Abstract
Purpose
This study was conducted to determine the effect of iCanDecide, an interactive and tailored breast cancer treatment decision tool, on the rate of high-quality patient decisions—both informed and values concordant—regarding locoregional breast cancer treatment and on patient appraisal of decision making.
Methods
We conducted a randomized clinical trial of newly diagnosed patients with early-stage breast cancer making locoregional treatment decisions. From 22 surgical practices, 537 patients were recruited and randomly assigned online to the iCanDecide interactive and tailored Web site (intervention) or the iCanDecide static Web site (control). Participants completed a baseline survey and were mailed a follow-up survey 4 to 5 weeks after enrollment to assess the primary outcome of a high-quality decision, which consisted of two components, high knowledge and values-concordant treatment, and secondary outcomes (decision preparation, deliberation, and subjective decision quality).
Results
Patients in the intervention arm had higher odds of making a high-quality decision than did those in the control arm (odds ratio, 2.00; 95% CI, 1.37 to 2.92; P = .0004), which was driven primarily by differences in the rates of high knowledge between groups. The majority of patients in both arms made values-concordant treatment decisions (78.6% in the intervention arm and 81.4% in the control arm). More patients in the intervention arm had high decision preparation (estimate, 0.18; 95% CI, 0.02 to 0.34; P = .027), but there were no significant differences in the other decision appraisal outcomes. The effect of the intervention was similar for women who were leaning strongly toward a treatment option at enrollment compared with those who were not.
Conclusion
The tailored and interactive iCanDecide Web site, which focused on knowledge building and values clarification, positively affected high-quality decisions largely by improving knowledge compared with static online information. To be effective, future patient-facing decision tools should be integrated into the clinical workflow to improve decision making.
INTRODUCTION
Patients who are newly diagnosed with curable breast cancer face a complicated treatment decision-making process. This context has motivated initiatives to improve decision quality to ensure patients are informed of the tradeoffs with regard to the risks and benefits of different treatments, that their values are understood and incorporated into treatment decisions, and that they are prepared to engage their clinicians in decision -making.1-3 Tools (ie, decision aids) have been developed to help patients manage treatment decision making after a diagnosis of breast cancer.4-15 Few trials have evaluated breast cancer treatment decision tools in surgical practice.5,15 Only one study has demonstrated the positive effect of a decision aid on a key aspect of decision quality, including knowledge and decision satisfaction,10 whereas others have produced mixed results. The effect of existing tools is also limited by small patient samples from a single academic or a few clinical practice sites, as well as by the lack of attention to the variable decision workflow found in community practice. Despite the existence of many cancer treatment information Web sites and some online decision aids, there remain large gaps in a patient’s level of knowledge about his or her treatment options, even after surgery.16,17 Furthermore, patients themselves report a strong desire for assistance with cancer treatment decisions.
To address these gaps, we conducted a large randomized controlled trial of an interactive and comprehensive version of a decision tool, called iCanDecide, which covered both locoregional and systemic treatment decision making for patients with breast cancer, and was tailored to their age, race, timing of surgical consult, and values clarification feedback compared with a static version that emulated contemporary, quality Web sites typical of those available to patients; the iCanDecide intervention Web site is available online.18 We hypothesized that patients with breast cancer who viewed the intervention version of iCanDecide would have higher rates of both aspects of a high-quality decisions, defined as a decision that is both informed and values concordant,19 would be more prepared to make their treatment decisions, and would appraise their decisions more positively than patients who viewed the control version that was similar to quality contemporary Web sites.3,20
METHODS
Overall Design
This study was based a conceptual framework for improving decision making, informed by our preliminary studies and theory.21-28 We performed a patient-level multisite randomized controlled trial in 22 surgical practices in four states from February 2014 to May 2016. Eligible consenting patients within each practice were randomly assigned to the intervention (tailored and interactive) or control (static information) version of the iCanDecide Web site. The study received institutional review board approval from the University of Michigan and the participating practices. The protocol was published before the completion of recruitment.28
Practice Recruitment
We recruited practices directly in geographic areas with diverse populations. Each practice, which ranged from one to five surgeons, received $1,000 for participation and was visited by the study team to provide training. Practices were responsible for having someone (eg, a surgeon or nurse) offer study information packets to eligible women and for providing monthly recruitment reports. Practices were given iPads to encourage participation from patients with limited access to the Internet.
Participants and Procedures
Eligible patients were women with a new diagnosis of early-stage (I to II) breast cancer between the ages of 21 and 84 years who had not yet received surgical treatment and who did not have a contraindication for either mastectomy or breast conservation therapy. Women who received neoadjuvant chemotherapy were eligible if actively considering surgical options. We used a flexible approach to enrollment—patients could enroll before or after their first surgical consultation visit—on the basis of extensive pilot work with surgical practices. Only one practice (n = 2 enrolled patients) opted to invite patients before surgical consultation.
The study packet included an introductory letter, Web site login information, and $20. Once logged in, participants consented online, completed a short survey, and were allocated to a study arm using random assignment stratified by site, age, race, education, and the timing of surgical consult. Two weeks after enrollment, women with invasive breast cancer were encouraged to log back into the tool to view a systemic treatment module. The first follow-up survey was mailed 4 weeks after enrollment, with a second survey mailed 9 months later. A modified Dillman method29 was used to encourage survey completion at each time point (mailed survey, telephone reminder, resending survey, and telephone option). This work reports the results of the first follow-up survey.
Intervention and Control Arms
Participants were randomly assigned online to either an interactive and tailored iCanDecide Web site (intervention) or to the static iCanDecide Web site (control). Both versions were based on an existing prototype3 that was developed according to criteria outlined by the International Patient Decision Aids Standards30 using user-centered design.31 Both versions were based on extensive piloting feedback from clinicians and patients with breast cancer. Patients were blinded to the true intervention arm as both versions had the same name and included quality information about the key content areas for locoregional and systemic treatment. The intervention included several innovative features designed to align with components of a high-quality decision, which included a knowledge-building module that systematically delivered information to participants about key content areas (survival outcomes, risk of local recurrence, radiation, recovery from surgery, need for additional surgery, genetic testing, reconstruction, and bilateral mastectomy), a values-clarification and feedback exercise that used conjoint analysis3 to assess the importance of four key attributes of treatment (radiation therapy, keeping the natural breast, need for additional surgeries, and cosmetic outcome),32,33 and a patient activation module that used testimonials that were tailored to age, race, and the timing of surgical consult. The control version emulated contemporary, quality Web sites that are typical of those available to patients.
Primary Outcome Measures
Outcomes were selected on the basis of our framework and their relevance to treatment decision making. The primary outcome, measured via patient report from the first follow-up survey, was a high-quality locoregional treatment decision that consisted of two components, which were accurate knowledge about the risks and benefits of treatment option tradeoffs, and that the chosen treatment was concordant with patient values.19 We assessed each component separately because they are distinctly different constructs with different clinical practice implications. Knowledge was measured by using a validated five-item knowledge scale for locoregional treatment19 that was adapted from a prior 12-item knowledge scale.32 We used a prespecified cutoff of > 80% to determine a clinically meaningful level of high knowledge. Values-concordant treatment was determined by using a validated five-item question set19 that assessed the importance to the patient to achieve certain outcomes (eg, keeping the natural breast and/or avoiding radiation) on a scale from 0 to 10. This question set was asked before the patient logged off the Web site. We modeled these attributes to generate a predicted probability of preferring breast conservation or unilateral or bilateral mastectomy. If the prediction aligned with the treatment received, this was considered concordant, otherwise it was considered nonconcordant.
Secondary outcomes included patient preparation for decision making, the extent of deliberation, and subjective decision quality (SDQ). Preparation for decision making was measured by using a validated scale that assessed the degree to which participants felt the Web site prepared them for making their treatment decision.34 We assessed deliberation (the degree to which patients spent time thinking through the options)35,38 and SDQ by using measures that were developed and validated by our team.36-38 All were measured on continuous scales and standardized to ranges from 1 to 5.
Patient Factors
Factors were obtained from the login survey and included age, race, education level, and partnered status, and whether the patient had seen her surgeon yet (yes or no). We also assessed the patient’s decision trajectory at enrollment by asking whether she was leaning toward a certain treatment option (mastectomy, lumpectomy, other, or not leaning) and whether she felt sure of the best treatment choice for her (yes or no). We combined these two questions into a binary measure—leaning strongly toward a treatment option (yes to leaning and to being sure) versus other combinations.
Web Site Use and Satisfaction
Patients were asked to indicate whether they thought the Web site was easy to use, helpful in decision making, helpful in thinking about pros and cons, and whether they would recommend it to other patients with breast cancer. Participants were also asked to rate their satisfaction with regard to the Web site’s length and the amount of information available. Finally, we asked whether patients contacted their surgeon’s office after viewing the Web site (yes or no).
Sample Size
Our prior research provided the assumptions with which to determine the sample size for this study.16,27,39,40 We estimated a need to recruit 222 patients per arm (N = 444). Assuming that 76% of participants would complete the primary outcome assessment, this would result in a final sample size of 340 (170 patients per arm), which would ensure 80% power (α = .05) to detect differences of 10% to 15% in high-quality decisions between patients in the intervention and control arms with an intracluster correlation of 0.01 to 0.04. We exceeded the recruitment goal to increase the power to detect differences in secondary outcomes. Data remained blinded and locked until after all data collection was completed.
Statistical Methods
All primary analyses were prespecified and followed the published protocol.28 We used an intention-to-treat analysis such that all patients who were randomly assigned were included in analyses.41 We first examined whether random assignment balanced the distribution of demographic and clinical factors across two arms. Because these covariates are balanced across two arms, we conducted unadjusted analyses of the primary outcome (high-quality decision) and its two components—knowledge and values-concordant treatment—using generalized linear mixed models with logit link function to evaluate the effect of intervention. We then conducted unadjusted analyses of secondary outcomes to evaluate the hypothesis that patients in the intervention arm would have higher rates of deliberation, decision preparation, and SDQ than patients in the control arm, using linear mixed models.
In adjusted analyses of primary and secondary outcomes using mixed models, we considered patient age, race, education, marital status at enrollment, cancer stage (ductal carcinoma in situ v not), and treatment received (mastectomy v not) as covariates in the models. Participants with missing items for outcomes or covariates (≤ 5%) were excluded from analyses.
In post hoc unadjusted analyses, we examined the association between the study arm (intervention v control) and the two outcomes significant in the primary and secondary outcomes analyses—knowledge and decision preparation—separately by baseline treatment decision trajectory, and we evaluated the interaction between decision trajectory and study arm. We used generalized linear mixed models for knowledge and linear mixed models for decision preparation. Detailed analyses can be found in the Data Supplement. Analyses were performed by using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
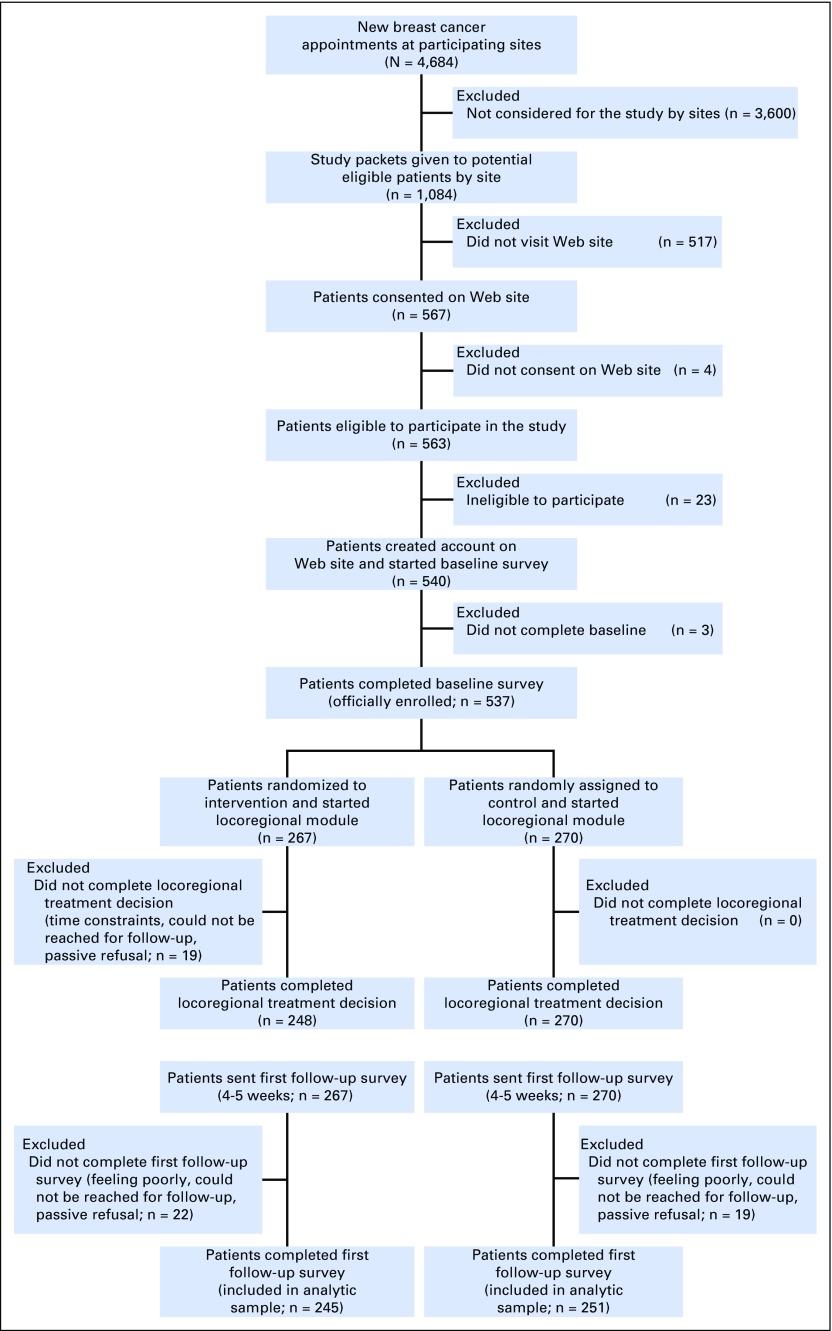
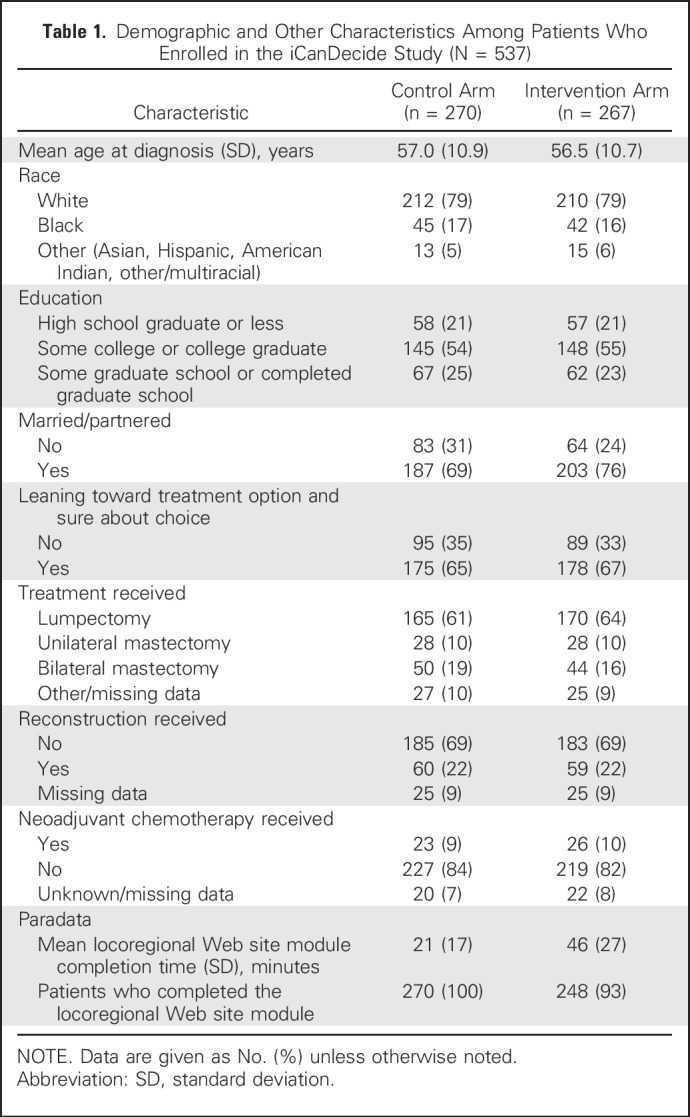
Study packets were distributed to 1,084 patients, of whom 567 (52.3%) visited the Web site and, of these, 537 (94.7%) were eligible, created an account, and completed an enrollment survey (Fig 1). The response rate to the first follow-up survey was 92% (n = 496) in both the intervention (n = 245) and control (n = 251) arms. Nonrespondents to the survey were more likely to be black and not married (P < .05), but similar with regard to other demographic factors. Study arms were balanced with regard to demographic and treatment factors. Approximately two thirds of patients were strongly leaning toward a treatment option at enrollment in each study arm. The majority of patients in both arms completed their respective Web site review, but completion rates in the intervention arm were higher in white patients than in minority patients (95.3% v 78.8%, respectively; P < .001). Patients in the intervention arm spent, on average, 46 minutes reviewing the Web site compared with 21 minutes for patients in the control arm (Table 1).
Fig 1.
Patient flow diagram.
Table 1.
Demographic and Other Characteristics Among Patients Who Enrolled in the iCanDecide Study (N = 537)
Outcomes Analyses
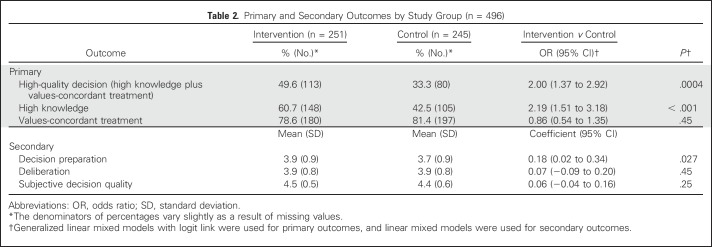
Overall, compared with patients in the control arm, more patients in the intervention arm achieved a high-quality decision (49.6% v 33.3%; P = .0004) and had high knowledge (60.7% v 42.5%; P < .001). There was no difference in values-concordant treatment outcome between arms (78.6% in the intervention arm, and 81.4% in the control arm; P = .45).
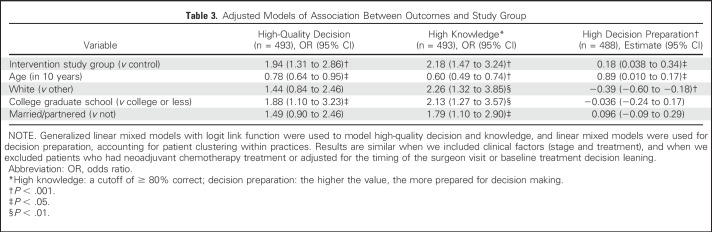
Primary outcomes analyses found that patients in the intervention arm had higher odds of making a high-quality decision than patients in the control arm (odds ratio [OR], 2.00; 95% CI, 1.37 to 2.92; P = .0004), as well as of high knowledge (OR, 2.19; 95% CI, 1.51 to 3.18; P < .001), but not of values-concordant treatment. Patients in the intervention arm also more often had high decision preparation than did patients in the control arm (estimate, 0.18; 95% CI, 0.02 to 0.34; P = .027). There were no differences in deliberation or SDQ by arm (Table 2). In multivariable analyses, patients in the intervention arm had significantly higher odds of a high-quality decision (OR, 1.94; 95% CI, 1.31 to 2.86), high knowledge (OR, 2.18; 95% CI, 1.47 to 3.24), and higher decision preparation (estimate, 0.18; 95% CI, 0.024 to 0.34; Table 3).
Table 2.
Primary and Secondary Outcomes by Study Group (n = 496)
Table 3.
Adjusted Models of Association Between Outcomes and Study Group
Post Hoc Analysis
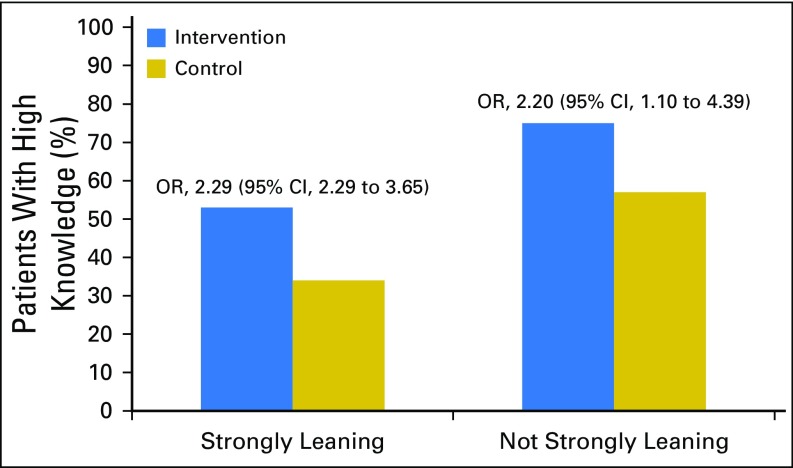
The effect of being in the tailored and interactive iCanDecide arm, relative to the control arm, on high knowledge was similar for women who strongly leaned toward a treatment at enrollment compared with those who did not (OR, 2.29; 95% CI, 1.44 to 3.65 v OR, 2.20; 95% CI, 1.10 to 4.39; Fig 2). There was a similar effect for decision preparation (estimate, 0.27; 95% CI, 0.02 to 0.52 for strongly leaning; estimate, 0.14; 95% CI, −0.06 to 0.34 for not strongly leaning; results are reported in the Data Supplement). No significant interactions were observed between decision trajectory and intervention arms for both knowledge and decision preparation.
Fig 2.
High knowledge by decision trajectory status and study group. Odds ratios (ORs) were obtained using generalized linear mixed models for knowledge comparing the intervention arm with the control group.
Patient Appraisal of the Web Sites
Nearly 90% of patients in both arms reported that the Web site was easy to use, 60% said it was helpful in their decision making, and most (85.1% in the intervention arm, and 79.4% in the control arm) would recommend it to others. More patients in the intervention arm reported that the Web site helped in thinking about the pros and cons that mattered most than did patients in the control arm (79.2% v 67.0%; P = .039). More patients in the control arm reported that the length was just right (85.6% v 75.0%; P = .04), but similar numbers reported that the amount of information was just right (79.9% in the intervention arm, and 75.8% in the control arm; P = .08). Finally, there was a trend toward more patients in the intervention arm contacting their surgeon’s office after using the Web site (31.2% v 20.9%; P = .12).
DISCUSSION
We found that an interactive and tailored breast cancer treatment decision tool improved decision quality and prepared patients for decision making compared with a high-quality static information Web site. This suggests that an interactive design is important to enhance the potential benefit of decision tools in practice. Most patients completed the components of the intervention Web site and positively appraised its ease of use and salience for treatment decision making.
The improvement in decision quality stemmed largely from improvements in knowledge about the risks and benefits of treatments. This is promising as the knowledge-building module was developed to address large deficits in knowledge about treatment after the diagnosis of curable breast cancer,16,43-45 and incorporated interactive learning principles. Our results are consistent with those reported by others who found that decision aids have a positive effect on knowledge.46 Of importance, we observed improvements in knowledge and decision preparedness regardless of the patient’s decision trajectory at the time of enrollment (strength of leaning toward a treatment). This suggests that tools can be deployed flexibly in practice even after the first visit, with positive effects on decision outcomes. At the same time, our study highlights persistent gaps in decision making about treatment; approximately 40% of patients in the intervention arm did not achieve high knowledge about treatment tradeoffs, and 60% did not achieve high decision preparation, which suggests that there is still considerable room for improving the decision-making process.
Being in the intervention arm did not affect values-concordant treatment, which was generally high across arms (> 80%). This is consistent with a recent review of decision aids that found little evidence that decision aids influence the receipt of values-congruent treatment.15 This supports the importance of knowledge as a key component of high-quality decisions, as uninformed values-driven choices have been shown to be associated with more extensive treatment.17
The high rate of values-concordant treatment that was observed in the study may be a result, in part, of patients already having clear treatment preferences after their surgical visit, when virtually all patients in our study viewed the Web sites. Whereas the ideal time to support shared decision making may be before consultations, we found that few surgeons are willing to provide patients with previsit materials about the treatment of breast cancer. Although electronic health systems may improve this process,47 our results underscore the importance of offering tools even after surgical consults, when patients are often still making these critical decisions.
As with other interactive decision aids, there was a potential patient burden associated with the intervention version of iCanDecide. Patients spent, on average, more than 40 minutes completing the tool, which included several interactive components. Patients had the option to participate at the clinic via an iPad, but nearly all chose to view it at home. Yet no patient expressed concerns about the length, and more patients in the intervention arm reported that the Web site helped them think about the pros and cons of treatment. Although the interactive nature of the intervention arm may have better personalized the Web site, interactivity can be confusing and cumbersome if not developed with high-quality standards as done in our study.31 Decision tools that engage patients at their desired level may be most effective. The race disparity in the completion rates in the intervention arm underscores the need to build tools that minimize cognitive burden and maximize usability across all types of patients.
Strengths of this study include the large sample size, high participation rates, the community-based deployment, and validated measures of decision quality. Yet there were some limitations. Whereas there was no true usual care arm in the study, many patients with breast and other cancers seek information online after their diagnosis to get information about treatment.48 Although we achieved good representation of patients across subgroups, there remain limits to generalizability to all racial and socioeconomic groups. Some women with limited access or lower facility with the Internet may not have enrolled in our study, despite providing practices with an iPad. Whereas the iCanDecide intervention included innovative features, it was not possible to include all patient values in the values clarification exercise. Finally, characteristics of the 48% of women who were invited but did not enroll are unknown.
Treatment decision making after a breast cancer diagnosis is complex and unfurls variably across patients and practices.49 We found that a tailored, interactive version of a patient-facing decision tool—iCanDecide—delivered flexibly in this context can improve key aspects of decision making. Our results underscore the demand for such tools on the part of patients with newly diagnosed breast cancer. Yet our study suggests important areas for future work to optimize informed decision making and patient appraisal of the process, including fully integrating the tools into clinical workflows to better engage clinicians in discussions with their patients, and comparing decision aids with other approaches, such as patient personal coaching.46,50 Implementation studies are needed to better determine how to deploy and evaluate such tools as iCanDecide in the context of surgeon and practice variation. Innovative strategies are needed to ensure that these tools can be broadly deployed to improve patient-clinician decision making.
ACKNOWLEDGMENT
We acknowledge the substantial contributions of our participating practices from Michigan, which include Beaumont Health System, Comprehensive Breast Care, Michigan Breast Specialists at St John Providence, St John Providence Macomb-Oakland, Rochester General Surgery, Pamela Johnson, MD, St Joseph Mercy Oakland, Hurley Medical Center, the University of Michigan Cancer Center, and St John Macomb-Oakland. Participating practices from Georgia include Breast Care Specialists BCS, Atlanta Breast Care, Grady Memorial Hospital/Emory, Georgia Cancer Specialists affiliated with Northside Hospital, Dekalb Surgical Associates, Coliseum Health System, Emory Healthcare, and Ruben, Luke, and Garcha Surgical Associates. Participating practices from Tennessee include Baptist Medical Group-Memphis Breast Care; and from California, the University of California at Los Angeles. We also acknowledge the outstanding work of our University of Michigan project staff—Ed Saunders, Viji Ramaswami, Holly Derry, Diane Egleston, Ben Hayward, Dennis O’Reilly, and Matthias Kirch from the Cancer Center Health Communication Core and Center for Health Communications Research; and Alexandra Jeanpierre, Stefanie Goodell, and Rose Juhasz from the Cancer Surveillance and Outcomes Research team (CanSORT) Research Management Team. Sarah T. Hawley and Yun Li had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We acknowledge with gratitude the patients with breast cancer who enrolled in our study.
Footnotes
Supported by Grant No. P01-CA163233 from the National Cancer Institute to the University of Michigan. Work conducted to develop iCanDecide was done using the University of Michigan Cancer Center Health Communication Shared Resource, which is supported by Grant No. P30-CA46592 from the National Cancer Institute.
Presented at the 55th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-6, 2017.
Clinical trial information: NCT01840163.
Listen to the podcast by Dr Egleston at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Sarah T. Hawley, Yun Li, Lawrence C. An, Kenneth Resnicow, Nancy K. Janz, Michael S. Sabel, Angela Fagerlin, Monica Morrow, Reshma Jagsi, Timothy P. Hofer, Steven J. Katz
Financial support: Sarah T. Hawley, Reshma Jagsi, Steven J. Katz
Administrative support: Steven J. Katz
Collection and assembly of data: Sarah T. Hawley, Yun Li, Kevin C. Ward, Steven J. Katz
Data analysis and interpretation: Sarah T. Hawley, Yun Li, Kenneth Resnicow, Reshma Jagsi, Timothy P. Hofer, Steven J. Katz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improving Breast Cancer Surgical Treatment Decision Making: The iCanDecide Randomized Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sarah T. Hawley
No relationship to disclose
Yun Li
No relationship to disclose
Lawrence C. An
No relationship to disclose
Kenneth Resnicow
No relationship to disclose
Nancy K. Janz
No relationship to disclose
Michael S. Sabel
No relationship to disclose
Kevin C. Ward
No relationship to disclose
Angela Fagerlin
No relationship to disclose
Monica Morrow
Honoraria: Company: Genomic Health
Consulting or Advisory Role: Genomic Health
Reshma Jagsi
Employment: University of Michigan
Consulting or Advisory Role: Amgen
Research Funding: AbbVie (Inst)
Travel, Accommodations, Expenses: Amgen
Timothy P. Hofer
No relationship to disclose
Steven J. Katz
No relationship to disclose
REFERENCES
- 1.Lee CN, Chang Y, Adimorah N, et al. : Decision making about surgery for early-stage breast cancer. J Am Coll Surg 214:1-10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler FJ, Jr, Levin CA, Sepucha KR: Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood) 30:699-706, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Hawley ST, Newman L, Griggs JJ, et al. : Evaluating a decision aid for improving decision making in patients with early-stage breast cancer. Patient 9:161-169, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholas Zdenkowski, Butow P, Tesson S, et al. : A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast 26:31-45, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Stacey D, Légaré F, Col NF, et al. : Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2014:CD001431, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Waljee JF, Rogers MA, Alderman AK: Decision aids and breast cancer: Do they influence choice for surgery and knowledge of treatment options? J Clin Oncol 25:1067-1073, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Goel V, Sawka CA, Thiel EC, et al. : Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making 21:1-6, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Street RL, Jr, Voigt B, Geyer C, Jr, et al. : Increasing patient involvement in choosing treatment for early breast cancer. Cancer 76:2275-2285, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Whelan T, Sawka C, Levine M, et al. : Helping patients make informed choices: A randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer. J Natl Cancer Inst 95:581-587, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Whelan T, Levine M, Willan A, et al. : Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: A randomized trial. JAMA 292:435-441, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Heller L, Parker PA, Youssef A, et al. : Interactive digital education aid in breast reconstruction. Plast Reconstr Surg 122:717-724, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Jibaja-Weiss ML, Volk RJ, Granchi TS, et al. : Entertainment education for breast cancer surgery decisions: A randomized trial among patients with low health literacy. Patient Educ Couns 84:41-48, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Lam WW, Chan M, Or A, et al. : Reducing treatment decision conflict difficulties in breast cancer surgery: A randomized controlled trial. J Clin Oncol 31:2879-2885, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Vodermaier A, Caspari C, Koehm J, et al. : Contextual factors in shared decision making: A randomised controlled trial in women with a strong suspicion of breast cancer. Br J Cancer 100:590-597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacey D, Légaré F, Lewis K, et al. : Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 4:CD001431, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagerlin A, Lakhani I, Lantz PM, et al. : An informed decision? Breast cancer patients and their knowledge about treatment. Patient Educ Couns 64:303-312, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Jagsi R, Hawley ST, Griffith KA, et al. : Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg 152:274-282, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Surveillance and Outcomes Research Team : Tools and resources.http://cansort.med.umich.edu/research/tools-and-resources/
- 19.Sepucha KR, Belkora JK, Chang Y, et al. : Measuring decision quality: Psychometric evaluation of a new instrument for breast cancer surgery. BMC Med Inform Decis Mak 12:51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Society of Clinical Oncology : Cancer.net. https://www.cancer.net/
- 21.Janz NK, Becker MH: The health belief model: A decade later. Health Educ Q 11:1-47, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Bandura A: Social Foundations of Thought and Action: A Social Cognitive Theory (ed 1). Englewood Cliffs, NJ, Prentice Hall, 1986 [Google Scholar]
- 23.Aday LA, Andersen R: A framework for the study of access to medical care. Health Serv Res 9:208-220, 1974 [PMC free article] [PubMed] [Google Scholar]
- 24.Green L, Kreuter M: Health Program Planning: An Educational and Ecological Approach (ed 4). New York, NY, McGraw-Hill Higher Education, 2005 [Google Scholar]
- 25.Hawley ST, Griggs JJ, Hamilton AS, et al. : Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst 101:1337-1347, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lantz PM, Janz NK, Fagerlin A, et al. : Satisfaction with surgery outcomes and the decision process in a population-based sample of women with breast cancer. Health Serv Res 40:745-767, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janz NK, Mujahid MS, Hawley ST, et al. : Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer 113:1058-1067, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawley ST, Li Y, Jeanpierre LA, et al. : Study protocol: A randomized controlled trial of a comprehensive breast cancer treatment patient decision tool (iCanDecide). Contemp Clin Trials Commun 5:123-132, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillman D, Smyth J, Christian L: Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method (ed 3). Hoboken, NY, John Wiley & Sons, 2009 [Google Scholar]
- 30.Elwyn G, O’Connor A, Stacey D, et al. : Developing a quality criteria framework for patient decision aids: Online international Delphi consensus process. BMJ 333:417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vito Dabbs A, Myers BA, Mc Curry KR, et al. : User-centered design and interactive health technologies for patients. Comput Inform Nurs 27:175-183, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CN, Dominik R, Levin CA, et al. : Development of instruments to measure the quality of breast cancer treatment decisions. Health Expect 13:258-272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins ED, Moore CP, Clay KF, et al. : Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol 27:519-525, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Bennett C, Graham ID, Kristjansson E, et al. : Validation of a preparation for decision making scale. Patient Educ Couns 78:130-133, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Elwyn G, Frosch D, Volandes AE, et al. : Investing in deliberation: A definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Making 30:701-711, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Resnicow K, Abrahamse P, Tocco RS, et al. : Development and psychometric properties of a brief measure of subjective decision quality for breast cancer treatment. BMC Med Inform Decis Mak 14:110, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez KA, Resnicow K, Williams GC, et al. : Does physician communication style impact patient report of decision quality for breast cancer treatment? Patient Educ Couns 99:1947-1954, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallner LP, Abrahamse P, Uppal JK, et al. : Involvement of primary care physicians in the decision making and care of patients with breast cancer. J Clin Oncol 34:3969-3975, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawley ST, Fagerlin A, Janz NK, et al. : Racial/ethnic disparities in knowledge about risks and benefits of breast cancer treatment: Does it matter where you go? Health Serv Res 43:1366-1387, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawley ST, Janz NK, Hamilton A, et al. : Latina patient perspectives about informed treatment decision making for breast cancer. Patient Educ Couns 73:363-370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Hopewell S, Schulz KF, et al. : CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 63:e1-e37, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Reference deleted. [Google Scholar]
- 43.Bleicher RJ, Abrahamse P, Hawley ST, et al. : The influence of age on the breast surgery decision-making process. Ann Surg Oncol 15:854-862, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Nold RJ, Beamer RL, Helmer SD, et al. : Factors influencing a woman’s choice to undergo breast-conserving surgery versus modified radical mastectomy. Am J Surg 180:413-418, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Molenaar S, Oort F, Sprangers M, et al. : Predictors of patients’ choices for breast-conserving therapy or mastectomy: A prospective study. Br J Cancer 90:2123-2130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agency for Healthcare Research and Quality: Decision Aids for Cancer Screening and Treatment. https://www.ncbi.nlm.nih.gov/books/NBK269405/ [PubMed]
- 47.DuBenske LL, Gustafson DH, Shaw BR, et al. : Web-based cancer communication and decision making systems: Connecting patients, caregivers, and clinicians for improved health outcomes. Med Decis Making 30:732-744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An LC, Wallner L, Kirch MA: Online social engagement by cancer patients: A clinic-based patient survey. JMIR Cancer 2:e10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz SJ, Hawley ST, Morrow M, et al. : Coordinating cancer care: Patient and practice management processes among surgeons who treat breast cancer. Med Care 48:45-51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epstein RM, Duberstein PR, Fenton JJ, et al. : Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncol 3:92-100, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]