. It is widely replicable and has the potential to improve quality of life for patients and families alongside optimising resource use. We have also tested this approach to integrated supportive and palliative care for people with heart disease.33
The liver nurses considered the palliative care training they received at the start of the study to be crucial in building their confidence in conducting sensitive conversations about deteriorating health and anticipatory care planning, and increasing their understanding of community care. However, our findings indicated that expertise in identifying and managing the complexities of caring for people with liver disease was more critical to this role than specialist palliative care experience. Following a period of targeted education, and with access to specialist palliative care advice where needed, the study nurses were able to tailor and apply palliative care principles in a complex patient group not routinely referred to palliative care services except close to death. The contribution of specialist nursing roles to the experience and outcomes of care has been recognised in relation to other non-malignant illnesses, cancer and palliative care.34,35 This study highlights the positive potential of the specialist nursing role in the care of people with advanced liver disease.
Limitations and strengths
The study was limited to a small number of white patients, largely with alcohol-related liver disease, attending one specialist centre in Scotland. The intervention lasted only 6 months and this limited our ability to fully test the validity of some of the questionnaires. Collection of community health and care service use data was more challenging than measuring hospital resource use, but new national datasets are likely to increase access to these outcome measures in future.
This is the first feasibility trial of integrating a palliative care approach within the role of a liver nurse specialist. We were able to identify and recruit patients who benefitted from early integrated supportive care, without them or the professionals involved being deterred using the term ‘palliative care’, which for many people is still associated with imminent death. A frequently cited trial providing early palliative care in oncology judged that a full RCT is feasible if at least 50% of recruited patients participated in an initial care review meeting and completed the study questionnaires.36 Using this criterion, our feasibility trial was successful; we achieved 64% participation, with no subsequent attrition except through death.
Evaluation of palliative care in an unpredictable and complex illness trajectory like liver disease is particularly challenging. Single index measures are unlikely to capture the breadth of important outcomes for patients, family carers, professionals and healthcare organisations.37,38 Palliative care interventions typically support multiple dimensions of need, and thus measures to assess these are indicated. It is also known in palliative care that access to a service may be appreciated, even if the service is not utilised. A wider range of measures used longitudinally over the course of the illness trajectory should be considered. We used a mixed-method evaluation which provided valuable, multi-faceted outcome data over time. In liver disease, the rapid onset of potentially life-threatening complications means that it is important not to focus too narrowly on place of death as a primary outcome.
Recommendations for a future RCT
We have presented a set of outcome measures and completion rates that can inform sample size calculations for a controlled trial. Future inclusion criteria should be broadened to include a first admission with complications of cirrhosis. Multiple sites should be considered to optimise recruitment and generalisability. The next trial should seek randomisation to the intervention against continuation of current best practice.
Late recruitment to the study and challenges with securing follow-up interviews due to patients’ fluctuating health indicate that a future trial operating a 6-month intervention could consider reducing interviews to two time points – baseline and 6 months.
The POS and the EuroQol-5D-5L are recommended as outcome measures. Supporting patients to complete these by phone, app, video call or in person should be considered to improve return rates. If carer questionnaires are to be used, such individual support may also be required. Alternatively, qualitative methods might be more appropriate to gather information on carer perspectives. Resource utilisation and health economic data and process measures such as the number of advance/anticipatory care plans or electronic summary care plans generated should also be collected. The choice of analytical strategy to compare functional outcomes in RCTs with high mortality where outcomes are ‘truncated due to death’ deserves special considerations.39 Each statistical approach relies on certain assumptions, and analysis must be mindful of the possible effects of the treatment on mortality.
The trial intervention might include a greater emphasis on educating and supporting primary care professionals to provide holistic care for people with advanced liver disease as they reported a lack of knowledge and confidence.
Conclusion
Further studies assessing palliative care interventions for people with end-stage liver disease are needed.41 This study provided valuable information to inform the design of a future RCT of a specialist supportive care nurse intervention to improve the care of people with advanced liver disease. It successfully identified recruitment processes, refined an acceptable intervention and tested the feasibility of collecting candidate outcome measures.
Acknowledgments
We are grateful to all patients and lay and professional carers who participated in this study, clinical colleagues who helped identify participants and the members of the PPI group. B.K., K.B., P.C.H., A.M., A.F. and S.A.M. conceived the study and contributed to its design. K.B. and P.B.Y. undertook the nurse training. H.B. and A.B. delivered the intervention. B.K. led the study, undertook all data collection and conducted analysis with A.F. R.E.O. advised on the choice of study instruments and C.J.W. on statistical tests and analysis. All authors were involved in the interpretation of the data and writing the final draft.
Footnotes
Availability of further data: Further information about all the instruments and interview schedules used, the training package developed for the nurses, more detailed findings of the instruments and resource use data may be available from the corresponding author.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval was obtained from South-East Scotland Research Ethics Committee 01 (reference 14/SS/1069). Governance approval was conferred by the local NHS Research & Development service on 6 November 2014.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Chief Scientist Office of Scotland (grant number CZH/4/1054). C.J.W. was also supported in this work by NHS Lothian via the Edinburgh Clinical Trials Unit. S.A.M. work was also supported by St. Columba’s Hospice, Edinburgh.
ORCID iD: Scott A Murray  https://orcid.org/0000-0002-3819-2912
https://orcid.org/0000-0002-3819-2912
References
- 1. Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014; 12: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National End of Life Care Intelligence Network. Deaths from liver disease: implications for end of life care in England, http://www.endoflifecare-intelligence.org.uk/resources/publications/deaths_from_liver_disease (2012, accessed 12 June 2017).
- 3. Volk ML, Tocco RS, Bazick J, et al. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kimbell B, Murray S. What is the patient experience in advanced liver disease? A scoping review of the literature. BMJ Support Palliat Care 2015; 5: 471–480. [DOI] [PubMed] [Google Scholar]
- 5. Roth K, Lynn J, Zhong Z, et al. Dying with end stage liver disease with cirrhosis: insights from SUPPORT. Study to understand prognoses and preferences for outcomes and risks of treatment. J Am Geriatr Soc 2000; 48: S122–S130. [PubMed] [Google Scholar]
- 6. Siouta N, Van Beek K, van der Eerden ME, et al. Integrated palliative care in Europe: a qualitative systematic literature review of empirically-tested models in cancer and chronic disease. BMC Palliat Care 2016; 15: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyd K, Kimbell B, Murray S, et al. Living and dying well with end-stage liver disease: time for palliative care? Hepatology 2012; 55: 1650–1651. [DOI] [PubMed] [Google Scholar]
- 8. Poonja Z, Brisebois A, van Zanten SV, et al. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014; 12: 692–698. [DOI] [PubMed] [Google Scholar]
- 9. Department of Health. End of life care strategy: promoting high quality care for all adults at the end of life, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/136431/End_of_life_strategy.pdf (2008, accessed 12 June 2017).
- 10. Scottish Government. Strategic framework for action on palliative and end-of-life care, http://www.gov.scot/Topics/Health/Quality-Improvement-Performance/peolc/SFA (2015, accessed 10 June 2017).
- 11. World Health Assembly. Strengthening of palliative care as a component of integrated treatment within the continuum of care, http://apps.who.int/gb/ebwha/pdf_files/EB134/B134_R7-en.pdf (2014, accessed 10 June 2017).
- 12. Williams MJ, Salmon C, Austin AS, et al. Services for liver disease in district general hospitals in the UK: a national questionnaire-based survey. Clin Med 2009; 9: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin Gastroenterol Hepatol 2013; 11: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaido T. Randomized controlled trials involving liver failure. Hepatogastroenterology 2008; 55: 1089–1092. [PubMed] [Google Scholar]
- 15. Baumann AJ, Wheeler DS, James M, et al. Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage 2015; 50: 882–886. [DOI] [PubMed] [Google Scholar]
- 16. Temel J, Greer J, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363: 733–742. [DOI] [PubMed] [Google Scholar]
- 17. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017; 6: CD011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Low J, Vickerstaff V, Davis S, et al. Palliative care for cirrhosis: a UK survey of health professionals’ perceptions, current practice and future needs. Frontline Gastroenterol 2016; 7: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higginson I, Evans C, Grande G, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013; 11: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010; 341: c4587. [DOI] [PubMed] [Google Scholar]
- 21. De J, Wand AP, Smerdely PI, et al. Validating the 4A’s test in screening for delirium in a culturally diverse geriatric inpatient population. Int J Geriatr Psychiatry 2016; 32: 1322–1329. [DOI] [PubMed] [Google Scholar]
- 22. Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative care core audit project advisory group. Qual Health Care 1999; 8: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gusi N, Olivares P, Rajendram R. The EQ-5D health-related quality of life questionnaire. In: Preedy VR, Watson RR. (eds) Handbook of disease burdens and quality of life measures. New York: Springer, 2010, pp. 87–99. [Google Scholar]
- 24. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the hospital anxiety and depression scale (HADS) in cancer and palliative settings: a meta-analysis. J Affect Disord 2010; 126: 335–348. [DOI] [PubMed] [Google Scholar]
- 25. Cohen SR, Mount BM, Strobel MG, et al. The McGill quality of life questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 1995; 9: 207–219. [DOI] [PubMed] [Google Scholar]
- 26. Weitzner MA, Jacobsen PB, Wagner H, Jr, et al. The caregiver quality of life index-cancer (CQOLC) scale: development and validation of an instrument to measure quality of life of the family caregiver of patients with cancer. Qual Life Res 1999; 8: 55–63. [DOI] [PubMed] [Google Scholar]
- 27. Kimbell B, Boyd K, Kendall M, et al. Managing uncertainty in advanced liver disease: a qualitative, multiperspective, serial interview study. BMJ Open 2015; 5: e009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McLoone P. Carstairs scores for Scottish postcode sectors from 2001 Census. Glasgow: University of Glasgow, 2004. [Google Scholar]
- 29. Zheng L, Finucane AM, Oxenham D, et al. How good is primary care at identifying patients who need palliative care? A mixed methods study. EJPC 2013; 20: 216–222. [Google Scholar]
- 30. Murray SA, Kendall M, Mitchell G, et al. Palliative care from diagnosis to death. BMJ 2017; 2017: 356. [DOI] [PubMed] [Google Scholar]
- 31. Tassinari D, Drudi F, Monterubbianesi MC, et al. Early palliative care in advanced oncologic and non-oncologic chronic diseases: a systematic review of literature. Rev Recent Clin Trials 2016; 11: 63–71. [DOI] [PubMed] [Google Scholar]
- 32. Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med 2014; 2: 979–987. [DOI] [PubMed] [Google Scholar]
- 33. Salamanca-Balen N, Seymour J, Caswell G, et al. The costs, resource use and cost-effectiveness of clinical nurse specialist–led interventions for patients with palliative care needs: a systematic review of international evidence. Palliat Med. Epub ahead of print 1 June 2017. doi: 10.1177/0269216317711570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Denvir MA, Cudmore S, Highet G, et al. Phase 2 randomised controlled trial and feasibility study of future care planning in patients with advanced heart disease. Sci Rep 2016; 6: 24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Begley CM. Evaluation of clinical nurse and midwife specialist and advanced nurse and midwife practitioner roles in Ireland (SCAPE). Dublin: National Council for the Professional Development of Nursing and Midwifery in Ireland, 2010. [Google Scholar]
- 36. National cancer patient experience survey, http://www.ncpes.co.uk/index.php/reports/national-reports/2489-cpes-2015-national-report-pdf/file (2015, accessed 12 June 2017).
- 37. Temel JS, Jackson VA, Billings JA, et al. Phase II study: integrated palliative care in newly diagnosed advanced non-small-cell lung cancer patients. J Clin Oncol 2007; 25: 2377–2382. [DOI] [PubMed] [Google Scholar]
- 38. Normand C. Measuring outcomes in palliative care: limitations of QALYs and the road to PalYs. J Pain Symptom Manage 2009; 38: 27–31. [DOI] [PubMed] [Google Scholar]
- 39. Normand C. Setting priorities in and for end-of-life care: challenges in the application of economic evaluation. Health Econ Policy Law 2012; 7: 431–439. [DOI] [PubMed] [Google Scholar]
- 40. Colantuoni E, Scharfstein DO, Wang C, et al. Statistical methods to compare functional outcomes in randomized controlled trials with high mortality. BMJ 2018; 2018: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhanji RA, Carey EJ, Watt KD. Review article: maximising quality of life while aspiring for quantity of life in end-stage liver disease. Aliment Pharmacol Ther 2017; 46: 16–25. [DOI] [PubMed] [Google Scholar]