Abstract
Purpose
Among individuals with advanced cancer, frequent hospitalization increasingly is viewed as a hallmark of poor-quality care. We examined hospitalization rates and individual- and hospital-level predictors of rehospitalization among individuals with advanced cancer in the year after diagnosis.
Methods
Individuals diagnosed with advanced breast, colorectal, non–small-cell lung, or pancreatic cancer from 2009 to 2012 (N = 25,032) were identified with data from the California Cancer Registry (CCR). After linkage with inpatient discharge data, multistate and log-linear Poisson regression models were used to calculate hospitalization rates and to model rehospitalization in the year after diagnosis, accounting for survival.
Results
In the year after diagnosis, 71% of individuals with advanced cancer were hospitalized, 16% had three or more hospitalizations, and 64% of hospitalizations originated in the emergency department. Rehospitalization rates were significantly associated with black non-Hispanic (incidence rate ratio [IRR], 1.29; 95% CI, 1.17 to 1.42) and Hispanic (IRR, 1.11; 95% CI, 1.03 to 1.20) race/ethnicity; public insurance (IRR, 1.37; 95% CI, 1.23 to 1.47) and no insurance (IRR, 1.17; 95% CI, 1.02 to 1.35); lower socioeconomic status quintiles (IRRs, 1.09 to 1.29); comorbidities (IRRs, 1.13 to 1.59); and pancreatic (IRR, 2.07; 95% CI, 1.95 to 2.20) and non–small-cell lung (IRR, 1.69; 95% CI, 1.54 to 1.86) cancers versus colorectal cancer. Rehospitalization rates were significantly lower after discharge from a hospital that had an outpatient palliative care program (IRR, 0.90; 95% CI, 0.83 to 0.97) and were higher after discharge from a for-profit hospital (IRR, 1.33; 95% CI, 1.14 to 1.56).
Conclusion
Individuals with advanced cancer experience a heavy burden of hospitalization in the year after diagnosis. Efforts to reduce hospitalization and provide care congruent with patient preferences might target individuals at higher risk. Future work might explore access to palliative care in the community and related health care use among individuals with advanced cancer.
INTRODUCTION
Hospitalization can be a distressing and unexpected event for individuals with cancer and their caregivers,1,2 particularly for those with advanced cancer. Among these individuals, treatment goals usually are palliative.3 Ideally, the care of individuals with advanced cancer whose disease is incurable4 should balance prolongation of survival and maximization of the quality of remaining life.3,5 Hospitalization and other aggressive medical interventions can work against these goals and are increasingly recognized as poor-quality cancer care.6,7
Hospitalization not only is often at odds with patient preferences but also contributes substantially to the high cost of cancer care. Among individuals with advanced cancer, inpatient care accounts for a majority of cancer-related spending8,9 and drives regional spending variation.10 Moreover, there is no apparent association between spending and survival.11 Taken together, the high cost of and excessive variability in inpatient cancer care suggest that interventions to reduce unnecessary hospitalizations may reduce costs and improve quality of life in this population.10,12
Indeed, a growing body of evidence suggests that community-based palliative care interventions substantially improve quality of life and potentially reduce health care use and costs for terminally ill individuals.13 Accordingly, recently updated national oncology guidelines support early palliative care, initiated soon after diagnosis and delivered concurrent with active treatment, for individuals with advanced disease.14 However, access to palliative care varies widely by care setting.15 Although most large hospitals now offer inpatient palliative care, similar community-based services are less accessible16 and may remain so for patients who receive care in fee-for-service systems and for-profit hospitals,15,17 which have less incentive to provide low-reimbursement outpatient services compared with other payment models (eg, accountable care organizations).18 A recent report by the Center to Advance Palliative Care concluded that hospital tax status is the strongest predictor of palliative care access after geography and hospital size and that for-profit hospitals are the least likely to offer palliative care services.19 Given the increasing clinical and policy attention to early palliative care, studies that examine health care use from the time of diagnosis and identify hospital attributes that may affect palliative care access and subsequent outcomes are needed.
In this study, prior knowledge is extended through estimation of hospitalization rates among individuals with advanced cancer from diagnosis forward and through examination of factors associated with rehospitalization by using population-based data sources. In addition, possible association of hospital characteristics—which include outpatient palliative care access and hospital ownership—with these outcomes is examined by using modeling techniques that account for clustering of patients within hospitals. To our knowledge, this is the first study that examines the independent and relative effects of these hospital characteristics on rehospitalization.20 The results may serve as benchmarks against which to compare trends in hospitalization over time and may provide insight for development of targeted clinical and policy efforts to reduce acute care use in this population.
METHODS
Study Design and Data Sources
A population-based, retrospective cohort design and two linked data sources were used for this study: (1) the California Department of Public Health California Cancer Registry (CCR), a population-based cancer surveillance system that collects clinical and sociodemographic information about individuals diagnosed with cancer in California21,22; and (2) the California Office of Statewide Health Planning and Development (OSHPD) death-linked patient discharge data (PDD) files that include patient discharge information for all acute-care hospitals licensed in the state of California (with Veterans Administration and military hospitals excluded).23 OSHPD and CCR records were matched with a probabilistic matching software and a combination of patient demographic variables, such as name, social security number, and date of birth.24-26
Sample
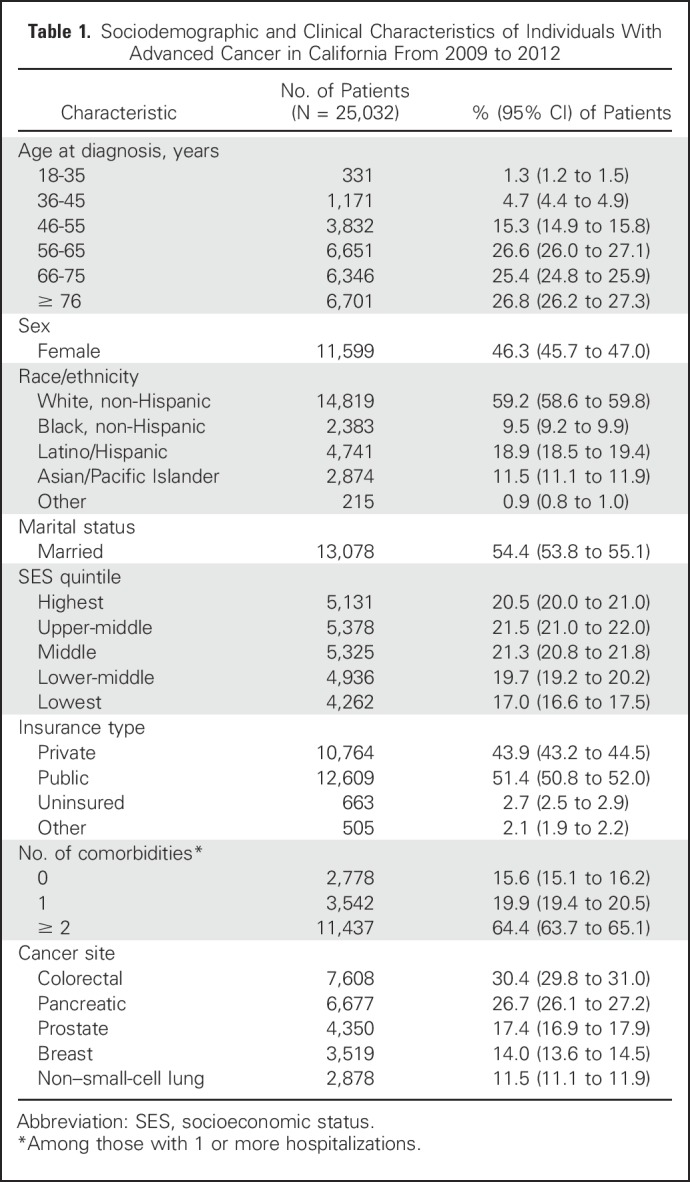
Among individuals with data in the CCR who were diagnosed with cancer between 2009 and 2012, a cohort with advanced breast, prostate, colorectal, non–small-cell lung, or pancreatic cancer (N = 27,477) was identified. Consistent with prior work10,11 and to identify individuals diagnosed with cancer at stages for which treatment is typically palliative,3,4 advanced stage was defined as stage IV for all cancers, stage IIIB for non–small-cell lung cancer (NSCLC), and stage III for pancreatic cancer (Appendix Table A1, online only). Data for individuals younger than 18 years old at diagnosis (n = 3), those who were missing or had duplicate variables needed for OSHPD linkage (n = 2,378), and those for whom date of death was missing or recorded as the same day as diagnosis (n = 58) were excluded. Our final sample included 25,032 adults with advanced cancer who met the stated criteria.
Outcomes and Variable Definitions
Any inpatient acute care admission that began on or after the day of cancer diagnosis to 365 days after diagnosis was included as a hospitalization. All hospitalizations, rather than a subset of potentially avoidable or unplanned hospitalizations, were included in the analysis for several reasons. Inpatient care drives regional variation in costs for advanced cancer care, which suggests that health system factors or local practice patterns, rather than clinical need alone, may drive care provision to some extent.10 In addition, planned hospitalizations for individuals with advanced cancer may be considered potentially avoidable in cases for which improved communication about prognosis, advanced care planning, and palliative care or hospice interventions lead patients to choose less aggressive treatment options.27
Covariates included the following: age, sex, race/ethnicity, insurance status, marital status, area-based socioeconomic status quintile (SES),28 and comorbidities (Table 1). Hospital characteristics, derived from the 2012 OSHPD Hospital Annual Utilization Report, included the following: hospital size, ownership type, presence of a teaching program, and presence of an outpatient palliative care program (described in the Appendix, online only).
Table 1.
Sociodemographic and Clinical Characteristics of Individuals With Advanced Cancer in California From 2009 to 2012
Statistical Analysis
Analyses were conducted with Stata 14 MP (STATA, College Station, TX). Descriptive statistics were calculated for all variables, and statistical significance was set at P < .05.
The mean and median number of hospitalizations were examined, and the proportions of patients who had cancer with any hospitalization, those with three or more hospitalizations, and those who died within the year after diagnosis were calculated. With the hospitalization as the unit of analysis, the mean and median length of stay were calculated, and the proportion of hospitalizations that originated in the emergency department (ED) was calculated. To account for clustering by hospital in these descriptive analyses, 95% CIs were calculated with the Stata svy command, and the hospital identifier was designated as the primary sampling unit.
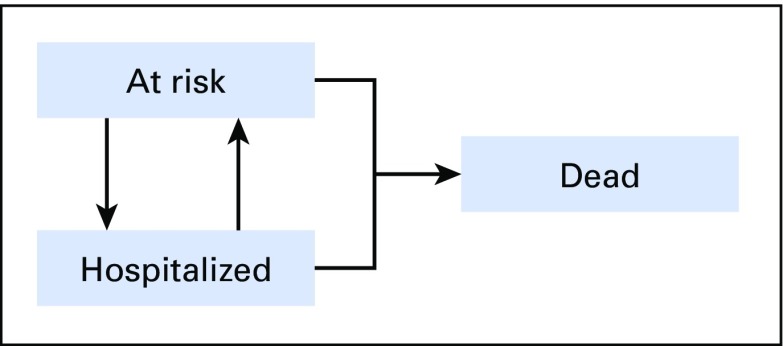
Multistate modeling captures multiple changes in the status of a patient during a period of time, and it includes both absorbing events (eg, death), and events that are not absorbing but that may occur multiple times per patient (eg, hospitalization).29 Because many individuals with cancer die or spend prolonged periods in the hospital, the hospitalization incidence rates that accounted for the duration each individual was at risk for being hospitalized within the observation period were estimated. To this end, a person-period data set was created by partitioning the follow-up data for each person into nonoverlapping periods, and each person-period was classified into one of three states in a multistate model: (1) at risk (alive, not hospitalized, and therefore at risk for hospitalization), (2) hospitalized (alive, but temporarily not at risk for hospitalization), or (3) dead (not at risk for hospitalization for the remainder of the observation period). Patients could transition in and out of the hospitalized or at-risk states until they either transitioned to the dead state or came to the end of the 365-day period after diagnosis (Fig 1).
Fig 1.
Multistate model to describe all possible states and between-state transitions.
The hospitalization incidence rate (ie, transition from the at-risk state to the hospitalized state) was modeled with a log-linear Poisson regression model for clustered data. Postestimation predictive margins were calculated to estimate the predicted age-adjusted hospitalization rate per year at risk for each cancer type.
In the subset of individuals with at least one hospital discharge, predictors of rehospitalization were modeled with a similar approach, but data were restricted to the at-risk person-periods after the initial hospital discharge of a patient. IRRs were modeled as functions of sociodemographic and hospital-level covariates by using a mixed-effects log-linear Poisson regression model with random intercepts for the patient and for the hospital to adjust for cluster effects. Each individual could have multiple at-risk person-periods for rehospitalization, and the values for hospital-level covariates in each person-period were based on the discharging hospital for the immediately preceding hospitalization. Sensitivity analyses were conducted with the subset of rehospitalizations that originated in the ED. (Details of the methodologic approach and analysis are in the Appendix.)
RESULTS
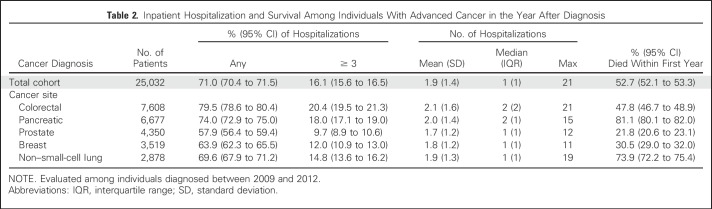
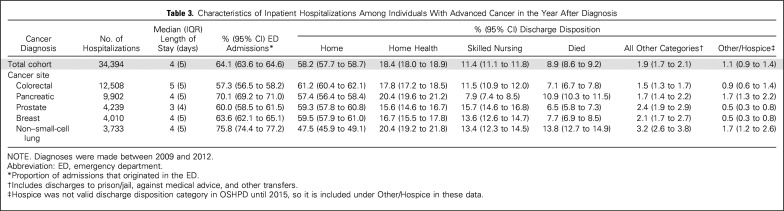
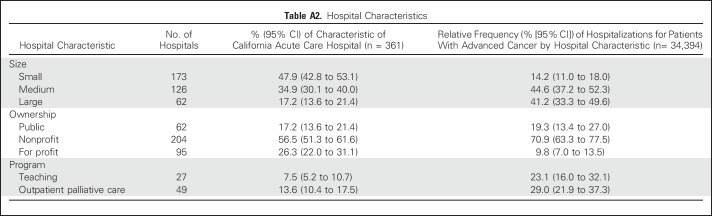
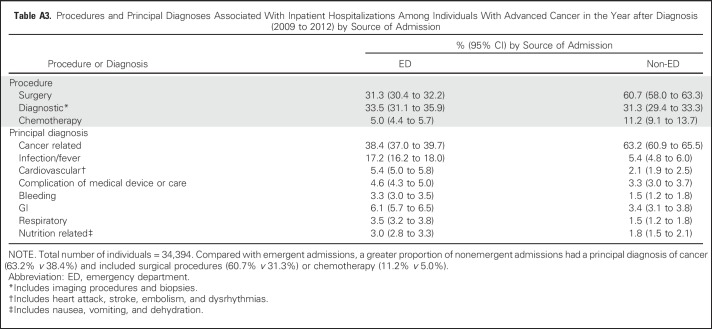
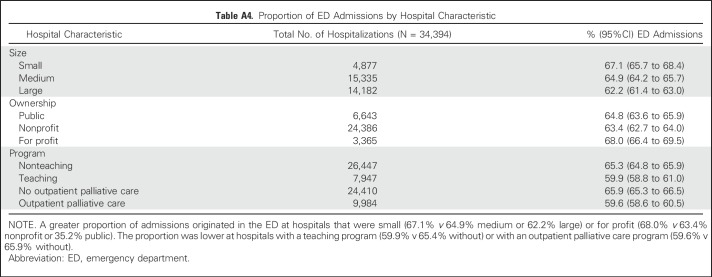
Individual sociodemographic and clinical characteristics are listed in Table 1, and hospital characteristics are listed in Appendix Table A2 (online only). In the year after diagnosis, 71.0% of individuals with advanced cancer had at least one hospitalization, and 16.1% had three or more. More than half (52.7%) died within the year (from 21.8% as a result of prostate cancer to 81.1% as a result of pancreatic cancer; Table 2). In the year after diagnosis, individuals with advanced cancer had 34,394 hospitalizations at 361 acute care hospitals in California. Across all hospitalizations, 64.1% originated in the ED (from 57.3% as a result of colorectal cancer to 75.8% as a result of NSCLC), and 58.2% were discharged to home (with only 1.1% discharged to the category that included hospice care; Table 3).Compared with admissions that originated in the ED, a greater proportion of non-ED admissions had a principal diagnosis of cancer (63.2% v 38.4%) and included surgical procedures (60.7% v 31.3%) or chemotherapy (11.2% v 5.0%; Appendix Table A3, online only). The proportion of admissions that originated in the ED was greater at for-profit hospitals (68.0% v 63.4% nonprofit or 35.2% public), and lower at hospitals that reported outpatient palliative care programs (59.6% v 65.9% without; Appendix Table A4, online only).
Table 2.
Inpatient Hospitalization and Survival Among Individuals With Advanced Cancer in the Year After Diagnosis
Table 3.
Characteristics of Inpatient Hospitalizations Among Individuals With Advanced Cancer in the Year After Diagnosis
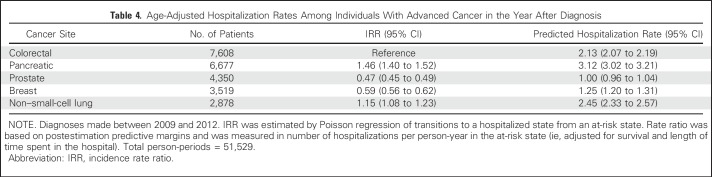
Age-adjusted hospitalization incidence rates (per person-year at risk) from postestimation predictive margins were highest for pancreatic cancer (3.12 predicted hospitalizations; 95% CI, 3.02 to 3.21) followed by NSCLC (2.45; 95% CI, 2.33 to 2.57), colorectal cancer (2.13; 95% CI, 2.01 to 2.19), breast cancer (1.25; 95% CI, 1.20 to 1.31), and prostate cancer (1.00; 95% CI, 0.96 to 1.54; Table 4.).
Table 4.
Age-Adjusted Hospitalization Rates Among Individuals With Advanced Cancer in the Year After Diagnosis
In the multistate model of rehospitalization, the incidence rate ratios (IRRs) describe relative rates. For example, women had an 8% lower rate of rehospitalization (IRR, 0.92; 95% CI, 0.86 to 0.97) than men given comparable time at risk (Table 5). Individual predictors associated with significantly higher rates included one comorbidity (IRR, 1.13; 95% CI, 1.04 to 1.23) or two or more comorbidities (IRR, 1.59; 95% CI, 1.47 to 1.73); black non-Hispanic race/ethnicity (IRR, 1.29; 95% CI, 1.17 to 1.42) or Hispanic race/ethnicity (IRR, 1.11; 95% CI, 1.03 to 1.20); and public insurance (IRR, 1.37; 95% CI, 1.23 to 1.46) or no insurance (IRR, 1.17; 95% CI, 1.02 to 1.35). There was a dose-response relationship for both age and SES with rehospitalization. The highest rehospitalization rates were seen in the youngest age group (18-35 years; IRR, 2.07; 95% CI, 1.71 to 2.51) and in the lowest SES quintile (IRR, 1.29; 95% CI, 1.18 to 1.42). Compared with colorectal cancer, pancreatic cancer (IRR, 2.07; 95% CI, 1.95 to 2.20) and NSCLC (IRR, 1.69; 95% CI, 1.54 to 1.86) were associated with higher rates; prostate cancer (IRR, 0.51; 95% CI, 0.46 to 0.57) and breast cancer (IRR, 0.72; 95% CI, 0.65 to 0.78) were associated with lower rates.
Table 5.
Multistate Poisson Regression of Rehospitalizations Among Individuals With Advanced Cancer in the Year After Diagnosis
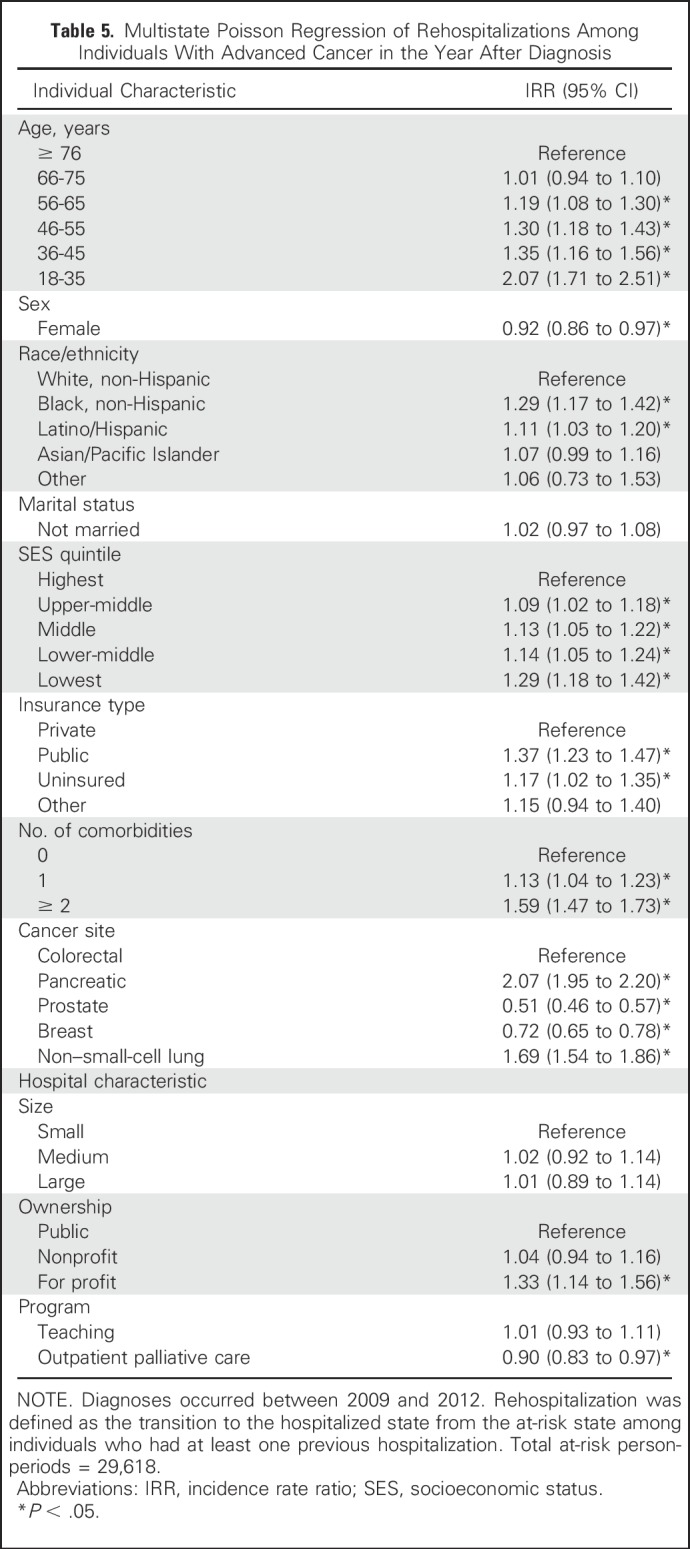
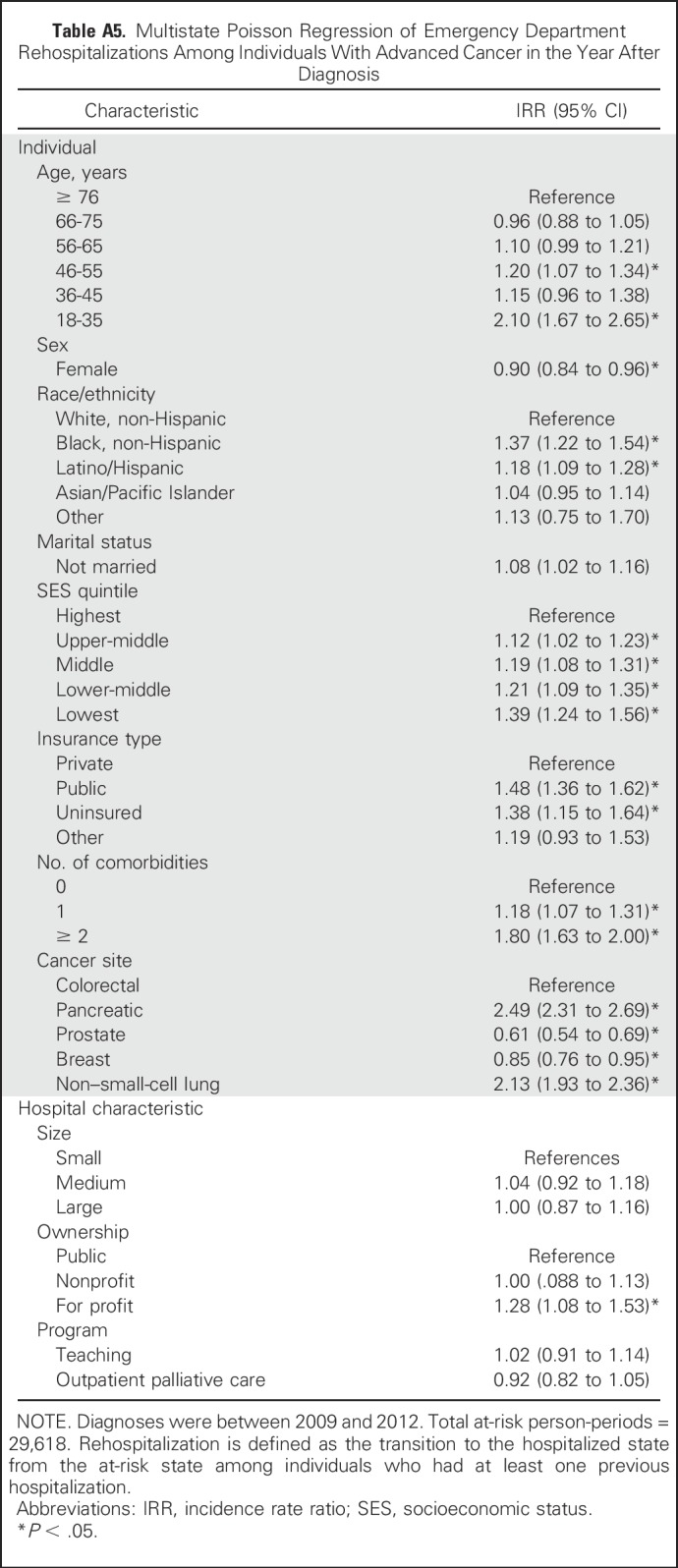
Hospital size and teaching status were not significantly associated with rehospitalization; however, discharge from a for-profit hospital was associated with a 33% increase in the rehospitalization rate (IRR, 1.33; 95% CI, 1.14 to 1.56) compared with discharge from a public hospital, and discharge from a hospital that reported an outpatient palliative care program was associated with an 10% reduction in the rehospitalization rate (IRR, 0.90; 95% CI, 0.83 to 0.97; Table 5). In a sensitivity analysis of hospitalizations that originated in the ED, these effects were attenuated (Appendix Table A5, online only).
DISCUSSION
In this population-based sample of individuals with advanced cancer, we found a heavy burden of hospitalization in the year after diagnosis; 71% had at least one hospitalization, and 16% experienced three or more hospitalizations. Importantly, a large proportion of hospitalizations (64.1%) originated in the ED, which suggests that they were not part of the planned trajectory of care. Studies of end-of-life (EOL) care intensity have found a similarly high prevalence of hospitalization in the last weeks of life (eg, 61.3% to 64.9%).20 The multistate modeling results demonstrated that hospitalization rates were even higher when modeling accounted for time at risk. For example, although individuals with pancreatic cancer had an average of two hospitalizations, the predicted rate was 3.11 hospitalizations per person-year at risk.
Sociodemographic characteristics associated with rehospitalization in this study were largely consistent with those reported elsewhere. For instance, women had lower odds of hospitalization and receipt of aggressive care in several EOL care studies, whereas individuals who reported a black race/ethnicity, a lower SES, and comorbidities had higher odds.28-32
Our finding that increased age was associated with lower rehospitalization rates was consistent with results of a Canadian study on EOL care for advanced pancreatic cancer33 and a US study of Medicare beneficiaries with poor-prognosis cancers in the last 6 months of life.28 However, this finding was inconsistent with one study, in which increased age was associated with more aggressive EOL care.31 Our multistate modeling approach fully accounted for time during which individuals were not at risk for hospitalization (because of either death or time spent in the hospital). Therefore, lower hospitalization rates in older age groups were not explained by differences in mortality and may reflect less aggressive treatment and/or earlier use of palliative care and hospice services among older individuals.
Individuals with prostate cancer experienced the lowest hospitalization rates in our study, whereas a previous decedent study suggested that this group had higher odds of aggressive EOL care than persons with other cancer types.31 This discrepancy may be due to differences in study design and outcome measures (ie, we examined health service use after diagnosis rather than EOL care specifically, and we analyzed only hospitalization rates, not other measures of aggressive care). Many individuals with prostate cancer in this study survived beyond the study period; we may have found evidence of more intensive health service use comparable to that of individuals with more aggressive cancers had we examined hospitalizations in the period that immediately preceded death.
We found that hospital characteristics were associated with rehospitalization; specifically, discharge from a for-profit hospital was associated with a 33% higher rehospitalization rate, whereas discharge from a hospital that reported an outpatient palliative care program was associated with a 10% reduction. Other work has found, similarly, that patients treated at for-profit hospitals receive higher-intensity EOL care20 and are more likely to be admitted from the ED.34 For-profit hospitals are highly responsive to market changes in profitability. Therefore, financial incentives to provide care in the inpatient setting may partly contribute to these findings in some contexts.35 The patient mix at for-profit hospitals is generally comparable to or healthier than that at nonprofit hospitals, perhaps because of more favorable market selection.36,37 Our analysis adjusted for sociodemographic characteristics and comorbidities, which suggests that differences in patient characteristics and illness severity did not confound these results. However, additional research is needed to better understand how and why hospital ownership might affect hospitalization rates.
Our finding that rehospitalization rates were lower after discharge from a hospital with an outpatient palliative care program adds to a growing body of evidence that palliative care may reduce acute care use.38-40 Outpatient access to such services may be particularly important, because receipt of community-based palliative care, residence in areas with greater hospice density, and hospital discharge with home hospice have been associated with lower EOL care intensity and readmission risk.18,31,38,41 However, one study reported that EOL care remained intensive despite use of hospice services,42 and more information is needed about the role that the timing and specific components of palliative care interventions play in the improvement of EOL care and the reduction of acute care use. In the sensitivity analysis (Appendix Table A5), the effect of treatment received at a hospital with outpatient palliative care was attenuated for ED rehospitalizations, which suggests the need for additional research on the role of palliative care in reducing planned versus emergent hospitalization (eg, through advance-care planning and prognostic communication v symptom management).
Study limitations include the observational design and use of administrative data, which limited the availability of clinical details (eg, patient preference for more aggressive treatment) needed to understand the preventability of hospitalizations. Congruence with patient preference is important in the assessment of quality of care in advanced cancer. However, prior work has suggested that patient preference does not explain regional variability in EOL care intensity.43 It is likely that many hospitalizations included in our analysis were clinically appropriate and beneficial to individuals with advanced cancer. The advanced cancer sites included in this study were diverse in terms of treatment modalities and expected disease trajectories; however, these findings were not meant to guide treatment decisions but rather to illustrate hospitalization patterns. We examined hospitalization in the year after diagnosis in light of recent recommendations for palliative care from the time of diagnosis. However, many individuals would be expected to pursue curative treatment options that result in hospitalization during this timeframe. The extent to which palliative care affects outcomes in the year after diagnosis, as opposed to the period that precedes death, is unclear and warrants future investigation. In addition, the hospital-reported variable that affirmed the presence of an outpatient palliative care program did not include any specific program elements, nor could we ascertain whether an individual received these services. This study was limited to individuals who were diagnosed with cancer, received care in California, and had valid social security numbers available for linkage with the OSHPD databases. As a result, we may have underestimated the burden of hospitalization for minority racial/ethnic groups, individuals of lower SES,44 and individuals who were diagnosed in California but received care in other states. Although we used the most recently available CCR data, these data were collected before full implementation of the Affordable Care Act, which may have influenced access to care and the resulting patterns of use.
In conclusion, this is the first study, to our knowledge, to use multistate models to estimate hospitalization rates and to identify individual and hospital predictors of rehospitalization in a population-based sample of individuals with advanced cancer while fully accounting for time at risk (ie, survival and time spent out of hospital). Our analysis suggests that traditional methods to estimate the incidence of hospitalization may underestimate the burden of hospitalization for individuals with advanced cancer. Future efforts to reduce avoidable hospitalizations might focus on subgroups at higher risk, including individuals with advanced pancreatic, lung, or colorectal cancer, younger age at diagnosis, public insurance, and multiple comorbidities, as well as patients who identify themselves as men and as black or Hispanic. Policy efforts might include improvements in access to outpatient palliative care and tests of payment models that reduce financial incentives to provide care in the inpatient setting.
ACKNOWLEDGMENT
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors. We thank Theodore Wun, MD, FACP, and Ann Brunson, MS, of the Center for Oncology Hematology Outcomes Research and Training (COHORT), Division of Hematology Oncology, UC Davis School of Medicine, for assistance with data acquisition for this study.
Appendix
Hospitalization
Any inpatient acute care admission that began on or after the day of cancer diagnosis to 365 days after diagnosis was included as a hospitalization. Multiple admission records for an individual patient within an overlapping timeframe (ie, hospital transfers) were considered a single episode, and information about that visit was obtained from the record associated with the discharging hospital. Death dates recorded in the California Cancer Registry (CCR) were used to obtain complete information about death dates for individuals never admitted to the hospital. When death dates in the CCR were missing, the date of death from Office of Statewide Health Planning and Development (OSHPD) files (linked with death records) was used.
Emergent hospitalization was defined as an inpatient hospitalization that originated in the emergency department of the admitting hospital, as documented in the source of admission–route variable in the OSHPD inpatient discharge data.
Covariates
Individual covariates (unless noted) were derived from CCR data and included the following: age (in years); sex (male as reference); race/ethnicity (black non-Hispanic, Latino/Hispanic, Asian/Pacific Islander, other, or white non-Hispanic as reference); insurance status (uninsured, public [Medicare or Medicaid], other, or private as reference); marital status (not married [divorced, single, widowed, separated], or married as reference); and socioeconomic status (SES) quintiles (lowest, lower-middle, middle, upper-middle, or highest as reference). SES quintiles were derived from the Yang SES index in the CCR. The Yang SES index is an area-based composite measure that uses census block and tract-level socioeconomic indicators from the American Community Survey.28a Comorbidities were assessed with the data from the earliest hospital visit in the OSHPD inpatient discharge data file and were tabulated by using International Classification of Diseases (ninth revision) clinical modification codes according to the categories developed by Elixhauser, et al (Med Care 36:8-27, 1998; with exclusion of principal diagnosis and cancer-related secondary diagnoses) according to the number of comorbidities present (≥ 2, 1, or 0 as reference).
Hospital covariates were derived from the OSHPD Hospital Annual Utilization Report from 2012 and included the following: hospital size (small [< 170 licensed beds], medium [171-379 licensed beds], or large [≥ 380 licensed beds] as reference); ownership type (nonprofit, for profit [any investor type], or public [state, county, University of California] as reference); teaching program (yes, or no as reference); and outpatient palliative care program (yes, or no as reference), defined by hospital-reported response to the question “Did your hospital have outpatient palliative care services during the report period?”
Modeling Approach and Statistical Analysis
Multistate modeling captures multiple changes in the status of a patient across a period of time, and it includes both absorbing events (eg, death) and events that are not absorbing but that may occur multiple times per patient (eg, hospitalization).29 This model posits three states for patients with cancer in the year after diagnosis: (1) at risk for hospitalization (alive, not hospitalized), (2) hospitalized (alive, but temporarily not at risk for hospitalization), or (3) dead. Patient can transition in and out of the hospitalized or at-risk states until either they transition to the dead state (Fig 1) or the end of the 365-day period after cancer diagnosis.
The incidence of transitions among the states of this model can be described by transition-intensity probability models, which can be defined in terms of transition-intensity functions, analogous to hazard functions in a two-state model (ie, survival analysis). The model assumes that each transition-intensity function has distinct parameters, which simplifies the estimation procedure by permitting estimation of the at-risk to hospitalized transition-intensity model separately for a given application (Kalbfleisch JD, Prentice RL: Hoboken, NJ, J Wiley, 2002). A multistate model was used in two separate applications.
In the first application, age-adjusted transitions from the at-risk to the hospitalized state were modeled by using a parametric constant hazards regression model; the natural logarithm (log) hazard for patient i was (i = 1,…, n). The model was as follows:
log hi (t) = B0 + B1 × agei + B2 × site_pancreatici + B3 × site_prostatei + B4 × site_breasti + B5 × site_non–small-cell lungi
for all t ≥ 0.
In this model, age is the patient age in years, and the four cancer site variables are dummy variables that correspond to use of reference cell coding; colorectal cancer is the reference group. As shown by Holford (Biometrics 36:299-305, 1980) and Laird and Olivier (J Am Stat Assoc 76:231-240, 1981), the maximum likelihood parameter estimates for this regression model can be obtained by applying the standard log-linear Poisson regression model to a person-period data set that consists of periods in which the person is at-risk for the event of interest, the outcome variable is a binary indicator for whether the event occurred in the person-period, and the logarithm of the length of the person-period is included as an offset term in the regression model. This approach was followed to estimate the first model. The regression coefficients, when exponentiated, have two interpretations, both as adjusted incidence intensity ratios (ie, adjusted hazards ratios) and as incidence rate ratios.
In the second application of the model, rehospitalizations were modeled. For the regression specification, additional covariates and random effects were included, but the model otherwise followed the same log-linear Poisson regression methodology. For this analysis, the relevant at-risk person-periods to include from each patient were all those that followed that first hospitalization discharge of the patient in the study time frame. The regression model was augmented with the covariates and random effects (described in the Methods) and was estimated by using mixed-effects Poisson regression with robust standard errors.
Table A1.
Advanced Cancer Groups by SEER Site and Stage Recodes
Table A2.
Hospital Characteristics
Table A3.
Procedures and Principal Diagnoses Associated With Inpatient Hospitalizations Among Individuals With Advanced Cancer in the Year after Diagnosis (2009 to 2012) by Source of Admission
Table A4.
Proportion of ED Admissions by Hospital Characteristic
Table A5.
Multistate Poisson Regression of Emergency Department Rehospitalizations Among Individuals With Advanced Cancer in the Year After Diagnosis
Footnotes
Supported by scholarships from the Gordon and Betty Moore Foundation, the Jonas Center for Nursing and Veteran’s Healthcare, and the Oncology Nursing Society (all to R.L.W.).
Presented in part at the ASCO Palliative Care in Oncology Symposium, San Francisco, CA, September 9-10, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Robin L. Whitney, Janice F. Bell
Data analysis and interpretation: Robin L. Whitney, Janice F. Bell, Daniel J. Tancredi, Patrick S. Romano, Jill G. Joseph
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hospitalization Rates and Predictors of Rehospitalization Among Individuals With Advanced Cancer in the Year After Diagnosis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Robin L. Whitney
No relationship to disclose
Janice F. Bell
No relationship to disclose
Daniel J. Tancredi
Research Funding: Merck (Inst)
Patrick S. Romano
No relationship to disclose
Richard J. Bold
No relationship to disclose
Jill G. Joseph
No relationship to disclose
REFERENCES
- 1. Health Care Utilization Project: Statistical Brief #125: Cancer hospitalizations for adults. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb125.pdf.
- 2.Manzano JGM, Luo R, Elting LS, et al. : Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J Clin Oncol 32:3527-3533, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppercorn JM, Smith TJ, Helft PR, et al. :American Society of Clinical Oncology statement: Toward individualized care for patients with advanced cancer. J Clin Oncol 29:755-760, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Kato I, Severson RK, Schwartz AG: Conditional median survival of patients with advanced carcinoma: Surveillance, epidemiology, and end results data. Cancer 92:2211-2219, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brooks GA, Cronin AM, Uno H, et al. : Intensity of medical interventions between diagnosis and death in patients with advanced lung and colorectal cancer: A CanCORS analysis. J Palliat Med 19:42-50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 21:1133-1138, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Wright AA, Keating NL, Ayanian JZ, et al. : Family perspectives on aggressive cancer care near the end of life. JAMA 315:284-292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren JL, Yabroff KR, Meekins A, et al. : Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst 100:888-897, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabroff KR, Lund J, Kepka D, et al. : Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiol Biomarkers Prev 20:2006-2014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks GA, Li L, Uno H, et al. : Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff (Millwood) 33:1793-1800, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks GA, Li L, Sharma DB, et al. : Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst 105:634-642, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clough JD, Patel K, Riley GF, et al. : Wide variation in payments for Medicare beneficiary oncology services suggests room for practice-level improvement. Health Aff (Millwood) 34:601-608, 2015 [DOI] [PubMed] [Google Scholar]
- 13. Greer JA, Jackson VA, Jacobsen JC, et al: Early palliative care for patients with advanced cancer, in Vranceanu AM, Greer JA, Safren SA (eds): The Massachusetts General Hospital Handbook of Behavioral Medicine: A Clinician’s Guide to Evidence-based Psychosocial Interventions for Individuals with Medical Illness. New York, NY, Humana Press, 2017, pp 277-296. [Google Scholar]
- 14.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 35:96-112, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Meier DE: Increased access to palliative care and hospice services: Opportunities to improve value in health care. Milbank Q 89:343-380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui D, Elsayem A, De la Cruz M, et al. : Availability and integration of palliative care at US cancer centers. JAMA 303:1054-1061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumanovsky T, Augustin R, Rogers M, et al. : The growth of palliative care in US hospitals: A status report. J Palliat Med 19:8-15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Institute of Medicine: Dying in America—Improving quality and honoring individual preferences near the end of life. Washington, DC, National Academies Press, 2014. [PubMed]
- 19. Center to Advance Palliative Care: Report card: Key findings. https://reportcard.capc.org/findings/
- 20.Morden NE, Chang C-H, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 31:786-796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.California Cancer Registry : Overview. http://www.ccrcal.org/Inside_CCR/FAQ.shtml
- 22.California Department of Public Health : https://archive.cdph.ca.gov/programs/ccr/Pages/default.aspx
- 23.Office of Statewide Health Planning and Development : Patient discharge data. https://www.oshpd.ca.gov/HID/Patient-Discharge-Data.html
- 24.California Cancer Registry : Data linkage procedures. http://www.ccrcal.org/Data_and_Statistics/Cancer_Data_for_Research.shtml
- 25.Zingmond DS, Ye Z, Ettner SL, et al. : Linking hospital discharge and death records: Accuracy and sources of bias. J Clin Epidemiol 57:21-29, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Chew HK, Wun T, Harvey D, et al. : Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 166:458-464, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Meisenberg BR, Hahn E, Binner M, et al. : ReCAP: Insights into the potential preventability of oncology readmissions. J Oncol Pract 12:153-154, e149-e156, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Miesfeldt S, Murray K, Lucas L, et al. : Association of age, gender, and race with intensity of end-of-life care for Medicare beneficiaries with cancer. J Palliat Med 15:548-554, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Yang J, Schupp C, Harrati A, et al. : Developing an area-based socioeconomic measure from American Community Survey data. Cancer Prevention Institute of California, Fremont, California, 2014 [Google Scholar]
- 29.Andersen PK, Keiding N: Multi-state models for event history analysis. Stat Methods Med Res 11:91-115, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kurtz ME, Kurtz JC, Given CW, et al. : Predictors of use of health care services among elderly lung cancer patients: The first year after diagnosis. Support Care Cancer 14:243-250, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Henson LA, Gomes B, Koffman J, et al. : Factors associated with aggressive end of life cancer care. Support Care Cancer 24:1079-1089, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legler A, Bradley EH, Carlson MD: The effect of comorbidity burden on health care utilization for patients with cancer using hospice. J Palliat Med 14:751-756, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang RW, Krzyzanowska MK, Zimmermann C, et al. : Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst 107: ju424, 2015 [DOI] [PubMed] [Google Scholar]
- 34. Carlos MB: Hospital ownership and admission through the ED. https://appam.confex.com/appam/sc17dc/webprogram/Paper19922.html. [Google Scholar]
- 35.Horwitz JR: Making profits and providing care: Comparing nonprofit, for-profit, and government hospitals. Health Aff (Millwood) 24:790-801, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger M, Gray BH: How nonprofits matter in American medicine, and what to do about it. Health Aff (Millwood) 25:W287-W303, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Shen Y-C: The effect of hospital ownership choice on patient outcomes after treatment for acute myocardial infarction. J Health Econ 21:901-922, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Goldenheim A, Oates D, Parker V, et al. : Rehospitalization of older adults discharged to home hospice care. J Palliat Med 17:841-844, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlson MD, Herrin J, Du Q, et al. : Impact of hospice disenrollment on health care use and Medicare expenditures for patients with cancer. J Clin Oncol 28:4371-4375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Earle CC, Neville BA, Landrum MB, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Wright AA, Hatfield LA, Earle CC, et al. : End-of-life care for older patients with ovarian cancer is intensive despite high rates of hospice use. J Clin Oncol 32:3534-3539, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnato AE, Herndon MB, Anthony DL, et al. : Are regional variations in end-of-life care intensity explained by patient preferences? A study of the US Medicare population. Med Care 45:386-393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohensky MA, Jolley D, Sundararajan V, et al. : Data linkage: A powerful research tool with potential problems. BMC Health Serv Res 10:346, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]