Abstract
Glucocorticoids have been widely used and exert pleiotropic effects on alveolar structure and function, but do not improve the long-term clinical outcomes for patients with bronchopulmonary dysplasia, emphysema, or interstitial lung diseases. Treatments that foster alveolar regeneration could substantially improve the long-term outcomes for such patients. One approach to alveolar regeneration is to stimulate and guide intrinsic alveolar progenitors along developmental pathways used during secondary septation. Other investigators and we have identified platelet-derived growth factor receptor-α–expressing fibroblast subpopulations that are alternatively skewed toward myofibroblast or lipofibroblast phenotypes. In this study, we administered either the glucocorticoid receptor agonist dexamethasone (Dex) or the antagonist mifepristone to mice during the first postnatal week and evaluated their effects on cellular proliferation and adoption of α-smooth muscle actin and lipid droplets (markers of the myofibroblast and lipofibroblast phenotypes, respectively). We observed that Dex increased the relative abundance of fibroblasts with progenitor characteristics, i.e., containing both α-smooth muscle actin and lipid droplets, uncoupling protein-1 (a marker of brown and beige adipocytes), delta-like ligand-1, and stem cell antigen-1. Dex enhanced signaling through the Smad1/5 pathway, which increased uncoupling protein-1 in a lung fibroblast progenitor cell line. We conclude that glucocorticoid receptor manipulation can sustain fibroblast plasticity, and posit that targeting downstream glucocorticoid responsive pathways could steer fibroblast progenitors along more desirable regenerative pathways.
Keywords: progenitor cells, pulmonary alveolar development, platelet-derived growth factor receptor-α, myofibroblast, lipofibroblast
Clinical Relevance
Current treatments for bronchopulmonary dysplasia, emphysema, and interstitial fibrosis do not restore damaged alveoli. This study shows how glucocorticoids regulate fibroblast progenitors, which contribute to alveolar septal formation during postnatal development. The findings identify glucocorticoid-responsive pathways that could be used to stimulate alveolar regeneration.
The unique contributions of individual pulmonary alveolar fibroblast subpopulations to air-space development and regeneration remain incompletely understood. Studies of hyperoxia-induced bronchopulmonary dysplasia in neonatal rats (1) or fibrotic injury in adult mice (2) have shown that structural abnormalities are ameliorated by steering fibroblasts toward a lipid-storage phenotype. Conversely, adoption of myofibroblast (MF) characteristics reduces the lipid-storage subpopulation (referred to as lipofibroblasts [LIFs]) (3). Recent studies using lineage tracing indicate that the fate of LIF progenitors is defined later than that of MFs, which arise from a prenatally defined population of sonic-hedgehog–responsive, Gli1-expressing progenitors (3). MF differentiation is dependent on transforming growth factor-β (TGF-β) signaling through TGF-β receptor 1 (Alk5), and disruption of Alk5 results in expansion of the LIF subpopulation (4). Manipulating the differentiation of one subpopulation influences that of the other, but it remains unclear whether this only occurs before birth or LIFs and MFs maintain postnatal plasticity. This is an important consideration when developing regenerative strategies for the treatment of destructive alveolar diseases such as bronchopulmonary dysplasia, emphysema, and interstitial fibrosis (5).
Mesenchymal progenitor cells with the potential to differentiate into adipocytes or another specialized cell (myocyte, oligodendrocyte, or adipocyte) express platelet-derived growth factor receptor-α (PDGFRα), which influences progenitor proliferation and differentiation (6–8). Furthermore, PDGFRα-expressing adipocyte precursors can assume characteristics of brown adipocytes and participate in the development of brown fat within deposits of white fat (beige fat) induced by cold exposure or β3-adrenergic stimulation (9). Using mice of different perinatal ages, investigators observed that PDGFRα is expressed in both LIFs (which stain for adipocyte-related differentiation protein [ADRP, also known as perilipin 2] and contain lipid droplets) and MFs (a larger proportion contain α-smooth muscle actin [α-SMA], and few contain ADRP) (10–12). Studies using flow cytometry to classify fibroblasts isolated from mice bearing green fluorescent protein (GFP) driven by the endogenous PDGFRα promoter (PDGFRα-GFP) identified fibroblasts with two levels of GFP intensity, which during alveolar development correlated with the abundance of PDGFRα molecules on the cell surface (13). During Postnatal Day 4 (P4) through P14, PDGFRα-GFPlow cells predominantly reside at the base of elongating secondary septa and store neutral lipids (14). PDGFR-GFPhigh fibroblasts congregate at the alveolar entry ring (secondary septal tip) and exhibit more α-SMA. Flow cytometry has also been used to sort lung fibroblasts from adult mice based on their intensity of GFP fluorescence. In adult mice, PDGFRα-GFPhigh cells demonstrated a gene expression profile for abundant production of extracellular structural proteins, whereas GFPlow lung fibroblasts showed higher expression of cell surface proteins involved in migration and cytoskeletal rearrangement (15). It remains unclear whether these differences in ontogeny and gene expression profiles result from divergent terminal differentiation along predefined pathways, adaptation to their current environment, or both.
To address this issue, we manipulated glucocorticoid signaling in mice and examined the effects on LIF and MF phenotypes. Glucocorticoids are used clinically but have shown limited efficacy in treating bronchopulmonary dysplasia and pulmonary fibrosis. A better understanding of the effects of glucocorticoids on fibroblast subpopulations may enable us to identify signaling pathways that could be specifically targeted to circumvent some of the undesirable effects of long-term corticosteroid administration.
Both augmentation and suppression of signaling have been used to define the effects of glucocorticoids on alveolar septation. However, the findings are enigmatic because they vary with the timing of the stimuli, the cell populations that were targeted, and which cellular properties were studied. The glucocorticoid agonist dexamethasone (Dex) delays capillary maturation and produces transient alveolar wall thinning, but enhances the expression of elastin (16–18). Targeted deletion of the glucocorticoid receptor (GR) in mesenchymal cells was shown to increase the volume density (VD) and proliferation of the airspace mesenchyme, diminish elastic fiber formation and epithelial maturation, and halt progression beyond the saccular stage, such that few mice survived after P1 (19). We hypothesized that the differential effects of glucocorticoids on the LIF and MF fibroblast subpopulations could explain why GR deletion increases alveolar mesenchymal cells but diminishes elastin, a major product of these cells.
Materials and Methods
A complete list of the materials used in this work and a more detailed explanation of the methods employed are provided in the online supplement.
Mice
Mice bearing the PDGFRα-GFP construct have been described previously (10). Production and nuclear localization of GFP are under the control of the endogenous pdgfrα promoter, and spatially and temporally recapitulate endogenous pdgfr-α expression (10). The mice were heterozygous for the pdgfrα-GFP allele and were phenotypically identical to wild-type (GFP−) mice. Members within 4 separate litters of mice were allocated to receive Dex or phosphate-buffered saline (in one litter, fibroblasts were isolated separately from two Dex-treated mice, yielding a total of five). In the Dex-treated mice, Dex was delivered subcutaneously at 100 ng/g body weight on P1 and at 50 ng/g on subsequent days. Mifepristone (Mfp) was dissolved in propylene glycol and 50 μg/g body weight was delivered subcutaneously to male mice from five separate litters, using other littermates as controls. To analyze proliferation, the pups were treated with 100 ng/g body weight of 5-ethynyl-2′deoxyuridine (EdU) 3 h before they were killed and lung fibroblasts were isolated (13). The protocols for animal use were approved by the Iowa City Veterans Affairs Medical Center Animal Use Committee (10).
Lung Inflation and Fixation
Lungs from mice bearing the PDGFRα-GFP insert at P8 and P12 were uniformly inflated (50 μl of fixative per gram body weight), fixed, and sectioned, and the Ki67antigen was analyzed by laser scanning confocal microscopy and enumerated using the optical fractionator stereological probe (14). Tissues used to analyze the gas-exchange surface area and alveolar wall thickness were embedded in London Resin (LR) White. They were analyzed using the Cycloids for Sv stereological probe to determine the gas-exchange surface area, and the Mertz probe to determine the arithmetic mean barrier thickness.
Isolation of Primary Mouse Fibroblasts and Flow Cytometry
Lung fibroblasts were isolated on P8 using a previously reported method involving digestion with collagenase and adherence to tissue-culture plastic for 1 h (10). Epithelial and endothelial cells comprised ∼ 2.5% and 1.6%, respectively, whereas macrophages were only detected in the PDGFRα-GFP− population (10). Fibroblasts stained for ADRP, α-SMA, phosphorylated Smad1/5 (pSmad1/5), or uncoupling protein-1 (UCP1) were permeabilized before immunostaining (10). When staining for LipidTOX red (LTR) and stem cell antigen-1 (Sca1), the cells were not permeabilized. CD45+ cells, which had adhered to the culture plastic after 1 h, were excluded from analysis. Virtually all of the PDGFRα-expressing fibroblasts were in the CD45− fraction. Forward scatter and side scatter were used to exclude small (presumably apoptotic) cells and aggregates. The background fluorescence from the corresponding immunoglobulin G isotype controls was subtracted to quantify the different fibroblast populations.
Isolation of Mitochondria for UCP1 Immunoblotting
Neonatal mouse lung fibroblasts (MLg) 2908 cells were subjected to adipogenic induction, and after a 16 h period without Dex, they were treated with 5 ng/ml TGF-β or 100 nM Dex, or remained untreated, and the mitochondria were isolated (20). After incubation with 0.5% n-octyl-B-D-glucopyranoside to solubilize UCP1, the samples were subjected to SDS-PAGE and immunoblotting. MLg 2908 cells were induced and similarly treated with TGF-β1 or Dex before immunoblotting for pSmad2, Smad2 pSmad1/5, Smad1/5, and β-tubulin.
Results
Glucocorticoid Signaling Regulates Alveolar Wall Thickness and Surface Area
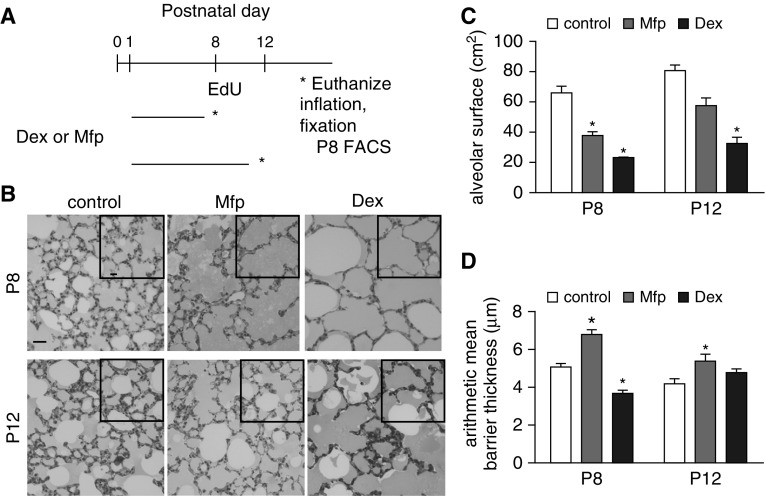
Dex, a potent GR agonist, or Mfp, a GR as well as a progesterone receptor antagonist, was administered during P1–P7 or P1–P11 and the mice were killed on P8 or P12 (Figure 1A). Images of lungs that had been uniformly inflated with agarose during fixation are shown in the figure. Residual agarose accounts for the gray background in the air spaces. Casual inspection showed that Dex increased the VD of the airspace at P8 and P12, and that the GR antagonist Mfp had the same effect at P8 (Figure 1B). Stereological analysis confirmed that the alveolar surface was smaller in both Dex- and Mfp-treated mice at P8 (Figure 1C). Mfp increased the mean barrier (alveolar wall) thickness at both ages, whereas wall thinning was only observed at P8 in Dex-treated mice (Figure 1D). Others have observed that pharmacological manipulation of the GR transiently alters the surface area, indicating that critical effects are manifest by P8, after which the GFPlow LIFs diminish (10, 16). The observation that both Dex and Mfp reduced the alveolar surface area indicates that septal outgrowth requires properly balanced glucocorticoid signaling, and that both excessive Dex and insufficient Mfp are detrimental.
Figure 1.
Perturbing glucocorticoid signaling disrupts alveolar structure. Dexamethasone (Dex) or mifepristone (Mfp) was administered, whereas controls were untreated, during the postnatal days shown in (A) (horizontal lines) and mice were killed (*) at Postnatal Day 8 (P8) or P12. (B) Lungs were uniformly inflated with low-melting-point agarose, fixed, embedded in London Resin White, sectioned, stained, and imaged (scale bars are 50 µm). Insets are lower magnification to show larger fields of view. (C) Combined alveolar surface (including both alveoli and alveolar ducts) for both lungs. Mean ± SEM, n = 4 mice per treatment. (D) Alveolar wall (arithmetic mean barrier) thickness was altered by both Mfp and Dex at P8, but only by Mfp at P12. Mean ± SEM, n = 4 mice for each treatment group, *P < 0.05 for Dex- or Mfp-treated mice compared with controls at the respective ages. EdU, ethinyl deoxyuridine; FACS, fluorescence-activated cell sorter.
Increased Proliferation Contributes to Increased Alveolar Wall Thickness in Mfp-Treated Mice
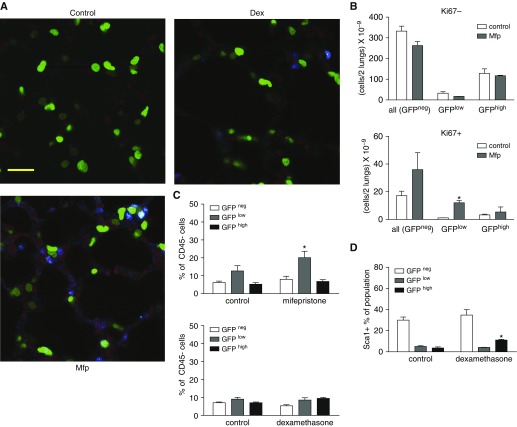
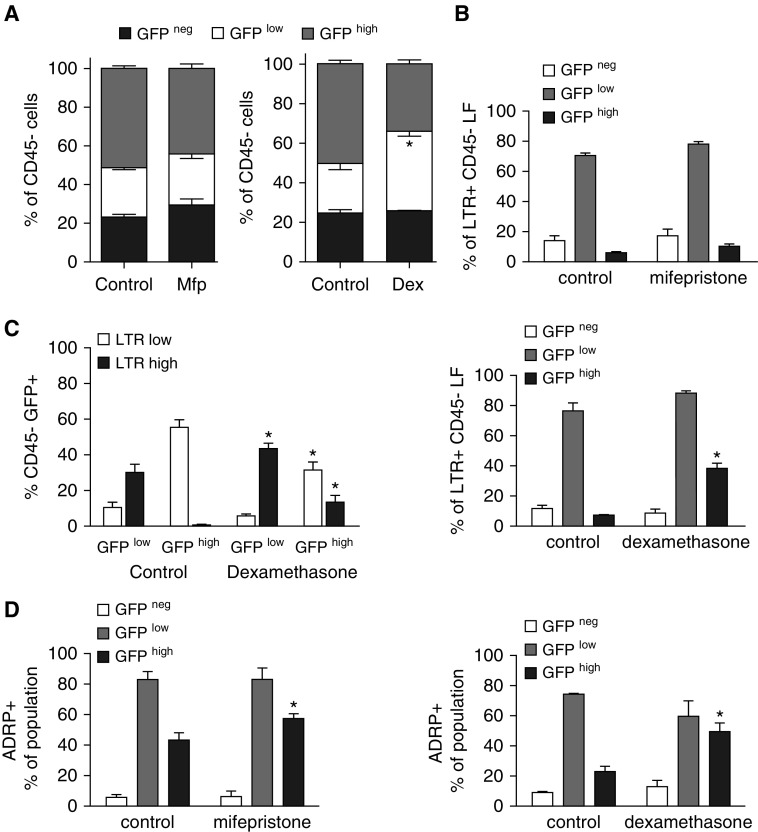
The increase in alveolar wall thickness after Mfp treatment could represent enhanced mesenchymal cell proliferation, as others have observed in GR-deleted mice (19). We examined proliferation in situ using the marker Ki67 or by pulse labeling with EdU before isolating fibroblasts at P8. Although the volume-corrected number of nonproliferating cells (GFP− cells included resident cells in the alveolar wall that were not fibroblasts) was not altered, we observed an increase in Ki67+ GFPlow, but not GFPhigh, fibroblasts after exposure to Mfp (Figures 2A and 2B). When we analyzed isolated fibroblasts using fluorescence-activated cell sorting, treatment with Mfp increased the proportion of EdU+ GFPlow fibroblasts at P8 (Figure 2C). Our prior studies showed that proliferation is sustained in PDGFRα-GFP fibroblasts, which bear the progenitor marker Sca1, and Dex increases the proportion of Sca1+ LFs within the GFPhigh population (Figure 2D) (10). However, Dex reduced the size of the entire GFPhigh population when expressed as a percentage of all CD45− fibroblasts (Figure 3A). These observations suggest that Dex retains GFPhigh fibroblasts as progenitors.
Figure 2.
Glucocorticoids differentially alter proliferation in PDGFRα-GFP–expressing subpopulations. Lung fibroblast proliferation was assessed by the abundance of Ki67 antigen or by flow cytometry at P8, using mice that were treated as shown in Fig. 1A. (A) Representative confocal images of lungs stained for Ki67 (blue) or the nuclear counterstain PoPo3 (red). PDGFRα-expressing cells exhibited GFP fluorescence (green, scale bar: 20 µm). (B) Ki67+ nuclei were enumerated within the GFPhigh and GFPlow populations in control or Mfp-treated mice on P8. GFP− includes alveolar cells other than fibroblasts, whereas only fibroblasts contained GFP. Mean ± SEM, n = 4 mice for each treatment group. *P < 0.05 for Mfp- or Dex-treated mice compared with controls. (C) EdU was administered to untreated (control) or Dex- or Mfp-treated mice on P8. Fibroblasts were isolated and subjected to flow cytometry, gating out CD45+ cells (macrophages or fibrocytes), so that the only GFP− cells analyzed were fibroblasts. Mean ± SEM of the EdU+ fibroblasts as percent of CD45− cells, n = 5 sets of 1 or 2 mice from 4 separate litters for each glucocorticoid treatment group. *P < 0.05 for Mfp-treated mice compared with controls. (D) Percent Sca1+, CD45− within each population stratified by GFP intensity. Mean ± SEM, n = 4 control and Dex-treated littermates derived from 4 separate litters. *P < 0.05 for Dex-treated mice compared with controls. GFP, green fluorescent protein; PDGFRα, PDGFR, platelet-derived growth factor receptor-α.
Figure 3.
Perturbing glucocorticoid signaling alters lipid storage in the GFPhigh population. (A) Flow cytometry showed that Dex, but not Mfp, altered the relative abundance of the three lung fibroblast (LF) populations defined by PDGFRα-GFP intensity: GFP−, GFPlow, and GFPhigh. Mean ± SEM, n = 5 control and treated littermates from 4 litters. *P < 0.05, size of GFPlow or GFPhigh component compared with control. *P < 0.05 for GFPhigh, comparing Dex-treated mice with controls. (B) Effects of Mfp and Dex on the proportions of neutral lipid–containing fibroblasts (stained with LipidTox red [LTR]) within each subpopulation stratified by intensity of GFP-fluorescence. Mean ± SEM, n = 5 mice, for each treatment, from 4 separate litters. *P < 0.05 comparing Mfp-treated mice with controls. (C) Using the cells shown in B, the LTR+ CD45− fibroblasts were gated into two populations based on the intensity of LTR fluorescence. The distribution of GFPlow and GFPhigh cells in LTRlow and LTRhigh populations is shown. Mean ± SEM, n = 5, same mice as in B. (D) Analysis similar to that shown in B, except that lipid droplets were identified by perilipin-2 (ADRP). Mean ± SEM, n = 5 mice, for each treatment, from 4 separate litters. *P < 0.05 comparing Mfp- or Dex-treated mice with littermate controls.
PDGFRα GFPhigh Fibroblasts Exhibit LIF Characteristics after Treatment with Mfp or Dex
PDGFRα+ mesenchymal progenitors retain a less differentiated, progenitor state in other organs; for example, both preadipocytes and myoblasts can assume either a lipid-storage or muscle-like phenotype (21). Although Mfp is a GR antagonist, its functional effects are not always converse to those of Dex (22). Our readouts were sensitive to the size (only LTR) and the abundance (both LTR and ADRP) of lipid droplets. These readouts reflect not only the immediate effects on GR but also differentiation (lipid droplets were observed both in GFPhigh progenitors and in mature GFPlow fibroblasts), the balance between fibroblast lipogenesis and lipolysis, and the response to hormones such as insulin (22). These factors are addressed in more detail in the Discussion.
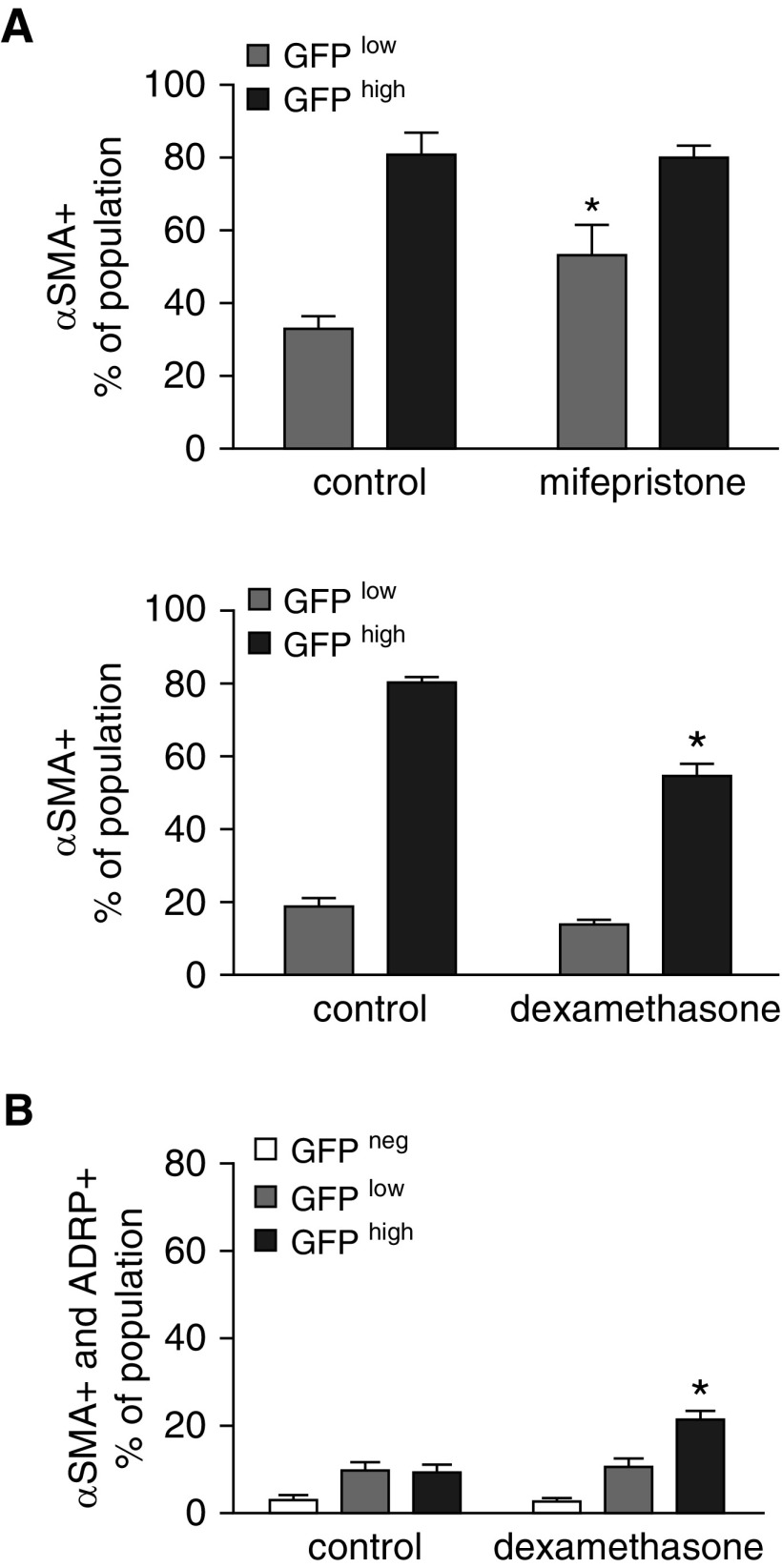
Mfp increased lipid storage in the GFPhigh population (Figures 3B and 3D). Mfp modestly increased the proportion of LTR+ GFPhigh fibroblasts (P = 0.045, a small but statistically significant difference). Gating on LTR fluorescence intensity, Dex increased the per-cell abundance of lipids in both the GFPlow and GFPhigh populations (Figure 3C). Mfp increased the proportion of GFPlow fibroblasts, whereas Dex decreased the proportion of GFPhigh fibroblasts that contained α-SMA (Figure 4A). This indicates that glucocorticoid signaling influences the relative abundance of lipid droplets and α-SMA in PDGFRα-expressing fibroblasts. Whereas Mfp did not alter the proportions of ADRP and α-SMA double-positive cells compared with controls (data not shown), Dex increased the proportion of GFPhigh fibroblasts that contained both ADRP and α-SMA (Figure 4B). This is consistent with GFPhigh fibroblasts retaining their bipotent progenitor status and delaying commitment to differentiated MFs.
Figure 4.
Perturbing glucocorticoid signaling alters the proportions of α-smooth muscle actin (α-SMA)-containing fibroblasts. Fibroblasts were isolated from mice treated with Mfp or Dex during P1–P7, or untreated littermate controls (see Fig. 1), and subjected to flow cytometry. (A) The proportions of CD45− GFPlow and GFPhigh fibroblasts that contained α-SMA are shown. (B) A minority of fibroblasts contained both α-SMA and ADRP, and Dex increased the proportion of double-positive cells in the GFPhigh population. Mean ± SEM, n = 5 treated mice from 4 litters each for Mfp and Dex with 5 littermate controls, which differed for Mfp and Dex exposures. *P < 0.05 comparing Dex- or Mfp-treated mice with the respective control populations stratified by GFP fluorescence intensity.
PDGFRα-Expressing Fibroblasts Exhibit Characteristics of Brown Adipocytes
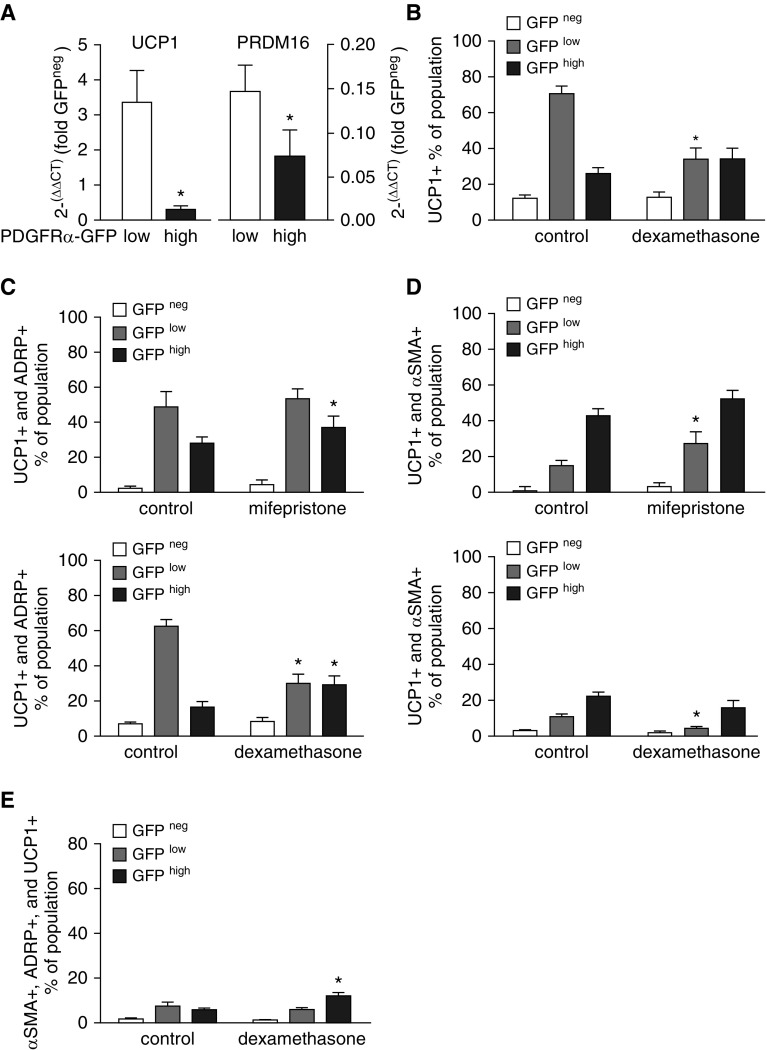
Others have shown that C3H10T1/2 mesenchymal progenitor cells alternatively differentiate to smooth-muscle– and brown-adipocyte–like phenotypes (23). The observation of a similar relationship in PDGFRα+ alveolar fibroblasts (10) prompted us to examine their similarity to brown adipocytes using the marker UCP1. In controls without manipulation of the GR, UCP1 gene expression was higher in the GFPlow population, which also exhibited higher expression of PRDM16 (Figure 5A), an important regulator of preadipocyte differentiation toward brown adipocytes. Flow-cytometric analysis showed that Mfp did not alter the proportions of UCP1+ cells within the three populations (data not shown), whereas Dex decreased the proportion of UCP1+ fibroblasts in the GFPlow GFPhigh population (Figure 5B). Both UCP1 mRNA (Figure 5A) and protein (Figure 5B) were highest in the GFPlow population of control fibroblasts. Both Mfp and Dex increased the proportion of ADRP, UCP1 double-positive cells in the GFPhigh population (Figure 5C), which resulted in part from the higher prevalence of ADRP+ cells in the GFPhigh population (Figure 3D). Dex diminished the proportion of ADRP, UCP1 double-positive cells in the GFPlow population (Figure 5C). We also observed that Mfp increased the proportion of GFPlow fibroblasts that contained both α-SMA+ and UCP1+ (Figure 5D) and increased the proportion of GFPhigh fibroblasts that contained α-SMA, ADRP, and UCP1 (Figure 5E). Therefore, as with beige adipocytes (24, 25), manipulation of glucocorticoids lessened the distinction between ADRP+ GFPlow and α-SMA+ GFPhigh fibroblasts, and conferred a more adipocytic phenotype to PDGFRα-expressing fibroblasts, which also contain α-SMA.
Figure 5.
GFPhigh fibroblasts that acquire more lipid droplets exhibit characteristics of brown adipocytes. (A) Fibroblasts were isolated from PDGFRα-GFP mice (control, not treated) and subjected to flow-cytometric sorting, gating on CD45− cells, and their GFP fluorescence intensity. Using quantitative RT-PCR, uncoupling protein-1 (UCP1, a marker of brown adipocytes) and PR domain containing 16 (Prdm16), mRNA from the GFPlow and GFPhigh populations was normalized to mRNA from the CD45−, GFP− population from the same fibroblast isolation. Mean ± SEM, n = 5 mice, all from separate litters. *P < 0.05 comparing GFPlow and GFPhigh. (B) Fibroblasts were isolated from mice treated with Dex during P1–P7 and subjected to flow cytometry after staining for UCP1, ADRP, and α-SMA. Mean ± SEM, n = 6 mice for each treatment group from 4 separate litters. *P < 0.05 for GFPhigh, comparing Dex-treated mice with untreated controls. (C) Dex and Mfp altered the proportions of fibroblasts that stained positively for ADRP as well as UCP1. Mean ± SEM, n = 5 mice for each treatment group from 4 separate litters. *P < 0.05 for GFPlow or GFPhigh, comparing Dex- or Mfp-treated mice with controls. (D) Dex and Mfp altered the proportions of fibroblasts that stained positively for both α-SMA and UCP1. Mean ± SEM, n = 5. Control and Mfp- or Dex-treated mice are the same as those shown in C. (E) Proportions of cells that stained positively for α-SMA, ADRP, and UCP1 within the GFP−, GFPlow, and GFPhigh populations in fibroblasts isolated from control and Dex-treated mice. Mean ± SEM, n = 5 using the same mice shown in D.
Dex Stimulates Phosphorylation of Smad1/5 in the Context of Increased UCP1
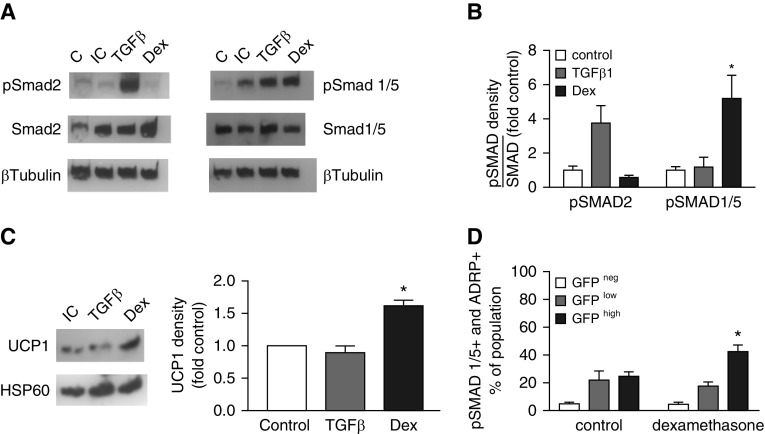
Dex augments Smad1/5 phosphorylation in NIH3T3 cells and adult human primary lung fibroblasts (26). Others have shown that that the TGF-β homolog BMP7 enhances phosphorylation of pSmad1/5, inducing C3H10T1/2 cells to acquire characteristics of brown adipocytes, including increased UCP1 (23). Therefore, we examined whether Dex augments Smad1/5 phosphorylation and induces UCP1 in the progenitor-like (bipotent differentiation to MF or adipocyte characteristics) MLg 2908 lung fibroblast cell line. Dex increased Smad1/5 phosphorylation (Figures 6A and 6B) and UCP1 protein in isolated mitochondria (Figure 6C). To determine whether Dex alters Smad1/5 signaling in vivo, we administered Dex to mice during P1–P8, and using immunostaining and flow cytometry, we observed that the proportion of pSmad1/5+ GFPhigh fibroblasts (73.6 ± 3.9, mean ± SEM, n = 5) was significantly higher than in controls (63.8 ± 3.0, P < 0.05). Gating on the CD45− fibroblasts, which were also ADRP+, demonstrated a more substantial effect of Dex on the proportion of GFPhigh fibroblasts containing pSmad1/5 (Figure 6D). Therefore, as in preadipocytes, Smad1/5 signaling was accompanied by a shift of differentiation toward the LIF phenotype.
Figure 6.
Dex increases UCP1 in cultured MLg 2908 cells and pSMAD1/5 both in culture and in vivo. (A) MLg 2908 neonatal mouse lung fibroblasts were induced to assume a lipid-storage phenotype and then exposed to medium alone (control) or supplemented with either transforming growth factor β 1 (TGF-β1) or Dex and then harvested for Western immunoblotting. The blots were probed for either pSmad2 and Smad2 or pSmad1/5 and Smad1/5, followed by β-tubulin. C, uninduced control; IC, induced control. (B) The density of pSmads in immunoblots is expressed relative to the abundance of the corresponding Smad for each sample and normalized to the ratio for the control. Mean ± SEM, n = 4 separate experiments, *P < 0.05 comparing Smad1/5 for Dex-treated MLg with controls. (C) MLg cells were induced and stimulated as in A before mitochondrial isolation and Western immunoblotting. The density of UCP1 relative to the respective induced control (set to a density of one) is shown for three separate experiments. Mean ± SEM, *P < 0.05 comparing Dex-treated mice with controls. (D) On P8, fibroblasts were isolated from a separate cohort of controls or mice that had been treated with Dex as shown in Fig. 1A. (A) After staining for pSmad1/5 and ADRP, flow cytometry was performed, gating on CD45− cells. Mean ± SEM, n = 5 mice for each treatment group from four different litters. *P < 0.05 comparing the GFPhigh population from Dex-treated mice with controls. HSP60, heat shock protein 60; pSMAD, phosphorylated form of a homolog of the Drosophila family of proteins including SMA (small body size) and mothers against decapentaplegia (MAD).
Dex Retains Lung Fibroblast Progenitors
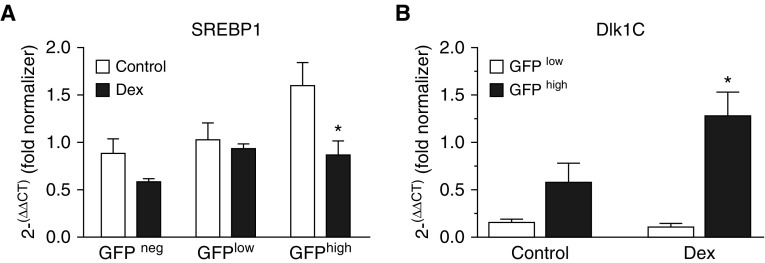
Dex increased the proportion of GFPhigh LF that contained both ADRP and α-SMA (Figure 4B), and a larger proportion were Sca1+ (Figure 2D), suggesting that GFPhigh cells retained progenitor characteristics with markers of both MF and LIF. We posited that Dex may restrain the divergence into LIF and MF phenotypes by holding more fibroblasts in a bipotent state. Therefore, we examined the expression of sterol receptor binding protein-1 (SREBP1), which indicates a transition to an adipocyte-like phenotype, and delta-like ligand-1, splice product-C (Dlk1C), a progenitor marker that is regulated by PDGFRα during mouse alveolar development (10, 27). Treatment with Dex increased Dlk1C and decreased SRBP1 mRNA relative to untreated controls, consistent with retention of progenitor characteristics (Figure 7).
Figure 7.
Dex increases the retention of fibroblasts as progenitors. Fibroblasts were isolated from control and Dex-treated PDGFRα-GFP mice and subjected to flow-cytometric sorting, gating on CD45− cells and their GFP fluorescence intensity. Using quantitative RT-PCR, (A) sterol receptor binding protein-1 (SREBP1) and (B) delta-like ligand-1, splice product-C (Dlk1C) mRNA was analyzed and the cycle threshold (CT) values were normalized to the CT value for a single normalizing RNA sample, which was used in all analyses. n = 5 separate cell isolations from control and Dex-treated mice, which were obtained from different litters. Mean ± SEM, *P < 0.05 comparing Dex-treated GFPhigh mice with controls.
Our findings illustrate how glucocorticoids influence the balance between LIFs and MFs, and sustain progenitors, which share characteristics of both phenotypes. Lung fibroblasts contain the beige adipocyte progenitor marker UCP1, which is augmented by Dex in the PDGFRα-GFPhigh population. They also illustrate how the effects of glucocorticoids depend on the differentiation status, and that timing and context are essential for targeting their effects on alveolar development or regeneration.
Discussion
Glucocorticoids Complexly Regulate Lipid-Droplet Metabolism
We used two different markers to identify lipid-storage cells by flow cytometry. LTR accumulates inside lipid droplets, where it binds to triglycerides and cholesterol esters. ADRP (perilipin 2) is located on the outer phospholipid coating rather than in the interior of lipid droplets. Therefore, the LTR intensity depends on the droplet volume, whereas the anti-ADRP fluorescence intensity depends on the droplet surface area. Like other cells, LIFs contain multiple droplets of various sizes (28). The size of the lipid droplets is controlled by the balance between lipid esterification and lipolysis, and therefore is sensitive to agents such as Mfp and Dex, which modulate lipolysis (28). We observed that both Dex and Mfp increased the proportions of ADRP+ (Figure 3D) and UCP1+, ADRP+ double-positive fibroblasts (Figure 5C) in the GFPhigh population. Dex increased the intensity of LTR fluorescence per cell (Figure 3C), which is consistent with an increased aggregate volume of intracellular lipid droplets. Dex also increased the proportion of LTR+ cells in the GFPhigh population (Figure 3B). Mfp increased the proportion of ADRP+ fibroblasts (dependent on increased surface area and not droplet volume) in the GFPhigh population (Figure 3B). Mfp decreased the lipid droplet size (with a corresponding increase in the surface/volume ratio) in adipocytes of mice receiving a high-fat diet (29, 30). If Mfp similarly affects fibroblast lipid droplets, one would expect an increased proportion of ADRP+, but not LTR+, fibroblasts, which is what we observed. Further complexity arises from differential effects of Dex on preadipocytes versus adipocytes. Dex increased proliferation, accelerated differentiation, and increased the UCP1 gene expression of brown preadipocytes (31). However, Dex antagonized the cyclic adenosine monophosphate (cAMP)-mediated stimulation of oxygen consumption and UCP1 expression in differentiated brown adipocytes (32), and increased lipolysis in mature white adipocytes (33). Although Dex and Mfp exert opposing effects on the GR, this does not consistently translate into obverse findings at the cellular and organ levels.
Glucocorticoids Have Time-Sensitive and Context-Dependent Effects on Alveolar Septation
The effects of glucocorticoids on alveolar development are influenced by the age at which they are administered. Massaro and coworkers (34) showed that administering 0.1 μg of Dex (which reduced body weight by 10%) to rats between P4 and P13 diminished the alveolar surface area at P14, and it remained lower than in saline-treated controls at P60. The alveolar wall interstitial thickness, the VD of lipid-laden and non–lipid-laden interstitial cells, and the VD of fibroblast lipid droplets were all reduced at P14 (18). Tschanz and colleagues (35), and later Roth-Kleiner and colleagues (17), showed that administering Dex from P1 through P4 produced septal thinning and diminished alveolar capillaries, both of which normalized after P13. This abbreviated administration of Dex only transiently interrupted the proliferation and apoptosis of septal cells at P4 and diminished elastin and tenascin-C at P6 (36). These studies identified a critical time during which Dex reversibly interrupts mesenchymal cell functions, and coincides with the postnatal period in which LIFs maximally proliferate and accumulate neutral lipids (14, 37). The study presented here explains some of these time-sensitive effects of glucocorticoids during secondary septation. First, proliferation of the GFPlow population was lower in control than in Mfp-exposed fibroblasts at P8 (Figure 2B). Therefore, the GFPlow population is particularly susceptible to manipulation of GR signaling before P5. Second, although GFPhigh fibroblasts diminished as a proportion of CD45− cells (Figure 3A), the GFPhigh, ADRP+, α-SMA+, and UCP1+ progenitor populations increased (Figure 5E). Retained progenitors may contribute to the “catch-up” septal formation that is observed when Dex is withdrawn before P5 (17, 35). Ntokou and coworkers (12) reported that only fibroblasts that had been lineage marked on or before P2 were observed in both lipid-laden and soma-expressing populations at P7. This suggests that by preserving PDGFRα-expressing progenitors, transient (P1–P4) glucocorticoid administration may enable alveolarization to recover.
Significance of UCP1 in Lung Fibroblast Progenitors
UCP1-expressing adipocytes arise from at least two progenitor populations. Classical brown adipocytes are defined embryonically and arise along myotomes from Pax7+, Myf5+ precursors, which may also differentiate into myocytes (38). Beige (sometimes termed brite) adipocytes arise within white adipose tissue from progenitors with characteristics of smooth muscle cells (i.e., they are PDGFRα+ and α-SMA+, and some are Myh11+). Whereas UCP1 is constitutively expressed by brown adipocytes, expression by beige adipocytes requires induction from cold exposure or β3-adrenergic stimulation (25). Adrenergic induction of UCP1 in beige adipocytes follows expansion of the adipose stromal vascular cell population and is dependent on vascular endothelial growth factor (VEGF)-A and vascular endothelial growth factor receptor-2 (VEGF receptor-2 or VEGFR2) (39). Relevant to our findings is the novel observation of Seki and associates (40) that the adipose stromal vascular endothelium produces PDGF-CC (a PDGFRα ligand), which stimulates UCP1 gene expression in PDGFRα+, CD34+, and Sca1+ perivascular mesenchymal cells. We previously found that some PDGFRα+ lung fibroblasts express CD34 and Sca1 at P8 (10), and have now shown that Dex expands the Sca1+, GFPhigh subpopulation and increases the proportion of these cells that express UCP1. Likewise, we previously showed that targeted pdgfrα deletion increased Dlk1C mRNA (10), and have now observed that Dex increases Dlk1C, consistent with their progenitor state. Therefore, like beige adipocyte precursors, PDGFRα-expressing lung fibroblast progenitors retain characteristics of both smooth-muscle and lipid-storage cells (41). Precisely what drives PDGFRα-expressing fibroblasts to express UCP1 remains unclear, because they are in a warm environment and β3-adrenergic receptors have not been observed in lung fibroblasts (42). UCP1 more likely marks progenitor cells, which have not fully committed to either an MF or a lipid-storage phenotype.
Contributions of Fibroblast Plasticity to Alveolar Repair and Regeneration
In mice and rats, neutral lipid droplets appear during the late canalicular stage and remain abundant through the first two postnatal weeks (14, 37, 43). Although their presence in the lungs of human newborns remains controversial, lipid-laden fibroblasts contribute to alveolar repair and regeneration in models of human disease (44). Other investigators have studied murine fibroblast subpopulations during compensatory right lung growth (CLG) after a left pneumonectomy. It was found that in adult mice, a larger proportion of PDGFRα-GFPlow fibroblasts contained α-SMA, and this population increased after a pneumonectomy (11). Administration of rosiglitazone, a peroxisome proliferator-activated recptor-γ (PPARγ) agonist that promotes lipid accumulation in LIFs, increased the abundance of GFPhigh, α-SMAlow fibroblasts. In a follow-up study, the same group explored differences between the GFPlow and GFPhigh populations in sham-operated and pneumonectomized mice (15). In the sham-operated mice, the lipid-laden cells also expressed CD34. Pneumonectomy reduced the proportion of lipid-laden cells and increased the proportion of α-SMA+ cells within the CD34+ population. Adrenalectomy influenced CLG much like targeted GR deletion did during the saccular stage of development. Adrenalectomized rats exhibited a thickened interstitium with more fibroblasts during CLG compared with pneumonectomized controls with intact adrenal glands (45). Therefore, pneumonectomy modifies the CD34+ fibroblast population by reducing the proportion that contains lipid droplets, increasing the proportion that contains α-SMA, and increasing the expression of the extracellular matrix proteins tenascin C and periostin, consistent with a more synthetic phenotype. Recently, El Agha and associates (46) lineage traced the lipid-storage and myofibroblastic phenotypes in adult mice. They demonstrated that after bleomycin administration, fibroblasts that were lineage labeled for α-SMA acquired lipid droplets, suggesting that adult lung fibroblasts retain plasticity and may have salutary effects after lung injury.
PDGFRα Marks Progenitors, but Does Not Exclusively Control their Differentiation
Our study also clarifies how PDGFRα signaling contributes to the balance between progenitors and differentiated alveolar septal MFs. Although alveolar fibroblast subpopulations can be distinguished by their apparent level of PDGFRα gene transcription (with GFP as a reporter of endogenous PDGFRα gene expression), it remains unclear how well GFP intensity correlates with PDGFRα signaling activity. During secondary septation, the GFP intensity correlates with the abundance of CD140a (PDGFRα) on the cell surface and the abundance of PDGFRα mRNA (10), and the PDGFR-kinase inhibitor imatinib primarily suppresses the proliferation of GFPhigh fibroblasts (10). However, factors in addition to gene expression may regulate PDGFRα signaling and impact the fibroblast phenotype. In murine neonates and adults, GFP intensity correlates with quantitative rather than qualitative differences in lipid storage or α-SMA, as both the GFPlow and GFPhigh populations contain cells exhibiting both lipid droplets and α-SMA (15). Gene expression profiling in adult mice characterized which genes are more highly expressed in GFPlow (vinculin and integrin α8, consistent with a contractile phenotype) and GFPhigh (the extracellular matrix proteins periostin, collagen 3a1, fibrillin 1, consistent with a synthetic phenotype) populations (15). However, PDGFRα signaling is also regulated post-transcriptionally. Studies using oligodendrocyte precursor cells (which are similar to cancer stem cells) from the periphery of glioblastoma multiforme tumors showed that PDGFRα signal transduction preserves “stemness” manifested as self-renewal, retention of markers of multipotency, and invasiveness (47). Other examples of post-transcriptional regulation include (1) the abundance of PDGFRα on the cell surface, which is regulated by endosomal recycling and exosomal shedding (48, 49); (b) the location on the cell surface (i.e., whether PDGFRα localizes to membrane lipid rafts) (48); and (3) the proximity to phosphatase inhibitors, which are also regulated by recycling and membrane location (50). These factors influence how PDGFRα impacts oligodendrocyte precursor cell stemness and differentiation, and could also influence PDGFRα function in alveolar fibroblasts (47). Additional experimentation is required to understand the impact of these various pathways on alveolar fibroblasts.
Potential Clinical Application
Lineage-tracing studies have demonstrated the importance of Smad2 signaling for the differentiation of Shh-responsive, PDGFRα+, mesenchymal progenitors destined to be MFs (3). A second population of PDGFRα+ cells, from the same mesenchymal lineage, can differentiate postnatally into a lipid-storage phenotype in the absence of Smad2 signaling (4). Our studies suggest that a portion of this second population maintains bipotency at least through P8, combining characteristics of both MFs (α-SMA) and LIFs (ADRP, UCP1). These less committed cells more likely retain the progenitor markers Dlk1C and Sca1, and contain both α-SMA and lipid droplets, if Dex is administered during this early postnatal time window. Administering glucocorticoids to newborns with bronchopulmonary dysplasia or adults with pulmonary fibrosis has not improved their clinical outcome. However, our studies suggest that Dex sustains plasticity, and that targeting additional downstream pathways could steer fibroblasts away from fibrogenesis toward a more salutary phenotype. Further investigation is required to identify and modify these incompletely defined pathways.
Footnotes
This study was funded by a Merit Review award from the Department of Veterans Affairs research service. Flow cytometry was performed at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center. The Aria flow cytometer was funded by a grant from the National Center for Research Resources of the National Institutes of Health under award number 1S10 RR027219.
Author Contributions: S.E.M. designed studies, acquired and analyzed data, and wrote the manuscript. D.M.M. acquired data and edited the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0376OC on May 21, 2017
References
- 1.Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. 2006;41:558–569. doi: 10.1002/ppul.20407. [DOI] [PubMed] [Google Scholar]
- 2.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. 2008;294:L891–L901. doi: 10.1152/ajplung.00333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Li M, Li S, Xing Y, Yang CY, Li A, Borok Z, De Langhe S, Minoo P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. 2015;33:999–1012. doi: 10.1002/stem.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li A, Ma S, Smith SM, Lee MK, Fischer A, Borok Z, Bellusci S, Li C, Minoo P. Mesodermal ALK5 controls lung myofibroblast versus lipofibroblast cell fate. 2016;14:19. doi: 10.1186/s12915-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeman KT, Fillmore CM, Kim CF. Lung stem and progenitor cells in tissue homeostasis and disease. 2014;107:207–233. doi: 10.1016/B978-0-12-416022-4.00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y-H, Petkova AP, Granneman JG. Identification of an adipogenic niche for adipose tissue remodeling and restoration. 2013;18:355–367. doi: 10.1016/j.cmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan SE, McCoy DM. Regulation of fibroblast lipid storage and myofibroblast phenotypes during alveolar septation in mice. 2014;307:L618–L631. doi: 10.1152/ajplung.00144.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Acciani T, Le Cras T, Lutzko C, Perl A-KT. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. 2012;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD, Szibor M, Braun T, Morty RE, Seeger W, et al. Characterization of the platelet-derived growth factor receptor-α-positive cell lineage during murine late lung development. 2015;309:L942–L958. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 13.McGowan SE, McCoy DM. Fibroblast growth factor signaling in myofibroblasts differs from lipofibroblasts during alveolar septation in mice. 2015;309:L463–L474. doi: 10.1152/ajplung.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-α-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. 2008;291:1649–1661. doi: 10.1002/ar.20764. [DOI] [PubMed] [Google Scholar]
- 15.Green J, Endale M, Auer H, Perl AK. Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor α kinase activity. 2016;54:532–545. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschanz SA, Makanya AN, Haenni B, Burri PH. Effects of neonatal high-dose short-term glucocorticoid treatment on the lung: a morphologic and morphometric study in the rat. 2003;53:72–80. doi: 10.1203/00006450-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Roth-Kleiner M, Berger TM, Tarek MR, Burri PH, Schittny JC. Neonatal dexamethasone induces premature microvascular maturation of the alveolar capillary network. 2005;233:1261–1271. doi: 10.1002/dvdy.20447. [DOI] [PubMed] [Google Scholar]
- 18.Massaro D, Massaro GD. Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. 1986;251:R218–R224. doi: 10.1152/ajpregu.1986.251.2.R218. [DOI] [PubMed] [Google Scholar]
- 19.Bird AD, Choo YL, Hooper SB, McDougall AR, Cole TJ. Mesenchymal glucocorticoid receptor regulates the development of multiple cell layers of the mouse lung. 2014;50:419–428. doi: 10.1165/rcmb.2013-0169OC. [DOI] [PubMed] [Google Scholar]
- 20.Clayton DA, Shadel GS. Isolation of mitochondria from cells and tissues. 2014;2014:pdb.top074542. doi: 10.1101/pdb.top074542. [DOI] [PubMed] [Google Scholar]
- 21.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 22.Magomedova L, Cummins CL. Glucocorticoids and metabolic control. 2016;233:73–93. doi: 10.1007/164_2015_1. [DOI] [PubMed] [Google Scholar]
- 23.McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. 2015;160:105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry DC, Jiang Y, Graff JM. Emerging roles of adipose progenitor cells in tissue development, homeostasis, expansion and thermogenesis. 2016;27:574–585. doi: 10.1016/j.tem.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartze JT, Becker S, Sakkas E, Wujak LA, Niess G, Usemann J, Reichenberger F, Herold S, Vadász I, Mayer K, et al. Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts. 2014;289:3262–3275. doi: 10.1074/jbc.M113.541052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida H, Kanamori Y, Asano H, Hashimoto O, Murakami M, Kawada T, Matsui T, Funaba M. Regulation of brown adipogenesis by the TGF-β family: involvement of Srebp1c in TGF-β- and activin-induced inhibition of adipogenesis. 2013;1830:5027–5035. doi: 10.1016/j.bbagen.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 28.Thiam AR, Beller M. The why, when and how of lipid droplet diversity. 2017;130:315–324. doi: 10.1242/jcs.192021. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T, Igarashi J, Hasan AU, Ohmori K, Kohno M, Nagai Y, Yamashita T, Kosaka H. Mifepristone promotes adiponectin production and improves insulin sensitivity in a mouse model of diet-induced-obesity. 2013;8:e79724. doi: 10.1371/journal.pone.0079724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammi C, Marzolla V, Armani A, Feraco A, Antelmi A, Maslak E, Chlopicki S, Cinti F, Hunt H, Fabbri A, et al. A novel combined glucocorticoid-mineralocorticoid receptor selective modulator markedly prevents weight gain and fat mass expansion in mice fed a high-fat diet. 2016;40:964–972. doi: 10.1038/ijo.2016.13. [DOI] [PubMed] [Google Scholar]
- 31.Park Y-K, Ge K. Glucocorticoid receptor accelerates, but is dispensable for, adipogenesis. 2017;37:e00260–16. doi: 10.1128/MCB.00260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barclay JL, Agada H, Jang C, Ward M, Wetzig N, Ho KK. Effects of glucocorticoids on human brown adipocytes. 2015;224:139–147. doi: 10.1530/JOE-14-0538. [DOI] [PubMed] [Google Scholar]
- 33.Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, Xu G. Direct effect of glucocorticoids on lipolysis in adipocytes. 2009;23:1161–1170. doi: 10.1210/me.2008-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massaro D, Teich N, Maxwell S, Massaro GD, Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. 1985;76:1297–1305. doi: 10.1172/JCI112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. 1995;68:229–245. doi: 10.1159/000244241. [DOI] [PubMed] [Google Scholar]
- 36.Luyet C, Burri PH, Schittny JC. Suppression of cell proliferation and programmed cell death by dexamethasone during postnatal lung development. 2002;282:L477–L483. doi: 10.1152/ajplung.00406.2000. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan NB, Grant MM, Brody JS. The lipid interstitial cell of the pulmonary alveolus. Age and species differences. 1985;132:1307–1312. doi: 10.1164/arrd.1985.132.6.1307. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Seale P. Control of brown and beige fat development. 2016;17:691–702. doi: 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.During MJ, Liu X, Huang W, Magee D, Slater A, McMurphy T, Wang C, Cao L. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. 2015;156:2059–2073. doi: 10.1210/en.2014-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seki T, Hosaka K, Lim S, Fischer C, Honek J, Yang Y, Andersson P, Nakamura M, Näslund E, Ylä-Herttuala S, et al. Endothelial PDGF-CC regulates angiogenesis-dependent thermogenesis in beige fat. 2016;7:12152. doi: 10.1038/ncomms12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. 2012;61:1112–1121. doi: 10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bossard F, Silantieff E, Lavazais-Blancou E, Robay A, Sagan C, Rozec B, Gauthier C. β1, β2, and β3 adrenoceptors and Na+/H+ exchanger regulatory factor 1 expression in human bronchi and their modifications in cystic fibrosis. 2011;44:91–98. doi: 10.1165/rcmb.2009-0372OC. [DOI] [PubMed] [Google Scholar]
- 43.Bruce MC, Honaker CE, Cross RJ. Lung fibroblasts undergo apoptosis following alveolarization. 1999;20:228–236. doi: 10.1165/ajrcmb.20.2.3150. [DOI] [PubMed] [Google Scholar]
- 44.Ahlbrecht K, McGowan SE. In search of the elusive lipofibroblast in human lungs. 2014;307:L605–L608. doi: 10.1152/ajplung.00230.2014. [DOI] [PubMed] [Google Scholar]
- 45.Rannels DE, Stockstill B, Mercer RR, Crapo JD. Cellular changes in the lungs of adrenalectomized rats following left pneumonectomy. 1991;5:351–362. doi: 10.1165/ajrcmb/5.4.351. [DOI] [PubMed] [Google Scholar]
- 46.El Agha E, Moiseenko A, Kheirollahi V, De LS, Crnkovic S, Kwapiszewska G, Kosanovic D, Schwind F, Schermuly RT, Henneke I, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. 2017;20:261–273.e3. doi: 10.1016/j.stem.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cenciarelli C, Marei HE, Felsani A, Casalbore P, Sica G, Puglisi MA, Cameron AJ, Olivi A, Mangiola A. PDGFRα depletion attenuates glioblastoma stem cells features by modulation of STAT3, RB1 and multiple oncogenic signals. 2016;7:53047–53063. doi: 10.18632/oncotarget.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pituch KC, Moyano AL, Lopez-Rosas A, Marottoli FM, Li G, Hu C, van Breeman R, Mansson JE, Givogri MI. Dysfunction of platelet-derived growth factor receptor α (PDGFRα) represses the production of oligodendrocytes from arylsulfatase A-deficient multipotential neural precursor cells. 2015;290:7040–7053. doi: 10.1074/jbc.M115.636498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen D, Zuo D, Luan C, Liu M, Na M, Ran L, Sun Y, Persson A, Englund E, Salford LG, et al. Glioma cell proliferation controlled by ERK activity-dependent surface expression of PDGFRA. 2014;9:e87281. doi: 10.1371/journal.pone.0087281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tibaldi E, Zonta F, Bordin L, Magrin E, Gringeri E, Cillo U, Idotta G, Pagano MA, Brunati AM. The tyrosine phosphatase SHP-1 inhibits proliferation of activated hepatic stellate cells by impairing PDGF receptor signaling. 2014;1843:288–298. doi: 10.1016/j.bbamcr.2013.10.010. [DOI] [PubMed] [Google Scholar]