Abstract
Introduction:
Hepatocellular carcinoma (HCC) is a poor-prognosis cancer with a high symptom burden. Multidisciplinary HCC care is complex and unique in cancer medicine. We sought to determine whether the distinct process affects hospice use and how hospice affects end-of-life acute care utilization.
Patients and Methods:
Patients dying after HCC diagnosed from 2004 to 2011 were identified within SEER-Medicare. Hospice use and associated factors were described using logistic regression. Coarse exact and propensity score matching created groups of hospice and nonhospice comparators balanced on clinical characteristics. Health care use from first hospice claim to death and the matched duration in the nonhospice group were compared.
Results:
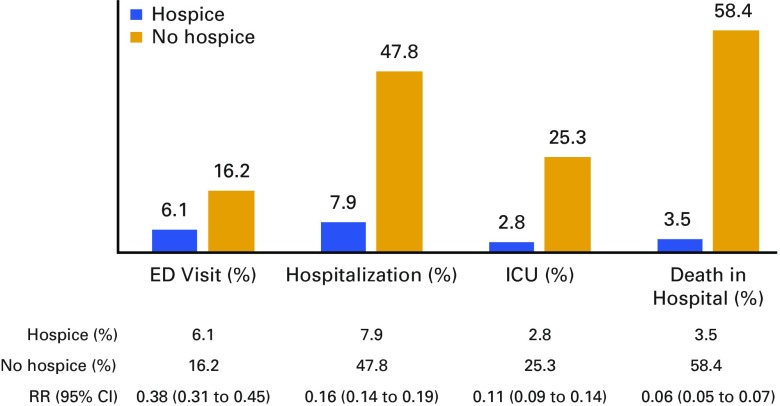
Of 7,992 decedent patients with HCC, 63% used hospice before death, with a median duration of 18 days (interquartile range, 5-51 days). Initial treatment with surgery and ablation (odds ratio [OR], 0.63; 95% CI, 0.53 to 0.74) or chemoembolization/radioembolization (OR, 0.71; 95% CI, 0.62 to 0.80) was associated with decreased odds of subsequent hospice use compared with untreated patients. Hospice use was more likely in those consulting hematology/oncology (OR, 1.33; 95% CI, 1.13 to 1.56) but not in those consulting gastroenterology (OR, 0.79; 95% CI, 0.65 to 0.95). Hospice patients had lower rates of hospitalization (7.9% v 47.8%; risk ratio [RR], 0.16; 95% CI, 0.14 to 0.19), intensive care unit stay (2.8% v 25.3%; RR, 0.11; 95% CI, 0.09 to 0.14), and in-hospital death (3.5% v 58.4%; RR, 0.06; 95% CI, 0.05 to 0.07).
Conclusion:
Processes of care influence which patients with HCC are referred to hospice. Hospice use has a marked effect on acute care use at the end of life in patients with HCC. Efforts to incorporate cancer-focused palliative care might improve the quality of end-of-life care in HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major cause of morbidity and mortality worldwide.1 In the United States, primary liver cancer—of which HCC is the predominant histopathology—ranks fifth in causes of cancer-related mortality.2 HCC is a particularly difficult cancer to treat because patients frequently present with advanced disease, and the nearly universal presence of comorbid cirrhosis among patients with HCC markedly limits treatment options.3 As such, for most patients, the diagnosis of HCC is a terminal one.
The offer of referral to expert-level palliative care and hospice are recognized by the Institute of Medicine as key components of quality end-of-life care.4 Hospice use in the United States has increased over the past few decades, such that approximately 60% of patients with terminal cancers enroll before death.5 Hospice participants have lower rates of hospitalization, intensive care unit (ICU) stays, and in-hospital death.5
For patients with HCC who often experience a high symptom burden related to cancer (eg, pain, anorexia, fatigue) and end-stage liver disease (ESLD; eg, ascites, hepatic encephalopathy, muscle cramps), such palliative referrals are likely paramount. However, HCC care is complex in a number of ways, which may present barriers to hospice referral. First, the prognosis of patients with HCC is determined just as much by the extent of cirrhosis as by the extent of cancer.6 Furthermore, although patients with ESLD have a high symptom burden that might benefit from hospice,7 it is challenging to determine which patients with ESLD will die within the 6-month hospice requirement.8,9 Second, liver transplantation overshadows the care of patients with ESLD and HCC.9 With survival among patients with HCC who underwent transplantation as good as that among patients who underwent transplantation for other indications,10 and as evidence emerges that patients with increasingly extensive cancers can be downstaged, undergo transplantation, and cured,11 it may be difficult for patients and providers to balance the reality of what will likely be a terminal disease with the possibility of curative transplantation. Finally, the process of care for HCC is unique in cancer medicine in that unlike most cancers where consultation is frequently undertaken with a medical or radiation oncologist after diagnosis, HCC treatment is often overseen by a hepatologist in conjunction with transplant surgery and interventional radiology, with varying involvement of medical oncology. Therefore, although these unique multidisciplinary HCC teams are unequivocally essential for optimal outcomes in HCC,12-14 they bring a different perspective to the care of HCC than do the multidisciplinary teams who care for patients diagnosed with other cancers.
In light of the unique process of HCC care combined with the exceptional burden of disease experienced by patients, we sought to evaluate the extent to which hospice services are used at the end of life in patients with HCC, what factors determine hospice use, and whether those enrolled in hospice were any less likely to require acute care services at the end of life.
PATIENTS AND METHODS
Patients
The cohort of patients with HCC was derived from the US National Cancer Institute’s SEER-Medicare linkage. The SEER program of cancer registries collects data on incident cancer cases diagnosed within 18 population-based registries, which encompass 28% of the US population.15,16 SEER cases have been linked to Medicare claims to facilitate research on cancer treatment.17
Patients with HCC diagnosed while alive between 2004 and 2011 were identified from SEER-Medicare using SEER code C22.0 and HCC histology codes 8170-8175 and 8180, regardless of reason for Medicare eligibility (eg, ≥ 65 years of age, disability, renal disease). To ensure that complete claims were available for our analyses, only patients with continuous enrollment in Medicare Parts A and B, those with fee-for-service Medicare for the 6 months before and after diagnosis, and those with at least one claim in the year before diagnosis were included. Because the intent of this project was to evaluate health care utilization at the end of life, we restricted this cohort to patients who died of any cause after the incident HCC diagnosis, as has been done by others evaluating end-of-life health care use.5,18 For the primary analysis, patients who died in the month of their diagnosis were excluded because of the possibility that these patients were incidentally found to have HCC during treatment of decompensated cirrhosis, which was the cause of their death. Such patients would be less likely to enroll in hospice and have a high rate of in-hospital death, and thus might be expected to inflate any observed hospice effect. As a sensitivity analysis, however, the entire analysis was also conducted without this restriction.
Covariables and Outcomes
Patient demographics, census tract socioeconomics, and tumor characteristics were derived from SEER. The underlying cause of liver disease and extent of liver comorbidity (defined by complications of cirrhosis: ascites, encephalopathy, varices, peritonitis, hepatorenal syndrome) were defined by the presence of two or more claims with that diagnosis code.19,20 Nonliver comorbidity was defined using the Klabunde modification of the Charlson Comorbidity Index,21 excluding codes from liver disease and cancer. All claims-based covariables were defined in the 12 months before diagnosis. Treatment group was defined by the initial treatment received, which were ascertained from claims after diagnosis as previously described (codes are available from the authors on request).20
Health care system covariables of interest were measured in the 6 months before the start of the hospice utilization window (see Analysis section). Provider specialty was assessed for primary care physicians, gastroenterologists (there is not a specific code for hepatology), and hematology/oncology. Hospital National Cancer Institute (NCI) designation and liver transplant status was determined from the hospital file. Patients seen at more than one hospital were categorized according to the highest level of subspecialty.
Hospice use was defined by at least one claim for hospice services from the time of diagnosis to death. Acute care use was defined by emergency department (ED) visits not leading to an admission, hospitalizations, and ICU stays during the hospice utilization or matched comparison window. The place of death was derived from the discharge destination and skilled nursing facility indicator variables in the Medicare Provider Analysis and Review file.
Analysis
First, we sought to describe the use of hospice in all decedent patients after an HCC diagnosis. To do so, rates of hospice utilization, time from diagnosis to hospice referral, and time from referral to death are presented descriptively. We used multivariable logistic regression to evaluate patient factors associated with a hospice referral among the entire cohort of decedent patients with HCC.
We next sought to (1) evaluate the health care system factors in the time around hospice decision making (eg, in the months before death) associated with hospice referral and (2) the effect of hospice referral on acute care services use. We used coarsened exact matching,5,22 to allow us to compare hospice patients with a comparison group of nonhospice patients with similar illness severity at a similar time course in their illness. To do this, we matched patients according to illness severity at diagnosis by matching on age, stage, use of prediagnosis alpha-fetoprotein screening (which we have previously found to be a strong predictor of survival in Medicare beneficiaries with HCC20,23), and initial treatment. Because there were residual imbalances in some covariables potentially associated with the outcome, we then applied propensity score matching using race, SEER region, and place of birth to generate the propensity score. Finally, we matched on utilization window, defined as the days from first hospice claim to death, such that for each hospice patient, the comparison group patient was alive for at least as long as the corresponding number of days before death. The start of this utilization window was also used as the anchor at which we began evaluating health care system factors predictive of hospice referral.
RESULTS
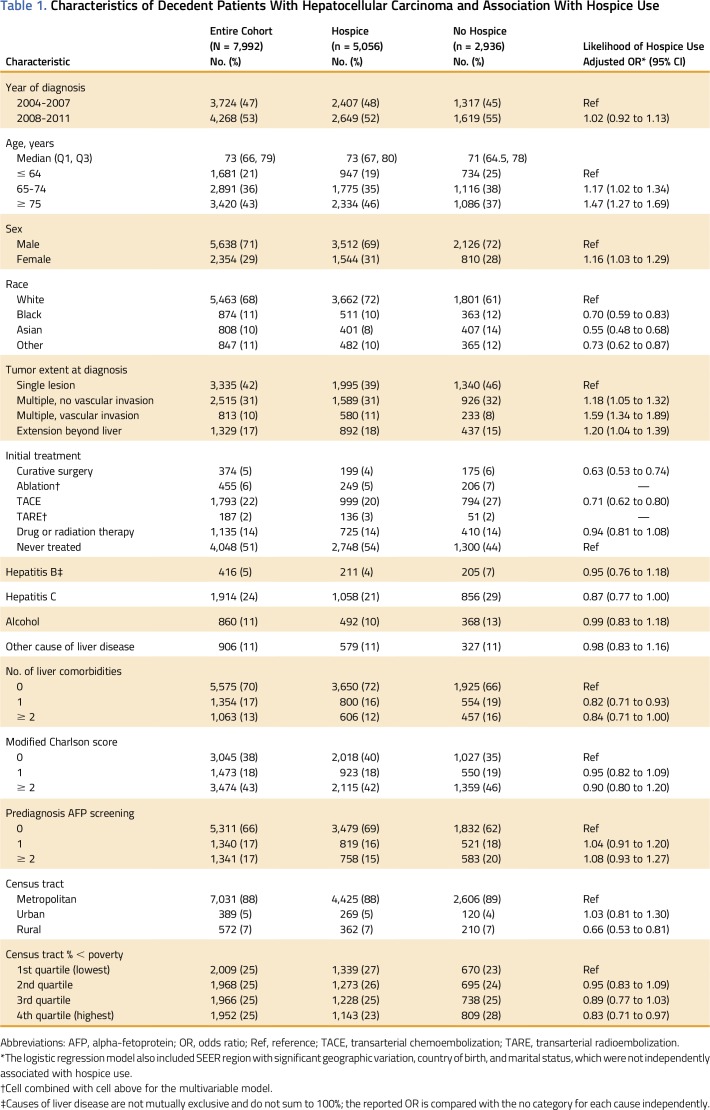
Of 11,130 patients with HCC diagnosed between 2004 and 2011 for whom complete claims were available, 9,656 (87%) died after their HCC diagnosis, 1,664 (15%) of whom died in the month of diagnosis and therefore were excluded from the primary analysis. In the 7,992 patients who survived the month of their diagnosis, the median age was 73 years (interquartile range [IQR], 66-79 years), with 1,681 patients (21%) younger than 65 years (Table 1). Only 374 patients (5%) underwent initial curative surgery (resection or transplantation) and 455 (6%) underwent ablation. Half of patients (4,048; 51%) were never treated for their HCC.
Table 1.
Characteristics of Decedent Patients With Hepatocellular Carcinoma and Association With Hospice Use
One or more claims for hospice were present in 5,056 of all patients (63%) between the time of cancer diagnosis and death. The median time from diagnosis to first hospice claim was 175 days (IQR, 59-500 days). The median time from first hospice claim to death was 18 days (IQR, 5-51 days).
Hospice use was more common in older patients (adjusted odds ratio [aOR] for patients ≥ 75 years of age v those < 65 years of age, 1.47; 95% CI, 1.27 to 1.69), but significantly less likely among nonwhites (aOR for blacks, 0.70; 95% CI, 0.59 to 0.83; aOR for Asians, 0.55; 95% CI, 0.48 to 0.68), as well as in men and those residing in the most rural and the poorest census tracts. A patient’s initial treatment received was significantly associated with subsequent hospice referral: compared with untreated patients, hospice use was less likely in patients treated with initial curative surgery (aOR, 0.63; 95% CI, 0.53 to 0.74) and in those with initial transarterial chemoembolization or radioembolization (aOR, 0.71; 95% CI, 0.62 to 0.80).
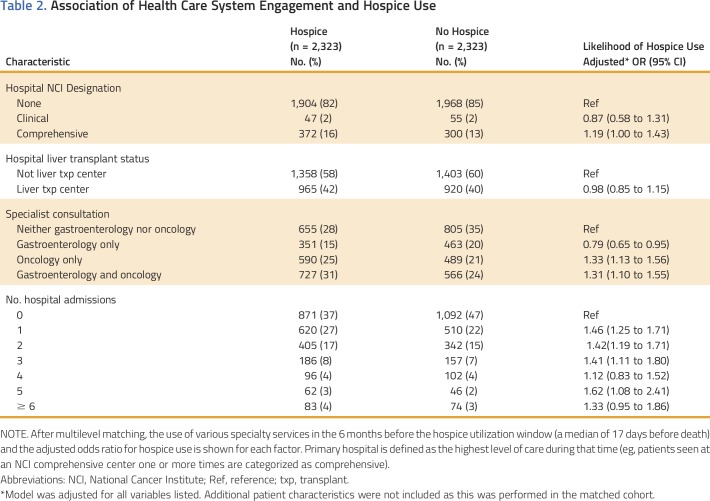
After coarsened exact matching and propensity score matching, 2,323 pairs (4,646 individuals) of hospice patients and comparison group patients were included. These groups were balanced on key covariables; however, because we did not directly match on survival time, the median survival from diagnosis was longer in hospice patients (9 months; IQR, 3-22 months), than comparison group patients (8 months; IQR, 3-21 months). This difference in survival reflects a difference in the time from diagnosis to the start of the hospice (and matched utilization window), which was a median of 229 days for the hospice group and 212 days for the comparison group (Appendix Table A1, online only). In the 6 months leading up to the start of the utilization window, patients referred to hospice were significantly more likely to be seen at an NCI-designated comprehensive cancer center (16% v 13%; aOR, 1.19; 95% CI, 1.00 to 1.43), but not more likely to be seen at a liver transplant center (42% v 40%; aOR, 0.98; 95% CI, 0.85 to 1.15; Table 2). Twenty-eight percent of hospice patients and 35% of comparison group patients did not see a gastroenterologist or hematologist/oncologist in the 6 months before the utilization window. Compared with these patients, consultation with an oncologist alone (aOR, 1.33; 95% CI, 1.13 to 1.56) or in addition to a gastroenterologist (aOR, 1.31; 95% CI, 1.10 to 1.55) was associated with increased odds of hospice referral. Consultation with a gastroenterologist without an oncologist, however, was associated with decreased odds of hospice use (aOR, 0.79; 95% CI, 0.65 to 0.95). Patients requiring multiple hospital admissions (excluding admissions for cancer-directed therapies) before the utilization window were significantly more likely to be referred to hospice.
Table 2.
Association of Health Care System Engagement and Hospice Use
Although the median duration of the exposure window was only 17 days before death, patients with a hospice claim were markedly less likely to use hospital-based acute care. At the end of life, hospice patients had fewer ED visits not resulting in admission (6.1% v 16.2%; risk ratio [RR], 0.38; 95% CI, 0.31 to 0.45), hospitalizations (7.9% v 47.8%; RR, 0.16; 95% CI, 0.14 to 0.19), and ICU stays (2.8% v 25.3%; RR, 0.11; 95% CI, 0.09 to 0.14; Fig 1). Of all hospital admissions, ascites (32% hospice, 44% comparison) and acute kidney failure (21% hospice, 28% comparison) were the most common admitting diagnoses. The following diagnoses were significantly less likely in hospice patient admissions: acute kidney failure (RR, 0.75; 95% CI, 0.61 to 2.17); acute respiratory failure (RR, 0.47; 95% CI, 0.32 to 0.68); sepsis (RR, 0.58; 95% CI, 0.45 to 0.76); and pneumonia (RR, 0.70; 95% CI, 0.50 to 0.98). A similar percentage of admissions had a comorbid severity modifier code for liver cancer, suggesting an equal distribution of active HCC during these admissions. Patients referred to hospice were markedly less likely to die in the hospital or a nursing facility, at 3.5%, than were patients who did not use hospice services, at 58.4% (RR, 0.06; 95% CI, 0.05 to 0.07).
FIG 1.
Health care utilization at the end of life according to hospice use. Percentage and associated risk ratio (RR) of health resource use during the exposure window before death (a median of 17 days before death) is shown for the patients in the matched cohort. ED, emergency department; ICU, intensive care unit.
For a sensitivity analysis, we retained patients dying in the month of their diagnosis in the analysis, which resulted in a sample of 9,656 patients and 2,511 pairs after multilevel matching. Doing so, NCI comprehensive status (OR, 1.11; 95% CI, 0.92 to 1.32) and gastroenterology consultation (OR, 1.03; 95% CI, 0.86 to 1.23) were no longer associated with hospice use. The reduction of acute health care utilization by hospice was not changed: hospice patients were significantly less likely to be hospitalized (7.3% v 44%; RR, 0.16; 95% CI, 0.14 to 0.16); have an ICU stay (2.8% v 22.0%; RR, 0.13; 95% CI, 0.10 to 0.16); and die in the hospital (3.7% v 59.7%; RR, 0.06; 95% CI, 0.05 to 0.08).
DISCUSSION
In this population-based study of Medicare beneficiaries, we found that 63% of patients with HCC used hospice services before death. Although the median time from initial hospice claim to death was only 17 days, hospice use over this short period was still associated with a marked decline in ED visits, hospitalizations, ICU stays, and in-hospital or nursing home deaths. In addition to confirming previously identified differences in use of hospice by sex, race, and rural residence,24 we found that the type of initial treatment received and consultation with an oncologist in the last months of life were strong predictors of hospice use.
Our findings in HCC should be taken in the context of two recently published articles, which thoroughly evaluated health care use (including hospice) and place of death among cancer patients with Medicare dying in the modern era.5,18 The 60% rate of hospice referral, short duration of hospice enrollment, and reduction in acute care services among patients with HCC with Medicare are in line with what was reported in a large cohort of Medicare patients who died in 2011 with poor-prognosis cancers.5 A notable difference in HCC, however, was the marked reduction for in-hospital death between hospice users and the comparison group, with a nearly 60% rate of in-hospital or nursing facility death for those patients not referred to hospice compared with only 3% of hospice users. For comparison, a recent international evaluation of the place of death found that only 26% of deaths among elderly US patients with cancer occur in the hospital or a skilled nursing facility.18
Because of the limitations of this observational data set, we do not have data on the severity of cancer nor the severity of liver disease in the time leading up to death; therefore, we are unable to determine whether patients died of cancer or ESLD. This is an important limitation because determining which patients with ESLD should be considered for hospice referral in the absence of HCC is difficult,8 and we would expect lower rates of hospice use and greater rates of hospitalization among patients dying predominantly from ESLD. An imbalance in ESLD deaths could partially account for the difference in hospitalizations and in-hospital deaths between the hospice and comparison groups, however, after multilevel matching, the hospice and nonhospice comparison groups were balanced on tumor extent at diagnosis and treatment also had similarly short median survivals, which suggests any such imbalance is likely to be small.
We must also consider that many patients with cirrhosis do not receive ongoing care for their liver disease or screening for HCC,25 and these patients may discover their cancer diagnosis at the time of decompensation from ESLD before death. This reality is likely reflected in the large proportion of patients in our cohort before restriction who were diagnosed in the month of their death (13% hospice, 24% nonhospice comparison group). Because such patients are often acutely ill with a high rate of in-hospital mortality,26 we excluded patients dying in the month of their diagnosis from our primary analysis of hospice use. By doing so, we narrowed the scope of our research question to a slightly better prognosis group of patients with HCC. However, in sensitivity analyses in which we retained these patients, the findings were remarkably similar. Thus, the in-hospital death imbalance is not solely attributable to a larger number of critically ill patients with decompensated ESLD too unstable for transfer to home hospice. Rather, we hypothesize that the marked difference in in-hospital deaths between groups might reflect that the considerable symptom burden of patients with advanced liver cancer is too complex for caregivers to manage at home without additional support such as that offered by hospice. Further work to elucidate the needs of patients with ESLD and HCC at the end-of-life—and the needs of their caregivers—is clearly warranted.
We hypothesized that because of the unique characteristics of the treating disciplines and the pivotal role of liver transplantation in HCC, health system characteristics would be associated with hospice use at the end of life. Despite the fact that all patients in this cohort died, patients initially treated with liver-directed therapy (regardless of subsequent treatment) were significantly less likely to be referred to hospice. Given that few of these patients had liver transplantation or resection, and the same was true of both resected patients and patients with transarterial chemoembolization/radioembolization, our finding is unlikely to represent unrelated death in patients cured of their HCC. Rather, we believe this finding supports our hypothesis that the unique process of care in HCC—in which providers whose specialty is not cancer specific take a leading role—does influence hospice referral at the end of life. Furthermore, receipt of care at an NCI-designated cancer center (regardless of provider seen) and consultation with a medical oncologist were strongly associated with hospice referral, whereas patients seen at liver transplant centers were not more likely to be referred to hospice. Further evaluation of how to increase cancer-focused palliative care in the unique multidisciplinary structure of HCC care is warranted.
In summary, we found that patients with HCC enrolled in hospice were less likely to undergo hospitalization or ICU stays at the end of life and markedly less likely to die in the hospital than patients with HCC who were never enrolled in hospice. Given the high cost of in-hospital care, efforts to expand hospice use to a greater proportion of patients with HCC are likely to be cost neutral or even cost saving.
ACKNOWLEDGMENT
Supported by the National Cancer Institute (NCI) of the National Institutes of Health under Award No. K07CA160722 (H.K.S.) and No. K12CA120780 (J.L.L.). Additional support was provided by the Integrated Cancer Information and Surveillance System, University of North Carolina Lineberger Comprehensive Cancer Center, with funding provided by the University Cancer Research Fund via the State of North Carolina. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Table A1.
Characteristics of Patients With Hepatocellular Carcinoma After Multilevel Matching
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Hanna K. Sanoff
Collection and assembly of data: Hanna K. Sanoff, YunKyung Chang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hospice Utilization and Its Effect on Acute Care Needs at the End of Life in Medicare Beneficiaries With Hepatocellular Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Hanna K. Sanoff
Research Funding: Bayer (Inst), Novartis (Inst), Merck (Inst), Precision Biologics (Inst), Immunomedics (Inst)
YunKyung Chang
No relationship to disclose
Melissa Reimers
No relationship to disclose
Jennifer L. Lund
No relationship to disclose
REFERENCES
- 1. Stewart BW, Wild C (eds): World cancer report 2014. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014.
- 2. American Cancer Society: Liver cancer. http://www.cancer.org/cancer/livercancer/
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4. Institute of Medicine: Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. http://www.nationalacademies.org/hmd/Reports/2014/Dying-In-America-Improving-Quality-and-Honoring-Individual-Preferences-Near-the-End-of-Life.aspx. [DOI] [PubMed]
- 5.Obermeyer Z, Makar M, Abujaber S, et al. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312:1888–1896. doi: 10.1001/jama.2014.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 7. Roth K, Lynn J, Zhong Z, et al: Dying with end stage liver disease with cirrhosis: Insights from SUPPORT. J Am Geriatr Soc 48:S122-S130, 2000 (5, suppl) [PubMed] [Google Scholar]
- 8.Fox E, Landrum-McNiff K, Zhong Z, et al. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. JAMA. 1999;282:1638–1645. doi: 10.1001/jama.282.17.1638. [DOI] [PubMed] [Google Scholar]
- 9.Medici V, Rossaro L, Wegelin JA, et al. The utility of the model for end-stage liver disease score: A reliable guide for liver transplant candidacy and, for select patients, simultaneous hospice referral. Liver Transpl. 2008;14:1100–1106. doi: 10.1002/lt.21398. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: A systematic review and pooled analysis. Liver Transpl. 2015;21:1142–1152. doi: 10.1002/lt.24169. [DOI] [PubMed] [Google Scholar]
- 12.Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford) 2008;10:405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21:1287–1295. doi: 10.1245/s10434-013-3413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gish RG, Lencioni R, Di Bisceglie AM, et al. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173–185. doi: 10.1586/egh.11.105. [DOI] [PubMed] [Google Scholar]
- 15. National Cancer Institute: About the SEER program. http://seer.cancer.gov/about.
- 16. National Cancer Institute: SEER-Medicare Linked Database. http://healthcaredelivery.cancer.gov/seermedicare/
- 17.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8) suppl:IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 18.Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315:272–283. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 19.Ulahannan SV, Duffy AG, McNeel TS, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology. 2014;60:1637–1644. doi: 10.1002/hep.27288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanoff HK, Chang Y, Stavas JM, et al. Effectiveness of initial transarterial chemoembolization for hepatocellular carcinoma among Medicare beneficiaries. J Natl Compr Canc Netw. 2015;13:1102–1110. doi: 10.6004/jnccn.2015.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Iacus S, King G, Porro G. Multivariate matching methods that are monotonic imbalance bounding. J Am Stat Assoc. 2011;106:345–361. [Google Scholar]
- 23.Sanoff HK, Chang Y, Lund JL, et al. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist. 2016;21:1113–1120. doi: 10.1634/theoncologist.2015-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy EP, Burns RB, Davis RB, et al. Barriers to hospice care among older patients dying with lung and colorectal cancer. J Clin Oncol. 2003;21:728–735. doi: 10.1200/JCO.2003.06.142. [DOI] [PubMed] [Google Scholar]
- 25.Davila JA, Morgan RO, Richardson PA, et al. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt ML, Barritt AS, Orman ES, et al: Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology 148:967-977 e2, 2015. [DOI] [PMC free article] [PubMed]