Abstract
Rationale: Video-assisted thoracoscopic surgery (VATS) and open lobectomy are both standard of care for the treatment of early-stage non–small cell lung cancer (NSCLC) because of equivalent long-term survival.
Objectives: To evaluate whether the improved perioperative outcomes associated with VATS lobectomy are explained by surgeon characteristics, including case volume and specialty training.
Methods: We analyzed the Surveillance, Epidemiology, and End Results–Medicare–linked registry to identify stage I–II NSCLC in patients above 65 years of age. We used a propensity score model to adjust for differences in patient characteristics undergoing VATS versus open lobectomy. Perioperative complications, extended length of stay, and perioperative mortality among patients were compared after adjustment for surgeon’s volume and specialty using linear mixed models. We compared survival using a Cox model with robust standard errors.
Results: We identified 9,508 patients in the registry who underwent lobectomy for early-stage NSCLC. VATS lobectomies were more commonly performed by high-volume surgeons (P < 0.001) and thoracic surgeons (P = 0.01). VATS lobectomy was associated with decreased adjusted odds of cardiovascular complications (odds ratio [OR] = 0.65; 95% confidence interval [CI] = 0.47–0.90), thromboembolic complications (OR = 0.47; 95% CI = 0.38–0.58), extrapulmonary infections (OR = 0.75; 95% CI = 0.61–0.94), extended length of stay (OR = 0.47; 95% CI = 0.40–0.56), and perioperative mortality (OR = 0.33; 95% CI = 0.23–0.48) even after controlling for differences in surgeon volume and specialty. Long-term survival was equivalent for VATS and open lobectomy (hazard ratio = 0.95; 95% CI = 0.85–1.08) after controlling for patient and tumor characteristics, surgeon volume, and specialization.
Conclusions: VATS lobectomy for NSCLC is associated with better postoperative outcomes, but similar long-term survival, compared with open lobectomy among older adults, even after controlling for surgeon experience.
Keywords: video-assisted thoracoscopic surgery lobectomy, open lobectomy, video-assisted thoracoscopic surgery lobectomy, surgeon volume, hospital volume
The primary treatment modality for stage I non–small cell lung cancer (NSCLC) is lobectomy (1), either via open thoracotomy or video-assisted thoracoscopic surgery (VATS). The safety of VATS was established by two phase II trials that were conducted over a decade ago (2–4). Since then, several observational cohort studies have reported outcomes after lobectomy via open thoracotomy or VATS (5–9). Meta-analyses (10) of these studies have shown that VATS lobectomy is associated with a decreased risk of postoperative complications (7, 9, 11) and a higher 5-year survival rate (12–14). Based on these findings, VATS has been increasingly adopted for the treatment of early-stage lung cancer, despite lack of evidence from randomized, controlled studies. However, VATS lobectomies are more frequently performed by high-volume surgeons with training in thoracic surgery (6). Thus, it is possible that improved outcomes after VATS may be explained, in part, by factors unrelated to the procedure itself.
Using population-based cancer data, we examined postoperative outcomes and long-term survival of older adults with stage I–II NSCLC treated by VATS lobectomy or open lobectomy, while accounting for surgeon volume and specialization.
Methods
We used data from the linked SEER (Surveillance, Epidemiology, and End-Results) cancer registries and Medicare claims files (15). SEER is a population-based network of registries that covers approximately 28% of the American population, collecting and reporting data on all incident cases of cancer in these regions (16). SEER data are merged with Medicare claims files, with successful linkages for 93% of patients of age 65 years or older (15, 17). These data can be also linked to information about the surgeons who operated on Medicare patients from the American Medical Association Physician Masterfile (17).
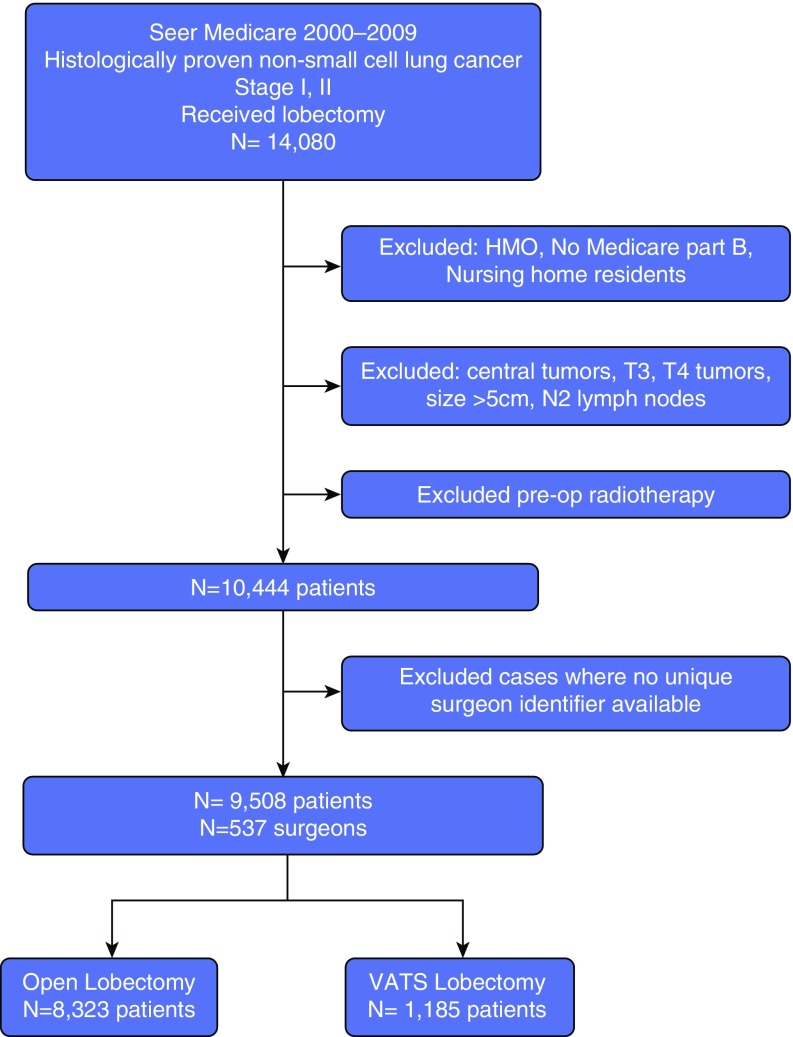
From SEER-Medicare data, we identified patients over 65 years of age with histologically confirmed, first/primary, stage I or II NSCLC diagnosed between 2000 and 2009 who underwent lobectomy. We excluded patients without Medicare Part B (outpatient) coverage or who were enrolled in Health Maintenance Organizations, as the linked database does not include complete claims for these individuals. We also excluded patients who received preoperative radiotherapy, those with missing data on tumor size, as well as patients with centrally located tumors, who may not be candidates for VATS. We also excluded patients diagnosed with lung cancer at autopsy and by death certificates. Nursing home residents were excluded from the study, as they likely are not candidates for resection due to poor functional status
Patients Characteristics
Sociodemographic characteristics (age, sex, race/ethnicity, and marital status) were obtained from SEER; estimated income was calculated based on census level data reported by Medicare. We assessed the burden of comorbidities from the International Classification of Diseases–Ninth Classification diagnostic codes included in Medicare claims using the Deyo adaptation of the Charlson comorbidity index, applying lung cancer–specific weights (18). We used data from the Hospice and Home Health Agency file to identify use of home health services (medical social service, home aid, physical therapy, intravenous therapy, and home oxygen) as a proxy for poor functional status (18).
Cancer characteristics (tumor site and histology) and stage (pathologic tumor, node, and metastasis status, and tumor size) were obtained from SEER. Using these data, patients were staged according to the seventh edition of the American Joint Commission on Cancer system (19). Use of cancer diagnostic tests (bronchoscopy, fine-needle aspiration, etc.), preoperative assessment (ventilation–perfusion scans and/or cardiac stress testing), and staging (positron emission tomography scan, mediastinoscopy, etc.) tests was ascertained from Medicare claims.
Patients who underwent lobectomy were identified using SEER (surgical codes 30–33) and Medicare (International Classification of Diseases ninth edition procedures code 32.4 or Current Procedural Terminology fourth edition code 32,480) (15). Patients who received segmentectomy, wedge resection, and pneumonectomy were excluded from the analysis. Use of VATS was determined from physician claims (International Classification of Diseases–Ninth Classification procedure code 32.41 or Current Procedural Terminology fourth edition code 32,663) (20). The number of lymph nodes removed during surgery was obtained from SEER. Data on postoperative use of radiation therapy was ascertained from combined SEER and Medicare records (21). Use of adjuvant chemotherapy is not captured by SEER and, thus, was determined from Medicare inpatient, physician, and outpatient claims applying validated algorithms (22).
Data regarding surgeons who operated on study patients were obtained from the Physician Masterfile maintained by the American Medical Association. This database includes sociodemographic information (physician age and sex), type of practice (private vs. government or academic), specialty (thoracic surgeon vs. others; also available in Medicare claims), and number of years in practice (16). Physician-specific procedure volume was extracted from inpatient, outpatient, and physician/carrier files. Consistent with prior studies, surgical case volume was defined as the volume of both VATS and open thoracic surgery cases performed in the year of the patient’s surgery (23). Using these data, surgeons were ranked into quintiles according to their volume.
Study Outcomes
Study outcomes included complications within 30 days of surgery. Presence of respiratory complications, extrapulmonary infections, cardiovascular complications, thromboembolic events, and need for transfusions were determined using Medicare claims and applying a published algorithm (24). Patients requiring postoperative inpatient care for greater than 2 weeks were classified as having a prolonged hospitalization (25). We also compared rates of 30-day reoperations and 30-day readmissions, two clinically relevant outcomes. Perioperative mortality was defined as deaths from any cause in the first 30 days after surgery, as captured in Medicare records.
We also evaluated long-term survival of study participants. Survival was computed as the period of time from the date of resection to the date of death or last follow-up; individuals alive at the last follow-up date (December 16, 2011) were treated as censored observations. Cause of death was ascertained from SEER and obtained from death certificate information.
Statistical Analysis
Baseline characteristics of patients who underwent open versus VATS lobectomies were compared using the t test, Wilcoxon test, or chi-square test, as appropriate. Differences in the characteristics of physicians performing open versus VATS procedures were assessed using generalized linear mixed models to account for the clustered nature of data from repeated measures within individual surgeons.
Allocation to open lobectomy versus VATS of study participants was not random. Thus, we used propensity score methods to adjust for measured determinants of treatment selection. The propensity for undergoing VATS was estimated using baseline patient characteristics, such as sociodemographic factors, tumor characteristics (location, histology, size, and tumor, node, and metastasis status), diagnostic, perioperative, and staging work-up. After fitting the model, we used regression analyses to assess the balance of covariates between the study groups while adjusting for propensity scores.
Surgical complications were compared among patients receiving VATS versus open lobectomy in progressively adjusted models. First, we fitted a model adjusting for baseline patient and tumor characteristics using propensity scores only. Second, we adjusted for propensity scores and surgeon volume. Finally, we adjusted for propensity scores, surgeon volume, and surgeon specialization. All comparisons used generalized linear mixed models (with a physician random intercept) to adjust for clustering within surgeons. In these models, propensity score adjustment was conducted using an inverse probability analysis weighting observations by the inverse of the probability of the treatment received.
Survival was compared using Cox proportional hazards models that were adjusted for propensity scores. We used robust standard errors to adjust for the correlated structure of the data.
Statistical analysis was performed using SAS 9.2 (SAS Institute). All tests were performed at a two-sided significance level of 0.05. This study was determined to be exempt from review by the Icahn School of Medicine at Mount Sinai’s Institutional Review Board.
Results
Overall, 1,185 (12%) study subjects underwent VATS. Patients treated with VATS were older and more likely to be female and have higher median income and fewer comorbidities (P < 0.001 for all comparisons; Table 1, Figure 1). VATS surgery was also associated with higher preoperative use of positron emission tomography scan, mediastinoscopy, and endobronchial ultrasound guided lymph node biopsy (P < 0.001 for all comparisons). Tumors resected by VATS were more often T1 or N0 disease (P < 0.001). VATS surgeries were more likely to be performed by surgeons specialized in thoracic surgery (P = 0.01) and with higher thoracic surgery procedural volume (P < 0.001). There was no difference in the age, sex, and practice location of surgeons performing open versus VATS procedures (P > 0.05 for all comparisons). All these differences in baseline characteristics were nonsignificant after adjustment for propensity scores (Table 1).
Table 1.
Baseline characteristics of surgeons and older patients with stage I–II non–small-cell lung cancer treated with video-assisted thoracoscopic surgery versus open thoracotomy
| Variable | Open Lobectomy | VATS Lobectomy |
P Value |
|
|---|---|---|---|---|
| (n = 8,323) | (n = 1,185) | Without Adjustment | With Adjustment* | |
| Patient characteristics | ||||
| Age, mean±SD, yr | 74 ± 5 | 75 ± 6 | <0.0001 | 0.99 |
| Female, n (%) | 4,414 (53) | 729 (61) | <0.0001 | 0.99 |
| Race/ethnicity, n (%) | 0.02 | 0.99 | ||
| White | 7,429 (88) | 1,060 (89) | ||
| African American | 426 (5) | >11† | ||
| Hispanic | 94 (1) | ≤11 | ||
| Other | 374 (5) | 71 (6) | ||
| Married, n (%) | 4,829 (58) | 649 (55) | 0.03 | 0.99 |
| Median income in area of residence, n (%) |
<0.0001 | 0.99 | ||
| Lowest quartile | 2,028 (24) | 154 (13) | ||
| Second quartile | 2,116 (25) | 226 (19) | ||
| Third quartile | 2,017 (24) | 281 (24) | ||
| Highest quartile | 2,162 (26) | 524 (44) | ||
| Comorbidity score, n (%) | <0.0001 | 0.99 | ||
| <1 | 2,504 (30) | 426 (36) | ||
| 1–1.5 | 2,679 (32) | 402 (34) | ||
| 1.5–2.5 | 1,029 (12) | 96 (8) | ||
| >2.5 | 2,111 (25) | 261 (22) | ||
| Cancer site, n (%) | 0.07 | 0.96 | ||
| Upper lobe | 5,157 (62) | 692 (58) | ||
| Middle lobe | 414 (5) | 71 (6) | ||
| Lower lobe | 2,658 (32) | 404 (34) | ||
| T status, n (%) | <0.001 | 0.99 | ||
| T1 | 6,524 (68) | 901 (76) | ||
| T2 | 2,700 (32) | 284 (24) | ||
| N status, n (%) | <0.001 | 0.99 | ||
| N0 | 7,204 (86) | 1,077 (91) | ||
| N1 | 1,119 (13) | 108 (9) | ||
| Stage, n (%) | <0.0001 | 0.99 | ||
| I | 7,204 (87) | 1,077 (91) | ||
| II | 1,119 (13) | 108 (9) | ||
| Histology, n (%) | <0.0001 | 0.99 | ||
| Adenocarcinoma | 4,943 (60) | 738 (62) | ||
| Squamous cell carcinoma | 2,616 (32) | 286 (24) | ||
| Large cell carcinoma | 271 (3) | 24 (2) | ||
| Other | 493 (6) | 137 (12) | ||
| Mediastinoscopy | 1,200 (14) | 226 (19) | <0.0001 | 0.99 |
| EBUS | ||||
| PET scan | 3,651 (44) | 623 (53) | <0.0001 | 0.99 |
| Characteristics of surgeons performing the procedure (n = 537) | ||||
| Age (years), mean ± SD | 52 ± 9 | 50 ± 7 | 0.015 | —‡ |
| Male, n (%) | 6,919 (96) | 1,037 (97) | 0.42 | |
| Private practice, n (%) | 5,238 (80) | 738 (79) | 0.92 | |
| Thoracic surgeon, n (%) | 6,022 (83) | 921 (91) | 0.01 | |
| Surgeon procedural volume7, n (%) | <0.001 | |||
| Lowest quintile | 1,679 (20) | 125 (11) | ||
| Second quintile | 1,551 (19) | 73 (6) | ||
| Third quintile | 1,787 (21) | 116 (9) | ||
| Fourth quintile | 1,836 (22) | 239 (20) | ||
| Highest quintile | 1,435 (17) | 631 (53) | ||
Definition of abbreviations: EBUS = endobronchial ultrasound; PET = positron emission tomography; VATS = video-assisted thoracoscopic surgery.
Adjusted for propensity scores
Exact number of patients not reported in cells with fewer than 11 individuals to maintain patient confidentiality.
Not included in the propensity score model.
Figure 1.
Study population. HMO = Health Maintenance Organization; VATS = video-assisted thoracoscopic surgery.
There were significant differences in the rates of complications between the two groups. Analyses adjusting for propensity scores showed that VATS was associated with lower rates of cardiovascular complications (odds ratio [OR] = 0.68; 95% confidence interval [CI] = 0.51–0.90), thromboembolic complications (OR = 0.60; 95% CI = 0.48–0.75), transfusion requirements (OR = 0.57; 95% CI = 0.47–0.70), and extrapulmonary infections (OR = 0.71; 95% CI = 0.59–0.85) (Table 2). Similarly, fewer VATS-treated patients required extended length of hospital stay (OR = 0.49; 95% CI = 0.41–0.58). There were no significant differences in the rates of respiratory complications (OR = 0.91; 95% CI = 0.81–1.02), 30-day reoperations (OR = 0.69; 95% CI = 0.40–1.21), or 30-day readmission (OR = 1.14; 95% CI = 0.88–1.47) across treatment groups. Perioperative mortality was also lower among patients treated with VATS compared with open lobectomy (OR = 0.30; 95% CI = 0.22–0.40). Similar results were found on analyses progressively adjusted for surgeon’s volume and specialization.
Table 2.
Surgical complications for older patients treated with video-assisted thoracoscopic surgery versus open lobectomy
| Complication | Open Lobectomy |
VATS Lobectomy |
Adjusted for Propensity Scores* |
Adjusted for Propensity Scores and Surgeon Volume† |
Adjusted for Propensity Scores, Surgeon Volume‡ and Specialization§ |
|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Respiratory | 2,675 (32) | 325 (27) | 0.91 (0.81–1.02) | 0.91 (0.81–1.02) | 0.90 (0.80–1.02) |
| Cardiovascular|| | 180 (2) | 31 (3) | 0.68 (0.51–0.90) | 0.67 (0.49–0.89) | 0.65 (0.47–0.90) |
| Thromboembolic|| | 349 (4) | 49 (4) | 0.60 (0.48–0.75) | 0.59 (0.47–0.73) | 0.47 (0.38–0.58) |
| Transfusion|| | 583 (7) | 63 (5) | 0.57 (0.47–0.70) | 0.59 (0.72–0.48) | 0.62 (0.49–0.77) |
| Extrapulmonary infection|| | 547 (7) | 52 (4) | 0.71 (0.59–0.85) | 0.71 (0.56–0.85) | 0.75 (0.61–0.94) |
| Extended length of stay|| | 1,307 (16) | 90 (8) | 0.49 (0.41–0.58) | 0.49 (0.41–0.58) | 0.47 (0.40–0.56) |
| 30-d readmission | 176 (2) | 26 (2) | 1.14 (0.88–1.47) | 1.14 (0.87–1.50) | 1.28 (0.94–1.76) |
| 30-d reoperation | 119 (2) | 13 (1) | 0.69 (0.40–1.21) | 0.70 (0.40–1.23) | 0.74 (0.40–1.37) |
| Perioperative mortality|| | 302 (4) | 20 (2) | 0.30 (0.22–0.40) | 0.31(0.23–0.41) | 0.33 (0.23–0.48) |
Definition of abbreviations: CI = confidence interval; OR = odds ratio for complications for patients treated with video-assisted thoracoscopic surgery versus open lobectomy; VATS = video-assisted thoracoscopic surgery.
OR for complications for patients treated with VATS versus open lobectomy.
Adjusted for patient propensity scores and clustering among surgeons.
Adjusted for surgeon volume in the year of the patient’s surgery.
Adjusted for surgeon specialization, age, sex, and volume in the year of the patient’s surgery.
Statistically significant difference between patients treated with VATS and lobectomy.
In analyses adjusting for propensity scores, overall risk of death was lower among VATS compared with open lobectomy-treated patients (hazard ratio [HR] = 0.89; 95% CI = 0.79–0.99) when adjusted for only patient and tumor characteristics using a propensity score model (Table 3). However, VATS was not associated with improved survival in analyses adjusting for surgeon volume (HR = 0.93; 95% CI = 0.83–1.05) or surgeon age, specialty, and volume (HR = 0.95; 95% CI = 0.83–1.08).
Table 3.
Hazard ratios for death of older patients with stage I–II treated with open lobectomy versus video-assisted thoracoscopic lobectomy
| Model | Overall Survival HR* (95% CI) |
|---|---|
| Adjusted for propensity scores | 0.89 (0.79–0.99) |
| Adjusted for propensity scores and surgeon volume rank | 0.93 (0.83–1.05) |
| Adjusted for propensity scores, surgeon volume and surgeon factors† | 0.95 (0.83–1.08) |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
Represents HR for death for patients treated with video-assisted thoracoscopic surgery versus open lobectomy.
Adjusted for surgeon age, sex, and specialization in thoracic surgery.
Discussion
VATS lobectomy has been increasingly adopted for the treatment of patients with early-stage NSCLC, and is now considered an established alternative to open resection. Most data supporting the advantages of VATS have been obtained from nonrandomized observational studies. Given that VATS procedures are more commonly performed by thoracic surgeons with high volumes of resections, it is possible that some of the benefits of this surgical approach are due to the technical skills of the operating surgeons. In this study, we found that VATS was associated with better postoperative outcomes even after adjusting for surgeon characteristics. These results suggest that continuous adoption of VATS lobectomy for older patients with NSCLC could lead to decreased perioperative morbidity and mortality.
Rates of VATS lobectomy for lung cancer treatment vary between 49% in the Society of Thoracic Surgeons database, which includes higher volume and more specialized surgeons, and 26% in the National Cancer Database, which represents a wider range of providers (26). Analysis of these multi-institutional registries shows that VATS is associated with shorter chest tube duration, less pain, and shorter length of hospital stay compared with thoracotomy (9, 10, 27). In addition, VATS has been associated with decreased hospital costs and decreased health care utilization (7, 28). Based on these results, current guidelines recommend VATS as an adequate approach for the treatment of early-stage NSCLC (19).
Despite these benefits, an ongoing concern is that VATS may compromise the oncologic principles of anatomic resection and complete lymphadenectomy. Adequate intraoperative lymph node evaluation is critical for determining lung cancer stage and for identifying potential candidates for adjuvant chemotherapy. A national study of the Danish Lung Cancer Registry found an increase in N0–N1 and N0–N2 upstaging associated with open when compared with VATS lobectomy (8). Despite these differences in nodal upstaging, adjusted analyses did not show a survival difference after VATS versus open surgery for clinical stage I NSCLC (8). A propensity-matched analysis among thoracic surgeons found no differences in N0–N2 upstaging between VATS and open groups, but showed that N0–N1 upstaging was less common with VATS when compared with open procedures (29). Decaluwé and colleagues (30) addressed this same question in a consecutive cohort of prospectively matched patients with clinical stage I NSCLC. All patients had extensive preoperative mediastinal staging, and tumor location was included in the multivariate model. In their cohort, the number of lymph node stations examined during VATS was similar to open resections, and surgical technique had no impact on the rate of nodal upstaging. We did not have access to information regarding upstaging and, thus, could not compare rates of this important outcome among patients in the two treatment groups. However, the equivalent survival after VATS versus open procedures suggests adequate oncological outcomes regardless of the treatment approach.
Surgeon operative volume and specialization have previously been associated with reduced 30-day mortality, improved postoperative morbidity, and better 5-year survival after lung cancer resection (28, 31). Lien and colleagues (32) studied the impact of surgeon volume of lung cancer resection on in-hospital mortality in a 4-year, nationwide, population-based study in Taiwan. The authors demonstrated an inverse relationship between surgeon volume and the odds of in-hospital deaths. In contrast, a U.K. study on individual surgeon volume did not find a relationship with in-hospital mortality (33). In the United States, lung cancer resections are performed by general surgeons, thoracic surgeons, and cardiac surgeons. Patients with lung cancer treated by thoracic surgeons have been shown to have higher long-term survival than those treated by general surgeons (34). In addition, thoracic surgeons perform preoperative and intraoperative staging more often than both general and cardiac surgeons (34). Similar to open lobectomies, VATS resections done by high-volume surgeons have been shown to have decreased postoperative complications and improved survival (35). Thus, the survival advantages associated with VATS may be explained, in part, by these factors. In this study, we showed that even after adjusting for surgeon’s volume and specialization, VATS was associated with decreased 30-day mortality of older patients with lung cancer. We also identified an overall survival benefit for VATS compared with open lobectomy in our unadjusted analysis. However, long-term survival after VATS and open resection was attenuated and no longer statistically different after controlling for physician volume and specialization in older patients.
There are some strengths and limitations in our study. The SEER-Medicare–linked registry includes information on patients with lung cancer and surgeons in multiple geographic areas, and in varied urban and rural settings across the United States. Consequently, our results should be less affected by local practice patterns and, thus, generalizable to other patients with lung cancer. However, our analyses were limited to patients above 65 years of age and to those patients without Medicare Part B or with health maintenance organization coverage. Although lung cancer is primarily a disease of older individuals, our results are not generalizable to younger patients. This analysis relied on the use of codes and claims in the SEER and Medicare databases to ascertain the type of surgical procedure performed and, thus, may be subject to misclassification. In particular, we were unable to capture whether VATS lobectomies were converted to open lobectomies. However, prior studies have shown that claims-based surgical coding is relatively accurate (15). Our cohort was relatively large compared with those of prior studies, and we had long-term follow-up on these patients, ensuring sufficient statistical power to compare outcomes after VATS versus open resection.
Observational data cannot provide definitive evidence regarding the benefits of VATS versus open lobectomy due to lack of randomization, creating the possibility of allocation bias. In our analyses, we used propensity score methods to attenuate the impact of measured confounders. However, propensity scores do not control for the impact of unmeasured variables. Thus, the possibility of residual bias remains as a potential threat of the validity of our findings. Despite these limitations, given that no randomized controlled trials have compared these surgical approaches, our results provide important information regarding the comparative effectiveness of VATS.
In summary, this study showed that VATS lobectomy is associated with better perioperative outcomes among older patients with stage I–II NSCLC, even after controlling for surgeon characteristics. These data suggest that continuous uptake of this surgical approach may lead to improved patient’s outcomes.
Supplementary Material
Footnotes
Supported by Agency for Healthcare Research and Quality (AHRQ) National Cancer Institute grant R01HS019670.
Author Contributions: N.E. and J.P.W. contributed to content, analysis, and drafting of the manuscript; M.K., K.S., and S.L. contributed to content and participated in manuscript review; G.M. and E.G. contributed to data analysis and collection, and reviewed the final manuscript; D.N., S.S., and A.N. contributed to content and drafting of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):7S–37S. doi: 10.1378/chest.12-2377. [DOI] [PubMed] [Google Scholar]
- 2.Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg. 1993;56:1248–1252. doi: 10.1016/0003-4975(93)90661-z. [Discussion, pp. 1252–1253.] [DOI] [PubMed] [Google Scholar]
- 3.Walker WS, Carnochan FM, Tin M. Thoracoscopy assisted pulmonary lobectomy. Thorax. 1993;48:921–924. doi: 10.1136/thx.48.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roviaro G, Varoli F, Rebuffat C, Vergani C, Maciocco M, Scalambra SM, et al. Videothoracoscopic staging and treatment of lung cancer. Ann Thorac Surg. 1995;59:971–974. doi: 10.1016/0003-4975(95)00029-k. [DOI] [PubMed] [Google Scholar]
- 5.Laursen LO, Petersen RH, Hansen HJ, Jensen TK, Ravn J, Konge L. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg. 2016;49:870–875. doi: 10.1093/ejcts/ezv205. [DOI] [PubMed] [Google Scholar]
- 6.Falcoz PE, Puyraveau M, Rivera C, Bernard A, Massard G, Mauny F, et al. Epithor Group. The impact of hospital and surgeon volume on the 30-day mortality of lung cancer surgery: a nation-based reappraisal. J Thorac Cardiovasc Surg. 2014;148:841–848. doi: 10.1016/j.jtcvs.2014.01.030. [Discussion, p. 848.] [DOI] [PubMed] [Google Scholar]
- 7.Swanson SJ, Meyers BF, Gunnarsson CL, Moore M, Howington JA, Maddaus MA, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg. 2012;93:1027–1032. doi: 10.1016/j.athoracsur.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Licht PB, Jørgensen OD, Ladegaard L, Jakobsen E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg. 2013;96:943–949. doi: 10.1016/j.athoracsur.2013.04.011. [Discussion, pp. 949–950.] [DOI] [PubMed] [Google Scholar]
- 9.Park HS, Detterbeck FC, Boffa DJ, Kim AW. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. Ann Thorac Surg. 2012;93:372–379. doi: 10.1016/j.athoracsur.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 10.Cai YX, Fu XN, Xu QZ, Sun W, Zhang N. Thoracoscopic lobectomy versus open lobectomy in stage I non–small cell lung cancer: a meta-analysis. PLoS One. 2013;8:e82366. doi: 10.1371/journal.pone.0082366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2009;138:11–18. doi: 10.1016/j.jtcvs.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Lee PC, Nasar A, Port JL, Paul S, Stiles B, Chiu YL, et al. Long-term survival after lobectomy for non–small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96:951–960. doi: 10.1016/j.athoracsur.2013.04.104. [Discussion, pp. 960–961.] [DOI] [PubMed] [Google Scholar]
- 13.Yang CF, Sun Z, Speicher PJ, Saud SM, Gulack BC, Hartwig MG, et al. Use and outcomes of minimally invasive lobectomy for stage I non–small cell lung cancer in the National Cancer Data Base. Ann Thorac Surg. 2016;101:1037–1042. doi: 10.1016/j.athoracsur.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ. 2014;349:g5575. doi: 10.1136/bmj.g5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV-43–IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Health: National Cancer Institute. Surveillance Epidemiology and End Results Database. [Updated 2017 Oct 4; accessed 2013 Dec 10]. Available from: http://seer.cancer.gov/
- 17.Baldwin LM, Adamache W, Klabunde CN, Kenward K, Dahlman C, L Warren J. Linking physician characteristics and medicare claims data: issues in data availability, quality, and measurement. Med Care. 2002;40(8 suppl):IV-82–IV-95. doi: 10.1097/00005650-200208001-00012. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non–small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 20.Smith CB, Kale M, Mhango G, Neugut AI, Hershman DL, Mandeli JP, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol. 2014;9:383–389. doi: 10.1097/JTO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 21.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare–linked data. Med Care. 2002;40(8 suppl):IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 22.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Earle C, Xu F, Panageas KS, Yabroff KR, Bristow RE, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98:163–171. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 24.Iezzoni LI, Daley J, Heeren T, Foley SM, Fisher ES, Duncan C, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Wisnivesky JP, Smith CB, Packer S, Strauss GM, Lurslurchachai L, Federman A, et al. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II–IIIA lung cancer: observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burt BM, Kosinski AS, Shrager JB, Onaitis MW, Weigel T. Thoracoscopic lobectomy is associated with acceptable morbidity and mortality in patients with predicted postoperative forced expiratory volume in 1 second or diffusing capacity for carbon monoxide less than 40% of normal. J Thorac Cardiovasc Surg. 2014;148:19–28. doi: 10.1016/j.jtcvs.2014.03.007. [Discussion, pp. 28–29.e1.] [DOI] [PubMed] [Google Scholar]
- 27.Whitson BA, et al. Surgery for early-stage non–small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2016. doi: 10.1016/j.athoracsur.2008.07.009. [Discussion, pp. 2016–2018.] [DOI] [PubMed] [Google Scholar]
- 28.David G, Gunnarsson CL, Moore M, Howington J, Miller DL, Maddaus MA, et al. Surgeons’ volume–outcome relationship for lobectomies and wedge resections for cancer using video-assisted thoracoscopic techniques. Minim Invasive Surg. 2012;2012:760292. doi: 10.1155/2012/760292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medbery RL, Gillespie TW, Liu Y, Nickleach DC, Lipscomb J, Sancheti MS, et al. Nodal upstaging is more common with thoracotomy than with VATS during lobectomy for early-stage lung cancer: an analysis from the National Cancer Data Base. J Thorac Oncol. 2016;11:222–233. doi: 10.1016/j.jtho.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decaluwé H, Stanzi A, Dooms C, Fieuws S, Coosemans W, Depypere L, et al. Central tumour location should be considered when comparing N1 upstaging between thoracoscopic and open surgery for clinical stage I non–small-cell lung cancer. Eur J Cardiothorac Surg. 2016;50:110–117. doi: 10.1093/ejcts/ezv489. [DOI] [PubMed] [Google Scholar]
- 31.Goodney PP, Lucas FL, Stukel TA, Birkmeyer JD. Surgeon specialty and operative mortality with lung resection. Ann Surg. 2005;241:179–184. doi: 10.1097/01.sla.0000149428.17238.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lien YC, Huang MT, Lin HC. Association between surgeon and hospital volume and in-hospital fatalities after lung cancer resections: the experience of an Asian country. Ann Thorac Surg. 2007;83:1837–1843. doi: 10.1016/j.athoracsur.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Treasure T, Utley M, Bailey A. Assessment of whether in-hospital mortality for lobectomy is a useful standard for the quality of lung cancer surgery: retrospective study. BMJ. 2003;327:73. doi: 10.1136/bmj.327.7406.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farjah F, Flum DR, Varghese TK, Jr, Symons RG, Wood DE. Surgeon specialty and long-term survival after pulmonary resection for lung cancer. Ann Thorac Surg. 2009;87:995–1004. doi: 10.1016/j.athoracsur.2008.12.030. [Discussion, pp. 1005–1006.] [DOI] [PubMed] [Google Scholar]
- 35.Smith CB, Wolf A, Mhango G, Wisnivesky JP. Impact of surgeon volume on outcomes of older stage I lung cancer patients treated via video-assisted thoracoscopic surgery. Semin Thorac Cardiovasc Surg. 2017;29:223–230. doi: 10.1053/j.semtcvs.2017.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.