Abstract
In this study we update the TDP-43 in Alzheimer’s disease staging scheme by assessing the topography of TDP-43 in 193 cases of Alzheimer’s disease, in 14 different brain regions (eight previously described plus six newly reported) and use conditional probability to model the spread of TDP-43 across the 14 brain regions. We show that in addition to the eight original regions we previously reported (amygdala, entorhinal cortex, subiculum, dentate gyrus of the hippocampus, occipitotemporal cortex, inferior temporal cortex, middle frontal cortex and basal ganglia (putamen/globus pallidum)), that TDP-43 is also deposited in the insular cortex, ventral striatum, basal forebrain, substantia nigra, midbrain tectum, and the inferior olive of the medulla oblongata, in Alzheimer’s disease. The conditional probability analysis produced six significantly different stages (P< 0.01), and suggest that TDP-43 deposition begins in the amygdala (stage 1), then moves to entorhinal cortex and subiculum (stage 2), then to the dentate gyrus of the hippocampus and occipitotemporal cortex (stage 3), then insular cortex, ventral striatum, basal forebrain and inferior temporal cortex (stage 4), then substantia nigra, inferior olive and midbrain tectum (stage 5), and finally to basal ganglia and middle frontal cortex (stage 6). This updated staging scheme is superior to our previous staging scheme, classifying 100 % of the cases (versus 94% in the old scheme), based on criteria provided, and better accounts for Alzheimer’s disease clinical and imaging features, such as Mini-Mental Status Examination score and hippocampal volume. We discuss the relevance of the updated staging scheme, as well as its impact on the prion-like hypothesis of protein spread in neurodegenerative disease. We also address the issue of whether frontotemporal lobar degeneration with TDP-43 could be the primary pathology in stage 6.
Keywords: TDP-43, Alzheimer’s disease, staging, brainstem, insular cortex, limbic
Introduction
The RNA binding protein TDP-43 has become important to our understanding of neurodegenerative diseases such as amyotrophic lateral sclerosis and some variants of frontotemporal lobar degeneration. TDP-43 was first shown to be one of the ubiquitinated proteins associated with both diseases in 2006[38]. Subsequently, TDP-43 has been shown to also be associated with Alzheimer’s disease[3]. TDP-43 is deposited in 30–70% of some Alzheimer’s disease case series[3,5,8,15,21,23,24,28,41], and has been found to be strongly associated with clinical and MRI features of Alzheimer’s disease, such as memory loss and hippocampal atrophy[21,24,37]. TDP-43 deposition in Alzheimer’s disease has been reported to have a stereotypic progression of spread which led to the development of the original TDP-43 in Alzheimer’s disease staging scheme[23]. Five stages have been described based on the frequency of TDP-43 deposition in eight brain regions (amygdala, entorhinal cortex, subiculum, dentate gyrus of the hippocampus, occipitotemporal cortex, inferior temporal cortex, middle frontal cortex and basal ganglia(putamen/globus pallidum)). Stage I involves only the amygdala, Stage II shows spread into entorhinal cortex and subiculum, Stage III involves the dentate gyrus of the hippocampus and occipitotemporal cortex, Stage IV the inferior temporal cortex, and Stage V shows TDP-43 deposition in frontal cortex and dorsal striatum.
Little is known however about the spread of TDP-43 into other brain regions. Regions that are uncommonly involved, such as the midbrain tectum, and regions that are commonly affected by neurofibrillary tangle pathology in Alzheimer’s disease, such as the basal forebrain, have not been analyzed in Alzheimer’s disease for the deposition of TDP-43. In addition, there is no data on the relationship of involvement of these, and other, important regions to the TDP-43 in Alzheimer’s disease staging scheme. That is, in Alzheimer’s disease it is unclear when these other regions become affected by TDP-43 relative to the eight regions that define the original TDP-43 in Alzheimer’s disease staging scheme. Having a comprehensive understanding of the topography of TDP-43 in Alzheimer’s disease is important, particularly since recent evidence suggests that proteins, including TDP-43, may propagate throughout the brain via a prion-like mechanism[39]. In addition, interpreting regional TDP-43 deposition in Alzheimer’s disease is important since TDP-43, in the form of neurites predominantly, has been found in approximately a third of brains from patients with normal cognition[6].
The main aim of this study was, therefore, to model the probable pattern of sequential spread of TDP-43 in Alzheimer’s disease across 14 brain regions (8 previously published (original) + 6 newly described (insular cortex, ventral striatum, basal forebrain, substantia nigra, midbrain tectum, and the inferior olive of the medulla oblongata)). These other six regions were chosen to expand on the number of limbic regions since limbic involvement is central to Alzheimer’s disease, and to determine whether brainstem regions are affected by TDP-43 in Alzheimer’s disease.
Material and Methods
In order to address our aim, we further analyzed our cases that were previously utilized for the development of the original TDP-43 in Alzheimer’s disease staging scheme[23], and for assessment of the effects of TDP-43 on Alzheimer’s disease clinical features[24]. The cohort consists of 342 cases that were prospectively recruited in the Mayo Clinic Alzheimer’s Disease Research Center and had died with autopsied brain tissue stored in the Brain Bank located in Rochester, MN. As previously described[23,24], all 342 cases had undergone pathological examination according to the recommendations of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD)[34] and each case had been assigned a Braak and Braak neurofibrillary tangle stage[9]. All 342 cases fulfilled NIA-Reagan criteria for Intermediate-high probability Alzheimer’s disease[43] (Braak and Braak stage IV or more and CERAD definite for beta-amyloid deposition). Demographics and clinical features of this cohort of 342 cases have been previously published[23,24]. Of these 342 cases, 195 (57%) were previously reported to show TDP-43 deposition. Of these 195 cases, two cases did not have paraffin blocks available for analysis of all regions of interest analyzed in this study, leaving 193 TDP-43 immunoreactive cases remaining for analysis. Of the 193 cases included in this study, 123 (64%) were female. The median education level attained was 14 years (range: 8, 20). One-hundred and sixteen cases (62%) were apolipoprotein E4 carriers. The median age at onset of the cohort was 77 years old (range: 50, 102), median age of death 88 years old (range: 56, 105) and median illness duration was 10 years (range: 2, 27). Ninety percent of the cases had a clinical diagnosis of dementia, 6% mild cognitive impairment, and 4% normal cognition, at the last evaluation prior to death. Of those with dementia, the final clinical diagnosis was Alzheimer’s dementia in all except five cases that had the following final diagnoses: behavioral variant frontotemporal dementia (n=1), corticobasal syndrome (n=3) and progressive supranuclear palsy (n=1). The median Mini-Mental Status Examination score for all 193 cases was 14 points (range: 0, 29).
This study was approved by the Mayo Clinic IRB. Prior to death, all participants or their proxies had provided written consent for brain autopsy examination.
Pathological analysis
For this study, paraffin blocks of 14 brain regions that included the eight original regions, as well as six newly reported regions (basal forebrain, insular cortex, ventral striatum, substantia nigra, midbrain tectum and inferior olive) were sectioned and immunostained for TDP-43 (polyclonal antibody MC2085 that recognizes a peptide sequence in the 25-kDA C-terminal fragment[44] with a DAKO-Autostainer (DAKA-Cytomaton, Carpinteria, CA) with 3,3’-diaminobenzidine as the chromogen. A region was considered TDP-43 positive if there were any TDP-43 immunoreactive neuronal cytoplasmic inclusions, dystrophic neurites, or neuronal intranuclear inclusions identified at 400× magnification. These lesion types were chosen as all three lesion types have been identified in amyotrophic lateral sclerosis[4,31,38], frontotemporal lobar degeneration[4,14,22,38] and Alzheimer’s disease[3,5,8,19,21,23,24,28,41], and are therefore considered to be abnormal. The definition of TDP-43 positivity used in this study is unchanged from that used to develop the original TDP-43 in Alzheimer’s disease staging scheme [23].
Conditional Probability analysis
We were interested in assessing the evidence that one region tended to have earlier TDP-43 involvement than another. Therefore, if we denote the two regions being compared as X and Y, and use a plus sign to denote positive for TDP-43 and minus sign to denote negative for TDP-43, we reasoned that cases who were (X−, Y−) or (X+, Y+) would not contribute any evidence of ordering because — at least relative to those two regions — concordant cases would be at the same stage. On the other hand discordant cases (X+, Y−) or (X−, Y+) would be informative because these cases were at different stages. We used McNemar’s test to assess the evidence against the null hypothesis that (X+, Y−) and (X−, Y+) were equally likely and therefore X and Y were part of the same stage. This testing was performed for all combinations of regions to generate a probability of sequential ordering for all 14 regions. We used p<0.01 as a conservative value to determine whether we had sufficient evidence to reject the null hypothesis that the two regions were part of the same stage.
To summarize how likely or probable it is that region X becomes TDP-43 positive before region Y we report the fraction of cases who were X+ among those who were Y−. We also report the fraction of cases who were Y+ among those who were X−. To be more concrete, if the cross-classification of regions X and Y is as follows
| Region Y | ||
|---|---|---|
| Region X | TDP-43+ | TDP-43− |
| TDP-43+ | a | b |
| TDP-43− | c | d |
we report the fraction of cases for whom X was before Y as b/(b + d) and the fraction of cases for whom Y was before X as c/(c + d). Expressed as conditional probabilities, we report and compare P(X+|Y−) and P(Y+|X−). We succinctly summarize the conditional probabilities by presenting them in a matrix-like graphical display where each cell in the matrix corresponds to a conditional probability that one region precedes another. Reading the plot from left to right, the entries show the estimated probability that the region on the left is TDP-43 positive before the region on the right. Reading the plot from top to bottom, the entries show the estimated probability that the region below is TDP-43 positive before the region above.
Staging cases in our cohort
We staged all 193 cases with the following criteria: Only one region from a specific stage needs to be involved in order for the case to attain that stage. The highest region that is involved determines the stage. In the event that a lower region is “skipped”, meaning no region from that stage is involved, but at least one region from a higher stage is involved, the case was given the highest stage, with one exception, the inferior olive. If the inferior olive was associated with stage X and was the only affected region in stage X and there were no affected regions from stage (X-1), inferior olivary involvement was ignored. If however, the inferior olive was the only affected region in stage X and at least one region from stage (X-1) was affected, the case was classified as stage X.
Analyses to help guide routine pathological assessment
In the event that the conditional probability analysis produced a stage that included more than three regions, and hence increasing the complexity of pathological assessments for that stage, we determine the frequencies of involvement of all combinations of regions within that stage. This data would be important to help provide a guide to pathologists in deciding which subset of regions to sample to provide the optimum trade-off between work load (i.e. number of regions to sample) and accuracy (i.e. the ability to correctly stage the case).
Clinical and imaging associations with the updated staging scheme
In order to assess whether cases with the highest TDP-43 stage might represent frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) we abstracted clinical and neuropsychological data to assess for clinical features suggestive of a frontotemporal dementia spectrum disorder. We also compare regional cortical grey matter volumes of cases in the highest stage to a TDP-43 negative control group, matched by age at death, sex and Braak stage. Clinical information abstracted included age at onset, sex, presenting symptom, final diagnosis, and the presence or absence of aphasia, disinhibition, apathy, loss of empathy, stereotyped behavior, hyperorality, executive deficits, resting tremor, cogwheel rigidity, limb bradykinesia, gait/postural instability and eye movement abnormality early in the disease course. Neuropsychological variables included Mini-Mental State Examination (MMSE),[18] Clinical Dementia Rating scale sum of Boxes (CDR-SB)[36], Dementia Rating Scale (DRS)[33], Boston Naming Test (BNT)[29], Wechsler Adult Intelligence Scale (WAIS) Block design,[42] Control Oral Word Association Test (COWAT),[7] the Auditory Verbal Learning Test (AVLT),[1] and the motor subscale of the Unified Parkinson’s disease Rating Scale (UPDRS).[17]
To determine whether TDP-43 deposition in the six newly added regions (insular cortex, ventral striatum, basal forebrain, substantia nigra, inferior olive and midbrain tectum) had any clinical significance we compared neuropsychological characteristics in cases with and without TDP-43 deposition, for each region of interest.
Results
TDP-43 deposition in the six new regions
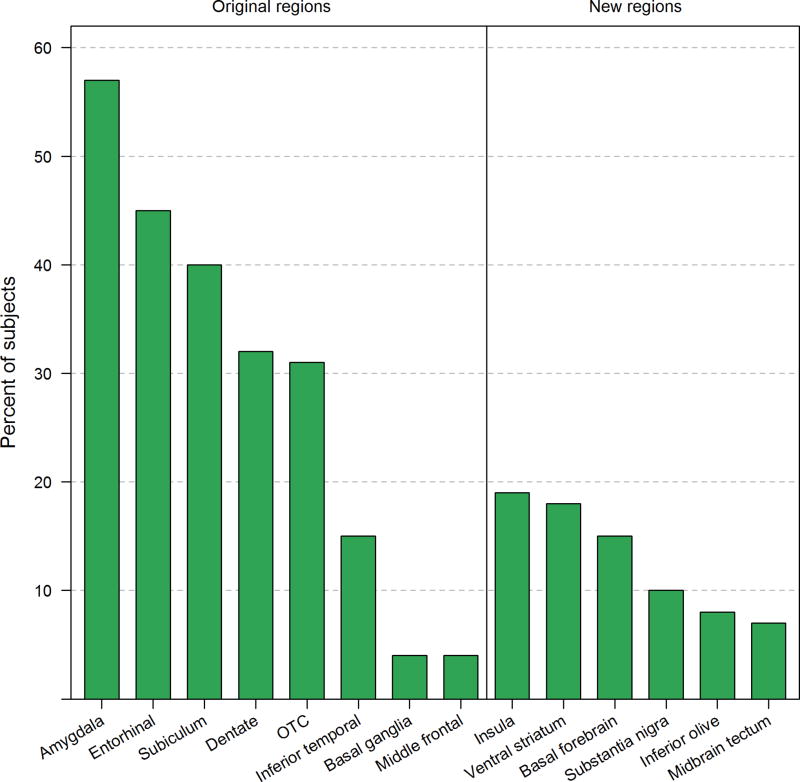
All six newly assessed regions (insular cortex, ventral striatum, basal forebrain, substantia nigra, midbrain tectum and the inferior olive) did show varying degrees of TDP-43 deposition (Figure 1). Morphological characteristics of the TDP-43 immunoreactive lesions in these six newly assessed regions were no different from the lesions observed in the eight original regions, although in the majority of instances we observed neuronal cytoplasmic inclusions; less commonly dystrophic neurites and only rarely intranuclear inclusions (Figure 1). In most cases, when TDP-43 immunoreactivity was present, lesion burden was observed to be scant to mild, with moderate to severe burden occurring much less frequently. The frequency of TDP-43 deposition in the six newly described regions varied, being most common in the insular cortex and ventral striatum, and least common in midbrain tectum. Limbic cortical regions were more frequently affected than brainstem regions (Figure 2). With the exception of the basal ganglia (putamen and globus pallidum) and middle frontal cortex, the six newly assessed regions were on average less commonly affected compared to the remaining regions from the original eight (Figure 2).
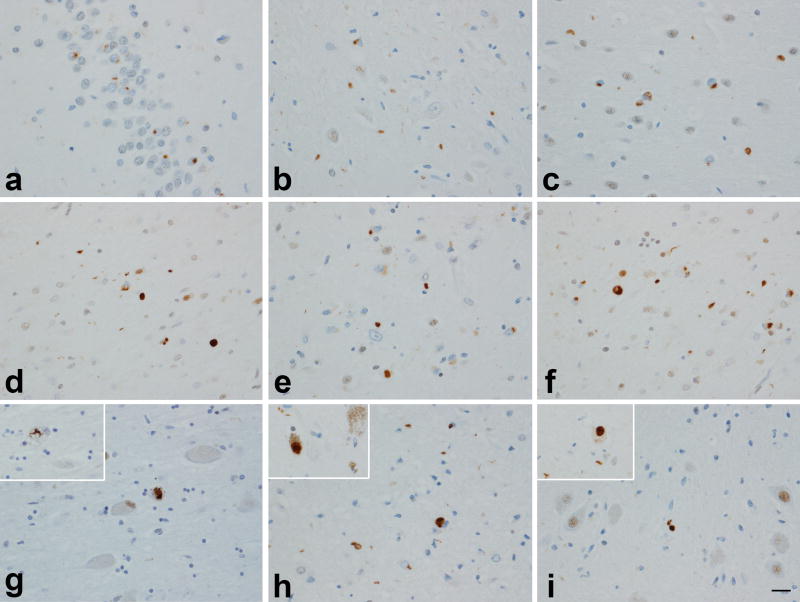
Figure 1.
TDP-43 deposition across different regions in cases with high probability Alzheimer’s disease: dentate fascia (a); subiculum (b); entorhinal cortex (c); amygdala (d); ventral striatum (e); insular cortex (f); basal nucleus (inset: NCI) (g); midbrain tectum (inset: substantia nigra) (h); medulla – inferior olivary nucleus (inset: NCI) (i). In most instances TDP-43 immunoreactive neuronal cytoplasmic inclusions were observed although dystrophic neurites can also be seen in many panels. Magnification × 200 (inset × 400).
Figure 2.
Bar plot showing the frequency of TDP-43 deposition in the eight original and six newly assessed regions among 340 cases. The most common affected region was the amygdala (frequency=56%).
The frequency of involvement of each of the six newly assessed regions within each of the original five stages is shown in Table 1. As can be seen, the six new regions analyzed appear to predominantly become involved after the original stage II but before the original stage V, hence somewhere in the middle of the original TDP-43 in Alzheimer’s disease staging scheme.
Table 1.
Frequency of regional TDP-43 deposition of all 14 regions for each of the original five stages among the 193 cases
| Region | Stage I (N = 34) |
Stage II (N = 48) |
Stage III (N = 59) |
Stage IV (N = 39) |
Stage V (N = 13) |
|---|---|---|---|---|---|
|
| |||||
| Amygdala | 34 (100%) | 48 (100%) | 59 (100%) | 39 (100%) | 13 (100%) |
| Entorhinal | 0 (0%) | 43 (90%) | 58 (98%) | 39 (100%) | 13 (100%) |
| Subiculum | 0 (0%) | 29 (60%) | 57 (97%) | 38 (97%) | 12 (92%) |
| Dentate | 0 (0%) | 0 (0%) | 57 (97%) | 38 (97%) | 13 (100%) |
| OTC | 0 (0%) | 0 (0%) | 53 (90%) | 39 (100%) | 13 (100%) |
| Insula | 0 (0%) | 1 (2%) | 30 (51%) | 24 (63%) | 10 (83%) |
| Ventral striatum | 1 (3%) | 2 (4%) | 24 (41%) | 27 (69%) | 7 (58%) |
| Basal forebrain | 0 (0%) | 0 (0%) | 19 (33%) | 21 (54%) | 12 (92%) |
| Inferior temporal | 0 (0%) | 0 (0%) | 0 (0%) | 39 (100%) | 12 (92%) |
| Substantia nigra | 1 (3%) | 0 (0%) | 10 (17%) | 13 (33%) | 10 (77%) |
| Inferior olive | 1 (3%) | 2 (4%) | 8 (14%) | 7 (18%) | 8 (67%) |
| Midbrain tectum | 0 (0%) | 0 (0%) | 5 (8%) | 10 (26%) | 10 (77%) |
| Basal ganglia | 1 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 11 (85%) |
| Middle frontal | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 13 (100%) |
Conditional probability analysis
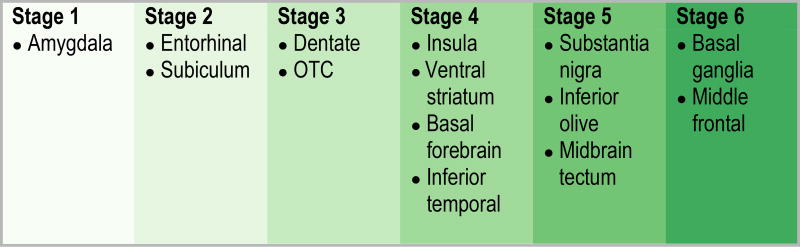
The probability analysis showing the likely sequential spread of TDP-43 in Alzheimer’s disease is shown in Figure 3. As can be seen, TDP-43 deposition spreads from the amygdala (stage 1) (not shown since it’s affected in all TDP-43 positive cases) to entorhinal cortex and subiculum (stage 2) without evidence to separate them, then to the dentate nucleus of the hippocampus and occipitotemporal cortex (stage 3) without evidence to separate them. TDP-43 deposition then spreads to the insula, ventral striatum, basal forebrain and inferior temporal cortex (stage 4) without evidence to separate them. The three brainstem regions (substantia nigra, inferior olive and midbrain tectum) appear to form a distinct stage (stage 5) without evidence to separate them. However, there was a striking difference between inferior temporal cortex and substantia nigra (p=0.01), inferior temporal and inferior olive (p < 0.001), and inferior temporal and midbrain tectum (P <0.001). All three brainstem regions were also significantly different from insular cortex, ventral striatum and basal forebrain (P < 0.001). Of all 14 regions assessed, the basal ganglia (putamen and globus pallidum) and middle frontal cortex were the most likely regions to be affected last (stage 6). The probability analysis therefore generated a sequential scheme involving six distinct groups of brain regions (Figure 4). In order to avoid confusion with our old staging scheme that used Roman numerals (stages I–V) the new staging scheme uses Arabic numerals (stages 1–6).
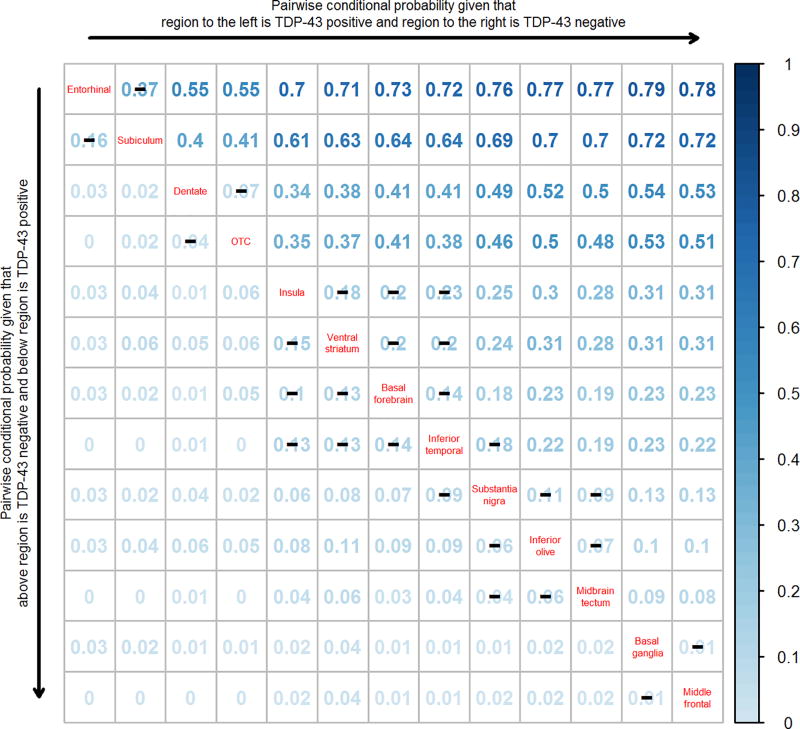
Figure 3.
A pairwise conditional probability matrix of the regions analyzed. Reading the plot from left to right, the conditional probability estimates show the estimated probability that the region on the left is TDP-43 positive before the region on the right. For example, the probability of entorhinal being TDP-43 positive given that subiculum is TDP-43 negative is 0.37. Reading the plot from top to bottom, the entries show the estimated probability that the region below is TDP-43 positive before the region above. For example, the probability of subiculum being TDP-43 positive given that entorhinal is negative is 0.16. Black lines (–) across conditional probability estimates indicate p-values are not statistically significant at the < 0.01 level. Note however, p-values between inferior temporal cortex and substantia nigra (p=0.01), between (insula, ventral striatum and basal forebrain) and substantia nigra (p<0.001), and between (insula, ventral striatum, basal forebrain, inferior temporal cortex) and (inferior olive and midbrain tectum (P<0.001). P-values were assessed using exact McNemar’s test.
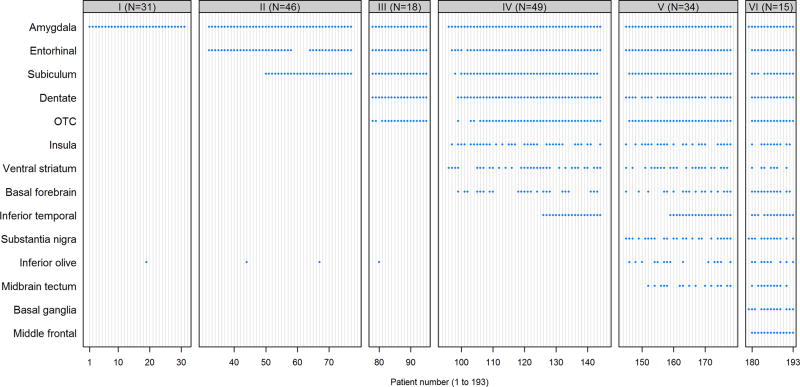
Figure 4.
Patterns of TDP-43 positivity across 14 regions for 193 cases. The vertical axis indicates regions and the horizontal axis indicates patients. A blue dot indicates the case was TDP-43 positive for that region. Patients are grouped by TDP-43 in Alzheimer’s disease stage.
We were able to classify 193 of our cases based on the criteria stipulated in the methods section. Of the 193 cases that were classified, six cases were observed to have had one or more skipped stage. All six cases had a clinical presentation of memory loss and all were given an antemortem diagnosis of Alzheimer’s dementia. Four of the six cases had one skipped stage, one had two skipped stages, and one had three skipped stages. Four additional cases were observed to have involvement of the inferior olive but no involvement of any regions from stage 4 or any other regions from stage 5. Based on our staging criteria, the inferior olive was ignored in these four cases which were classified as stage 1 (n=1), stage 2 (n=2) and stage 3 (n=1). Six cases had involvement of at least one stage 4 region and only inferior olive from stage 5. Based on our staging criteria, these six cases were classified as stage 5. Table 2 shows the frequency of cases classified in the old staging scheme compared to the new staging scheme. Based on the new classification scheme, two cases previously classified as stage I were reclassified as stages 4 and 5. No stage II cases changed classification. Twenty-nine of 61 cases (48%) previously classified as stage III were reclassified as stage 4, while 14 (23%) were reclassified as stage 5. Twenty of the 39 (51%) previously classified stage IV cases were reclassified as stage 5. All previously classified stage V cases were reclassified as stage 6.
Table 2.
Classification frequency of old staging scheme versus updated staging scheme
| Updated six stage staging scheme | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Old scheme | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 6 |
| Stage I | 31 | 1 | 1 | |||
| Stage II | 46 | |||||
| Stage III | 18 | 29 | 14 | |||
| Stage IV | 19 | 20 | ||||
| Stage V | 14 | |||||
Analyses to help guide routine pathological assessment
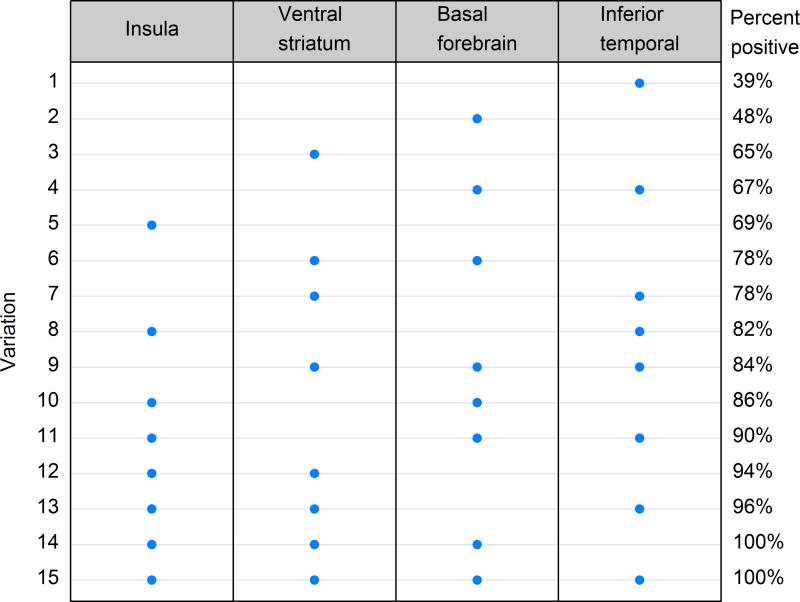
The conditional probability analysis placed four regions into stage 4 (Figure 5). Hence, we calculated the frequencies of involvement of combinations of all four regions for stage 4. If only one region is selected for screening, the insula gives the best opportunity for staging since 65% of stage 4 cases would be correctly classified. This percentage increases as more regions are added (Figure 6). Figure 6 could be used a guide to select regions for pathological sampling for stage 4. For example, if one wanted to sample only two regions, the highest percentage of captured cases would be 94% and would require sampling the insula and ventral striatum. Based on the old staging scheme, in which only the inferior temporal cortex would have been sampled, only 39% of cases would be correctly classified as stage 4 in the updated scheme (Figure 6).
Figure 5.
Diagram illustrating the TDP-43 in Alzheimer’s disease stage progression.
Figure 6.
A plot of percent of stage 4 cases with TDP-43 deposition in 15 combinations of all four regions (insula cortex, ventral striatum, basal forebrain and inferior temporal cortex). The plot is ordered by percent TDP-43 positive from smallest to largest.
Clinical and imaging associations with the updated staging scheme
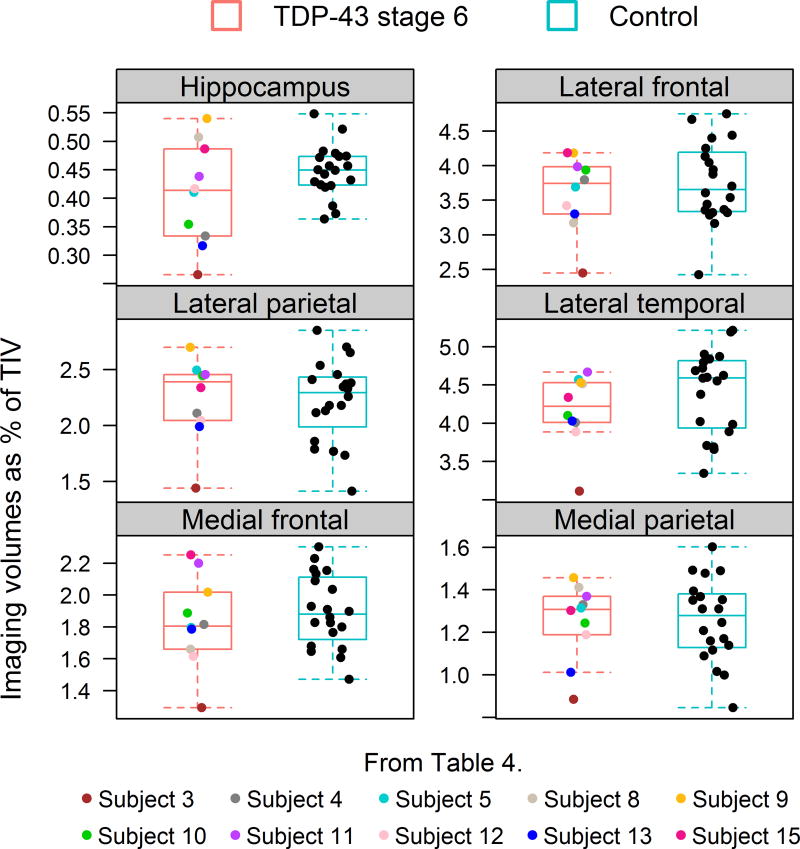
Demographic and neuropsychological data for each stage is shown in Table 3. There were trends for decline in performance on neuropsychological tests as stage increases. Neuropsychological data for each of the 15 stage 6 cases is shown in Table 4. No stage 6 case, except for one case (subject 8), had any clinical features suggestive of an FTLD spectrum disorder. Of the clinical features abstracted, only aphasia was noted to be present and was present in five cases with one case (subject 1) having a very low score on the Boston Naming Test. No case had any behavioral or personality change or Parkinsonism. Fourteen cases had a final clinical diagnosis of Alzheimer’s dementia. The remaining case (subject 8) had presented with word finding difficulties and was initially diagnosed as progressive non-fluent aphasia. Later in the disease course the diagnosis was changed to corticobasal syndrome after asymmetric parkinsonian features developed. This case also showed severe executive dysfunction on COWAT 4 years after onset. Ten stage 6 cases had a volumetric MRI. While hippocampal atrophy was greater in four stage 6 cases compared to the Alzheimer’s controls with TDP-43, there was no evidence for greater frontal or temporal lobe atrophy in any stage 6 cases compared to controls (Figure 7). The one case (subject 3) with medial frontal and lateral temporal lobe atrophy outside the range of controls also showed great hippocampal atrophy and also severe medial and lateral parietal lobe atrophy.
Table 3.
Demographic, pathological and clinical data stratified by TDP-43 stage
| Stage 1 (N = 31) |
Stage 2 (N = 46) |
Stage 3 (N = 18) |
Stage 4 (N = 49) |
Stage 5 (N = 34) |
Stage 6 (N = 15) |
Trend test P-value |
|
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. Female (%) | 20 (65) | 29 (63) | 12 (67) | 31 (63) | 21 (62) | 10 (67) | 0.96 |
| Education, yrs | 14 (12, 20) | 14 (8, 20) | 15 (8, 20) | 12 (8, 20) | 13 (8, 20) | 13 (9, 20) | 0.26 |
| APOE ε4 carrier (%) | 25 (81) | 25 (57) | 11 (61) | 29 (62) | 16 (50) | 10 (67) | 0.13 |
| Age at onset, yrs | 74 (56, 91) | 76 (58, 102) | 82 (50, 96) | 78 (63, 97) | 80 (60, 94) | 76 (54, 96) | 0.10 |
| Time from onset to death, yrs | 8 (2, 27) | 10 (2, 20) | 8 (3, 13) | 11 (2, 19) | 11 (3, 27) | 9 (6, 18) | 0.14 |
| Age at death, yrs | 85 (71, 100) | 87 (65, 105) | 88 (56, 101) | 88 (76, 104) | 90 (70, 101) | 85 (65, 104) | 0.01 |
| Cognitively abnormal at death (%) | 30 (97) | 42 (93) | 18 (100) | 48 (100) | 34 (100) | 15 (100) | 0.06 |
| Diagnosis (%) | 0.22 | ||||||
| CN | 2 (6) | 5 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| MCI | 3 (10) | 1 (2) | 1 (6) | 2 (4) | 3 (9) | 2 (13) | |
| Other Dementia | 26 (84) | 39 (87) | 17 (94) | 46 (96) | 30 (91) | 13 (87) | |
| Braak stage (%) | 0.84 | ||||||
| 4 | 6 (19) | 7 (15) | 4 (22) | 8 (16) | 5 (15) | 3 (20) | |
| 5 | 6 (19) | 15 (33) | 6 (33) | 13 (27) | 11 (32) | 4 (27) | |
| 6 | 19 (61) | 24 (52) | 8 (44) | 28 (57) | 18 (53) | 8 (53) | |
| CERAD Frequent (%) | 22 (71) | 28 (62) | 11 (61) | 31 (63) | 18 (53) | 12 (80) | 0.64 |
| Infarction (%) | 11 (35) | 9 (20) | 3 (17) | 16 (33) | 9 (26) | 5 (33) | 0.80 |
| Hippocampal sclerosis positive (%) | 4 (13) | 3 (7) | 4 (22) | 29 (60) | 25 (74) | 13 (87) | <0.001 |
| Lewy bodies positive (%) | 7 (23) | 18 (40) | 8 (44) | 15 (31) | 13 (38) | 5 (33) | 0.59 |
| MMSE | 26 (16, 29) | 24 (13, 30) | 24 (7, 30) | 23 (11, 29) | 25 (12, 29) | 23 (13, 30) | 0.22 |
| Total UPDRS | 0 (0, 6) | 0 (0, 13) | 2 (0, 28) | 2 (0, 10) | 0 (0, 8) | 0 (0, 8) | 0.56 |
| Boston Naming Test | 47 (2, 58) | 46 (12, 59) | 43 (22, 54) | 39 (10, 59) | 38 (9, 57) | 40 (8, 59) | 0.06 |
| WAIS-Block Design | 17 (2, 36) | 14 (0, 35) | 12 (0, 22) | 10 (0, 35) | 12 (0, 28) | 14 (5, 23) | 0.048 |
| COWAT total raw | 33 (5, 66) | 24 (7, 59) | 30 (15, 58) | 24 (9, 54) | 30 (6, 43) | 24 (0, 51) | 0.06 |
| AVLT Delayed Recall | 2 (0, 9) | 0 (0, 8) | 0 (0, 3) | 0 (0, 6) | 0 (0, 6) | 0 (0, 5) | 0.01 |
MMSE = Mini-Mental State Examination; UPDRS = Unified Parkinson’s Disease Rating Scale; WAIS = Wechsler Adult Intelligence Scale; COWAT = Control Oral Word Association Test; AVLT = Auditory Verbal Learning Test.
Unless otherwise specific data shown as median (range). P-values were from Spearman's and Wilcoxon rank sum tests.
Table 4.
Clinical data for the 15 stage 6 cases at examination closest to onset
| Subject | Duration onset and testings (yrs.) |
MMSE /30 |
DRS total /144 |
DRS Attention /37 |
DRS Initiation /Perseveration /37 |
DRS Construction /6 |
DRS Conceptualization /39 |
DRS Memory /25 |
WAIS- Block Design /51 |
COWAT total raw |
AVLT Delayed Recall /15 |
Boston Naming Test /60 |
Total UPDRS /108 |
NPI Total Severity /36 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| 1 | 1.6 | 23 | 109 | 36 | 21 | 6 | 28 | 18 | 23 | 15 | 0 | 8 | NA | NA |
| 2 | 4 | 22 | 119 | 37 | 31 | 6 | 30 | 15 | 6 | 51 | 0 | 28 | 0 | NA |
| 3 | 1.8 | 30 | 141 | 35 | 37 | 6 | 39 | 24 | 12 | 45 | 4 | 40 | NA | NA |
| 4 | 2.1 | 24 | 110 | 37 | 20 | 6 | 36 | 11 | NA | 10 | 0 | 27 | 7 | 2 |
| 5 | 2.2 | 24 | 118 | 32 | 28 | 6 | 37 | 15 | NA | 28 | 2 | 34 | 0 | 4 |
| 6 | 0.25 | 22 | 107 | 31 | 26 | 6 | 32 | 12 | 10 | 28 | 0 | 26 | NA | NA |
| 7 | 2.5 | 28 | 139 | 35 | 37 | 5 | 39 | 23 | 19 | 30 | 5 | 57 | 1 | NA |
| 8 | 4.3 | 23 | 109 | 33 | 17 | 6 | 35 | 18 | 17 | 0 | 1 | 44 | 8 | 9 |
| 9 | 1.2 | 29 | 130 | 34 | 37 | 5 | 30 | 24 | NA | 29 | 1 | 55 | 3 | 1 |
| 10 | 1.4 | 18 | 104 | 33 | 21 | 5 | 33 | 12 | 5 | 24 | 0 | NA | NA | 2 |
| 11 | 1.7 | 26 | 121 | 36 | 25 | 6 | 37 | 17 | NA | 21 | 5 | 44 | 0 | 0 |
| 12 | 5.6 | 29 | 134 | 34 | 35 | 6 | 38 | 21 | NA | 22 | 2 | 48 | 5 | 0 |
| 13 | 4.2 | 18 | 89 | 29 | 18 | 4 | 25 | 13 | NA | 10 | 0 | NA | 0 | 2 |
| 14 | 1.2 | 13 | 103 | 36 | 20 | 6 | 27 | 14 | 16 | 9 | 1 | 32 | NA | NA |
| 15 | 5.9 | 20 | 117 | 33 | 30 | 5 | 36 | 13 | NA | 39 | 0 | 59 | 0 | 3 |
MMSE = Mini-Mental State Examination; DRS = Dementia Rating Scale; UPDRS = Unified Parkinson’s Disease Rating Scale; WAIS = Wechsler Adult Intelligence Scale; COWAT = Control Oral Word Association Test; AVLT = Auditory Verbal Learning Test; NPI-Q = brief questionnaire version of the Neuropsychiatric Inventory.
Figure 7.
A comparison of regional volumes between 10 stage 6 cases that had antemortem volumetric head MRI and 20 age, gender, and NFT Braak stage matched Alzheimer’s disease cases without TDP-43.
Comparison of neuropsychological and motor features between cases with and without TDP-43 in the 6 new regions is shown in Tables 5 and 6. There was evidence that TDP-43 deposition in the ventral striatum and basal forebrain has clinical significance with poorer performance on memory, language, and executive tests in those with TDP-43 compared to those without TDP-43.
Table 5.
Comparisons of clinical data between stages 1–3 cases without TDP-43 and stage 4 cases with TDP-43 at examination closest to onset
| Insula | Ventral striatum | Basal forebrain | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes (N=33) | No (N=94) | Yes (N=32) | No (N=94) | Yes (N=23) | No (N=94) | |
|
| ||||||
| MMSE | 23 (11, 29) | 25 (7, 30) | 23 (11, 29) | 25 (7, 30) | 23 (17, 29) | 25 (7, 30) |
| Total UPDRS | 2 (0, 10) | 1 (0, 28) | 2 (0, 10) | 1 (0, 28) | 2 (0, 10) | 1 (0, 28) |
| Boston Naming Test | 42 (10, 59) | 46 (2, 59) | 37 (10, 52)** | 46 (2, 59) | 36 (10, 58)** | 46 (2, 59) |
| WAIS-Block Design | 11 (0, 35) | 14 (0, 36) | 10 (0, 29) | 14 (0, 36) | 10 (2, 20)* | 14 (0, 36) |
| COWAT total raw | 24 (9, 54) | 28 (5, 66) | 21 (9, 48)* | 28 (5, 66) | 21 (9, 33)** | 28 (5, 66) |
| AVLT Delayed Recall | 0 (0, 6) | 1 (0, 9) | 0 (0, 4)* | 1 (0, 9) | 0 (0, 2)** | 1 (0, 9) |
| NPI-Q Total Severity | 3 (0, 14) | 2 (0, 11) | 2 (0, 14) | 2 (0, 11) | 2 (1, 14) | 2 (0, 11) |
MMSE = Mini-Mental State Examination; UPDRS = Unified Parkinson’s Disease Rating Scale; WAIS = Wechsler Adult Intelligence Scale; COWAT = Control Oral Word Association Test; AVLT = Auditory Verbal Learning Test; NPI-Q = brief questionnaire version of the Neuropsychiatric Inventory.
Data shown as median (range). P-values are from Wilcoxon Rank Sum test.
p < 0.05,
p < 0.01,
p < 0.001.
Table 6.
Comparisons of clinical data between stages 1–4 cases without TDP-43 and stage 5 cases with TDP-43 at examination closest to onset
| Substantia nigra | Inferior olive | Midbrain tectum | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes (N=23) | No (N=144) | Yes (N=14) | No (N=139) | Yes (N=15) | No (N=144) | |
|
| ||||||
| MMSE | 24 (12, 29) | 25 (7, 30) | 24 (18, 28) | 25 (7, 30) | 22 (12, 26) | 25 (7, 30) |
| Total UPDRS | 1 (0, 8) | 1 (0, 28) | 0 (0, 5) | 1 (0, 28) | 0 (0, 7) | 1 (0, 28) |
| Boston Naming | 44 (16, 57) | 44 (2, 59) | 33 (9, 54) | 44 (2, 59) | 39 (16, 57) | 44 (2, 59) |
| WAIS-Block Design | 14 (0, 28) | 14 (0, 36) | 12 (1, 26) | 14 (0, 36) | 12 (2, 26) | 14 (0, 36) |
| COWAT total raw | 31 (7, 43) | 26 (5, 66) | 27 (6, 38) | 26 (5, 66) | 30 (12, 36) | 26 (5, 66) |
| AVLT Delayed Recall | 0 (0, 6) | 0 (0, 9) | 0 (0, 6) | 0 (0, 9) | 0 (0, 6) | 0 (0, 9) |
| NPI-Q Total Severity | 1 (0, 6) | 3 (0, 14) | 3 (0, 6) | 2 (0, 14) | 1 (0, 6) | 3 (0, 14) |
MMSE = Mini-Mental State Examination; UPDRS = Unified Parkinson’s Disease Rating Scale; WAIS = Wechsler Adult Intelligence Scale; COWAT = Control Oral Word Association Test; AVLT = Auditory Verbal Learning Test; NPI-Q = brief questionnaire version of the Neuropsychiatric Inventory.
Data shown as median (range). P-values are from Wilcoxon Rank Sum test.
p < 0.05,
p < 0.01,
p < 0.001
Discussion
In this study we demonstrate that TDP-43 deposition in Alzheimer’s disease goes beyond involvement of the eight regions previously reported. We show that TDP-43 deposition also occurs in other limbic regions such as the insular cortex, ventral striatum and basal forebrain, as well as in brainstem regions such as the substantia nigra, inferior olive of the medulla and midbrain tectum. Previously, we assessed the frequency of TDP-43 deposition in eight regions in order to propose a sequence of spread[23]. In this study we go one step further. We use conditional probability to take into account what two regions are doing jointly. This probability analysis was performed on 14 different regions. As a result, we are able to expand upon our understanding of the topography of TDP-43 and the likelihood of regional spread across the brain in Alzheimer’s disease.
The probability analysis helps us to better understand the sequence that TDP-43 spreads across brain regions in Alzheimer’s disease and suggest that the first region to be involved is the amygdala. In addition, as in our previous study in which we were unable to determine whether the subiculum or entorhinal cortex was affected first[23], the probability analysis also did not find sufficient evidence to separate both regions. Similarly, the probability analysis did not find evidence to separate the dentate gyrus of the hippocampus and the occipitotemporal cortex, hence agreeing with our previous designation of both regions as stage III. The probability analysis did find evidence to combine the inferior temporal cortex with the dentate and occipitotemporal cortex, and instead suggest that the inferior temporal cortex is affected after the dentate gyrus of the hippocampus and the occipitotemporal cortex, but before the basal ganglia and middle frontal cortex, as we had previously suggested in our original staging scheme. Surprisingly, there was little evidence to separate the inferior temporal cortex from the insular cortex, ventral striatum and basal forebrain. Hence, it appears that some limbic regions (those in stage 4) are affected after other limbic regions (those in stage 3), and that those that are affected later are affected around the same time as the inferior temporal cortex. Interestingly, the probability analysis placed all three brainstem regions together with very strong evidence to separate them from the insular cortex, ventral striatum, basal forebrain and inferior temporal cortex. It therefore appears that brainstem regions are involved later, rather than early, in the process of TDP-43 deposition in Alzheimer’s disease, but before TDP-43 spreads to the frontal cortex and basal ganglia.
Taking all these findings into account, we propose an update to our original staging scheme, expanding the number of stages from five to six. In addition, in order to avoid confusion between the older stage and the updated stage we now use Arabic numerals instead of Roman numerals for staging. The updated staging scheme is includes Stage 1 that involves only the amygdala, Stage 2 that shows spread into entorhinal cortex and the subiculum, Stage 3 that involves the dentate gyrus of the hippocampus and occipitotemporal cortex, Stage 4 that involves the insular cortex, ventral striatum, basal forebrain and inferior temporal cortex, Stage 5 that involves the substantia nigra, inferior olive and midbrain tectum, and Stage 6 that involves the basal ganglia and middle frontal lobe (Figure 5).
When we applied our staging criteria to this staging scheme we were able to classify all cases. In order to do so we selected the highest region of involvement, with the exception of the inferior olive, as discussed below. One-hundred eighty-three cases (95%) showed a pattern of sequential spread without having skipped any stages. The six cases with skipped stages are interesting and somewhat reminiscent of what has been observed with staging alpha-synuclein deposition in Lewy body disease in which some cases do not show the typical sequential spread of alpha-synuclein pathology[16,20]. There are at least two possible explanations for skipped regions in our cohort. The first is that cases with skipped regions are unique and hence may represent a different "TDP-43 strain" of disease, or a fundamentally different pattern of disease. The second is that skipped regions are simply due to a sampling bias and if additional sections were sampled we would in fact find pathology, and hence eliminate the skipped regions. Further work is needed to understand such cases. In this series, the inferior olive was found to be involved in 10/14 (71%) of stage 6 cases versus 4/126 (3%) stage 1–3 cases (p=0.0001) demonstrating that inferior olivary involvement is strongly associated with higher stages. Hence, we conclude that it is reasonable to use the inferior olive to classify a case as stage 5 as long as there is involvement of stage 4 regions but to ignore the inferior olive if stage 4 is skipped. Ignoring the inferior olive when stage 4 is skipped is not unreasonable given that it is rarely involved in stages 1–3, as well as the fact that published data shows the inferior olive to have TDP-43 immunoreactivity in about 10% of brains from normal control patients[40].
TDP-43 deposition in Alzheimer’s disease was observed in limbic regions that have not been previously discussed in the literature. These regions, the insula cortex, basal forebrain and ventral striatum are commonly affected by other proteins in Alzheimer’s disease. The basal forebrain and insula cortex, for example, are well known to be affected by tau immunoreactive neurofibrillary tangle pathology in Alzheimer’s disease[10,35]. In fact, TDP-43 spread in Alzheimer’s disease is somewhat reminiscent of tau spread as defined by the Braak neurofibrillary tangle stage. In the Braak neurofibrillary tangle stage, the entorhinal cortex and subiculum are affected the hippocampus proper followed by occipitotemporal cortex, followed by isocortex including inferior temporal and middle frontal cortices[9]. Similarly, in Alzheimer’ disease, TDP-43 spreads from entorhinal and subiculum to hippocampus and occipitotemporal, then isocortex including inferior temporal and middle frontal. Interestingly, involvement of the amygdala differs in both schemes. The amygdala is involved early in the Braak neurofibrillary tangle scheme [9] but only scant to minimal, and becomes progressive more involved over Braak stages. On-the-contrary, TDP-43 deposition in the amygdala can be moderate-severe at stage 1. It is unclear why deposition in the amygdala differs between proteins in Alzheimer’s disease. Another interesting difference between tau and TDP-43 deposition in Alzheimer’s disease is that tau deposition have been shown to begin in brainstem regions, such as the locus ceruleus, in the form of pretangles, prior to deposition of neurofibrillary tangles in transentorhinal cortex (NFT Braak stage 1) and beyond[11]. Therefore, it appears that TDP-43 deposition occurs after tau deposition in Alzheimer’s disease, more with the later argyrophilic deposition of tau rather than the phosphorylation of tau. This would be in keeping with our recent study showing that tau, but not TDP-43, drives early clinical presentation in Alzheimer’s disease[25].
The probability analysis and resulting TDP-43 in Alzheimer’s disease stages gives us a platform to briefly discuss the mechanism of how TDP-43 likely spreads across brain regions in Alzheimer’s disease. Currently, there is some evidence that abnormal proteins including beta-amyloid, tau, alpha-synuclein and TDP-43 spread across brain regions in a “prion-like” manner in neurodegenerative diseases[2,13,26,30,39]. Some researchers have suggested a direct cell to cell mechanism of spread between contiguous cells[2,26,32] while others have suggested a mechanism of spread via cell to cell transmission along the axon[12]. The TDP-43 in Alzheimer’s disease staging scheme is difficult to explain via proximal/radiating cell to cell transmission given the distance between some of the regions in consecutive stages; spread via distant cell to cell transmission through anterograde axonal transport would be more likely. Many of the regions that are involved in the early stages are considered limbic regions and are intimately interconnected. One could therefore easily envision spread from stage 1 to 2 and from stage 2 to stage 3 and so forth via a mechanism involving anterograde axonal transport. TDP-43 deposition was observed only in neurons, as cytoplasmic inclusions or dendritic processes, which would support a mechanism of neuron to neuron spread, although we cannot entirely exclude glial cells playing a role in the spread. With-that said, our study was not designed to determine the mechanism of spread of TDP-43 in Alzheimer’s disease, and hence our discussion on the mechanism of spread is mainly speculative.
The deposition of TDP-43 in Alzheimer’s disease has spawned debate as to whether deposition represent the co-existence of two diseases; Alzheimer’s disease and frontotemporal lobar degeneration with TDP-43 (FTLD-TDP). We specifically address this issue in this study with a detailed clinico-imaging assessment of the individual cases in stage 6, since stage 6 cases have the most widespread TDP-43 deposition as well as a pattern reminiscent of FTLD-TDP. One school of thought is that stage 6 cases represent FTLD-TDP, with Alzheimer’ disease being a secondary process. This is certainly possible, and would be supported by our data showing a high frequency of APOE4 gene carriers in stage 6 cases that could be argue to “drive” the Alzheimer’s disease pathology. On-the-other hand, we found no clinical or imaging evidence of involvement of frontal or temporal lobe to suggest an FTLD process, as we have previously reported[27], and the high APOE4 frequency was not unique to stage 6 cases. In addition, one must not forget that superficial cortical microvacuolation and neuronal loss of the frontal and/or temporal lobes is typical of FTLD yet was not present in our stage 6 cases. A limitation of our study however is the absence of quantitative data. Given our experience with TDP-43 deposition in FTLD, and TDP-43 deposition in stage 6 Alzheimer’s disease cases, we hypothesize that there is a striking difference in the amount of TDP-43 that is deposited in FTLD versus deposited in Alzheimer’s disease; being much less in Alzheimer’ disease than in FTLD. Therefore, until a specific biomarker for FTLD is identified to definitively answer the question, current data does not support stage 6 cases being FTLD-TDP.
The updated TDP-43 in Alzheimer’s disease staging scheme has one more stage than the original mainly due to the addition of a brainstem stage. The biggest differences between both stages are: a) we have added three regions to stage 4 (insula cortex, ventral striatum and basal forebrain); b) stage 5 is now a brainstem stage; and c) the basal ganglia/frontal cortex stage is now stage 6. This updated staging scheme is superior to the original staging scheme as the updated set of regions for stage 4 for example, better reflect the biological dynamics. In restaging everyone the inferior temporal lobe alone captures 39% of the cases that should be classified as stage 4. In other words, the inferior temporal cortex as the sole region for stage 4 is not sensitive enough to capture all stage 4 cases. As one moves across this updated staging scheme we observe a decline in clinical and imaging measures which furthers supports the updated staging scheme.
Conclusion
By applying conditional probability analysis to 14 regions of interest we have updated our original TDP-43 in Alzheimer’s disease staging scheme to incorporate the involvement of additional limbic and brainstem regions. The updated staging scheme has six stages.
Acknowledgments
We wish to thank Kris Johnson, Linda Rousseau, Virginia Phillips and Monica Casey Castanedes for pathological support. The work was supported by grants from the National Institutes of Health (R01 AG037491-06 (KAJ) and P50-AG016574 (RCP)).
References
- 1.A R. L'examen clinique en psychologie. Presses Universitaires de France; City: 1964. [Google Scholar]
- 2.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 5.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 6.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol. 2013;126:51–57. doi: 10.1007/s00401-013-1110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benton A, Hamsher K. Multilingual Aphasia Examination. University of Iowa; City: 1989. [Google Scholar]
- 8.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120:43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 12.Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavaguera F, Hench J, Goedert M, Tolnay M. Invited review: Prion-like transmission and spreading of tau pathology. Neuropathol Appl Neurobiol. 2015;41:47–58. doi: 10.1111/nan.12197. [DOI] [PubMed] [Google Scholar]
- 14.Davidson Y, Kelley T, Mackenzie IR, et al. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 15.Davidson YS, Raby S, Foulds PG, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer's disease, late onset Alzheimer's disease and Down's syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 16.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 17.Fahn S, Elton R . Members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden C, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. 2. Macmillan Healthcare Information; City: 1987. [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Hu WT, Josephs KA, Knopman DS, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 21.Josephs KA, Whitwell JL, Knopman DS, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josephs KA, Murray ME, Whitwell JL, et al. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol. 2014;127:441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephs KA, Whitwell JL, Tosakulwong N, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer's disease impact clinical features. Ann Neurol. 2015;78:697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung Y, Dickson DW, Murray ME, et al. TDP-43 in Alzheimer's disease is not associated with clinical FTLD or Parkinsonism. J Neurol. 2014;261:1344–1348. doi: 10.1007/s00415-014-7352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009;29:566–573. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan E, Goodglass H, Weintraubb S. The Boston Namiing Test. Lea & Febiger; City: 1983. [Google Scholar]
- 30.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwong LK, Neumann M, Sampathu DM, Lee VM, Trojanowski JQ. TDP-43 proteinopathy: the neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 2007;114:63–70. doi: 10.1007/s00401-007-0226-5. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattis S. Dementia Rating Scale (DRS) Psychologial Assessment Resources; City: 1998. [Google Scholar]
- 34.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 35.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 37.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77:942–952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka T, Masuda-Suzukake M, Arai T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Uchino A, Takao M, Hatsuta H, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Scale-III. Physiological Corporation; City: 1997. [Google Scholar]
- 43.Working group. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–2. [PubMed] [Google Scholar]
- 44.Zhang YJ, Xu YF, Cook C, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]