Abstract
Purpose
Cisplatin-associated acute kidney injury (C-AKI) is common. We sought to develop and validate a predictive model for C-AKI after the first course of cisplatin.
Methods
Clinical and demographic data were collected on patients who received cisplatin between 2000 and 2016 at two cancer centers. C-AKI was defined as a 0.3 mg/dL rise in serum creatinine within 14 days of receiving cisplatin. Using multivariable logistic regression models with C-AKI as the primary outcome, we created a scoring model from the development cohort (DC) and tested it in the validation cohort (VC).
Results
C-AKI occurred in 13.6% of 2,118 patients in the DC and in 11.6% of 2,363 patients in the VC. Factors significantly associated with C-AKI included age 61 to 70 years (odds ratio [OR], 1.64 [95% CI, 1.21 to 2.23]; P = .001) and 71 to 90 years (OR, 2.97 [95% CI, 2.06 to 4.28]; P < .001) compared with ≤ 60 years; cisplatin dose 101 to 150 mg (OR, 1.58 [95% CI, 1.14 to 2.19]; P = .007) and > 150 mg (OR, 3.73 [95% CI, 2.68 to 5.20]; P < .001) compared with ≤ 100 mg; a history of hypertension (OR, 2.10 [95% CI, 1.54 to 2.72]; P < .001) compared with no hypertension; and serum albumin 2.0 to 3.5 g/dL (OR, 2.21 [95% CI, 1.62 to 3.03]; P < .001) compared with > 3.5 g/dL. The baseline estimated glomerular filtration rate was not significantly associated with the risk of C-AKI. The c-statistics of the score-based model in the DC and the VC were 0.72 (95% CI, 0.69 to 0.75) and 0.70 (95% CI, 0.67 to 0.73), respectively. Scores of 0, 3.5, and 8.5 were associated with a probability of C-AKI of 0.03 (95% CI, 0.03 to 0.05), 0.12 (95% CI, 0.11 to 0.14), and 0.51 (95% CI, 0.43 to 0.60), respectively.
Conclusion
A score-based model created by using the patient’s age, cisplatin dose, hypertension, and serum albumin is predictive of C-AKI.
INTRODUCTION
Since its introduction in 1978, cisplatin has led to a dramatic survival advantage for patients with a variety of cancers.1 Although it is an effective chemotherapeutic agent, cisplatin causes nephrotoxicity in approximately 30% of patients at some point during treatment.2-4 Cancer centers and clinical trials use various renal function measures such as serum creatinine (SCr), creatinine clearance,5 or, less commonly, the estimated glomerular filtration rate (eGFR)6-8 as one of the primary criteria for determining candidacy for cisplatin use. Although prudent, it may not be sufficient to identify patients at risk of cisplatin-associated acute kidney injury (C-AKI) using baseline renal function alone. Other risk factors identified from observational studies have not been incorporated routinely into candidacy determination. One reason might be that most studies of C-AKI have involved a small number of patients,9,10 typically with a single type of cancer.11-14
A prediction model developed from readily available clinical characteristics and risk factors for C-AKI could transform counseling and patient selection. Prediction models are becoming increasingly recognized as useful tools, and policymakers recommend their use routinely in clinical practice guidelines.15-18 Therefore, we sought to use large-scale data repositories of patients with cancer treated with cisplatin to identify risk factors for C-AKI after the first course. We then used these risk factors to develop a clinically useful prediction model and validated the model in an independent sample.
METHODS
The methods described in this article are in accordance with the Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) statement.19
Source of Data
We conducted an observational study of two cohorts assembled using two large but independent patient data repositories—the Partners Research Patient Data Registry (RPDR), which collects data from all Partners-affiliated institutions including Massachusetts General Hospital (MGH) and Brigham and Women’s Hospital, and the Oncology Data Retrieval System (OncDRS) at Dana-Farber Cancer Institute (DFCI). The study was approved by the DFCI institutional research board.
Development cohort (MGH).
We obtained a list of all patients who had received cisplatin at MGH between 2005 (the earliest year that the pharmacy data were recorded electronically) and 2014. Demographic and clinical information was obtained from the RPDR.
Validation cohort (DFCI/Brigham and Women’s Hospital).
We obtained demographic and clinical data from the OncDRS for patients treated at DFCI who had received cisplatin between 2000 and 2015.
Participants and Exposure
We included patients ≥18 years of age who had been treated with cisplatin, who had had at least one SCr measurement within the month before the first course of cisplatin, and at least one measurement within 14 days after this first course. Patients who had had cisplatin administered in the setting of allergic desensitization and those with a baseline creatinine level of > 1.5 mg/dL were excluded. Patients with covariates with a prevalence of < 5% and those with > 50% missing data were also excluded.
Cisplatin may have been administered in a single infusion or in fractionated infusions over consecutive days, in which case the dose was the sum of all cisplatin administered over the first course. Patients received hydration before and after chemotherapy, as per provider discretion, with normal saline or an electrolyte solution with potassium chloride and magnesium sulfate.
Covariates
We collected data for potential predictors of interest from the month before the date of an individual’s first course of cisplatin (the index date). Variables were chosen on the basis of a previous literature review of risk factors for C-AKI and additional known risk factors for acute kidney injury (AKI) from other causes. Demographic information included age, sex, ethnicity, height, and weight at the time of initial cisplatin administration. Clinical information included cisplatin dose (milligrams); fractionated versus single infusion; date of infusion; concurrent use of mannitol or amifostine; baseline laboratory values, including blood urea nitrogen, creatinine, magnesium, and albumin; use of diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers; and history of diabetes or hypertension recorded before index date using diagnosis codes (International Classification of Diseases, Ninth Revision, Clinical Modification).20
Outcome
We defined C-AKI as a ≥ 0.3 mg/dL rise in SCr from baseline to peak measurements as per the National Cancer Institute’s nephrotoxicity criteria after the first course of cisplatin.21 For the purpose of comparison, we also calculated the frequency of AKI by using additional definitions, including a 1.5-fold increase in creatinine or a doubling of creatinine. We defined the severity of C-AKI using the Acute Kidney Injury Network criteria; stage I was a ≥ 0.3 mg/dL rise in creatinine or an increase of 1.5- to two-fold from baseline; stage II was a SCr increase > 2.0- to 3.0-fold from baseline; stage III was an SCr increase > 3.0-fold from baseline or an SCr ≥ 4.0 mg/dL with an acute increase of at least 0.5 mg/dL or need for renal replacement therapy.
Creatinine checked within the month before the index date was used as the baseline. If there were multiple values, then the value closest to the index date was chosen to be the most representative of renal function at the time of cisplatin exposure. Peak creatinine was defined as the highest creatinine value within 14 days after the index date. This was intended to accommodate the variability in the time until follow-up laboratory measurements after administration of chemotherapy as well as the known window of toxicity.
Statistical Analysis
Continuous variables are presented as mean (standard deviation) or median (interquartile range [IQR]), whereas categorical variables are described by frequency. Unadjusted associations between the covariates and the primary outcome were evaluated using χ2 tests for categorical data, the t test for normally distributed variables, and the Kruskal-Wallis test for nonparametric variables. We targeted variables commonly available in the clinical setting with the goal of developing a readily applicable clinical model. Variables considered were age, sex, ethnicity, history of diabetes, history of hypertension, mannitol use, body mass index (BMI), eGFR calculated by the Chronic Kidney Disease Epidemiology equation, cisplatin dose, and fractionated versus single infusion. All continuous variables were examined linearly as well as in multiple categories, and nonsignificant categories in the same direction were collapsed where appropriate for parsimony.22 We used multivariable logistic regression with backward elimination, setting a P value > .05 for the removal of variables. Once the primary variables were selected by backward elimination, we added clinically relevant interaction terms to examine any potential effect modification among age, ethnicity, eGFR, and cisplatin dose. Data for serum albumin, height, and weight were missing for some participants in the development cohort (DC). Sensitivity analysis was performed with and without the use of the missing categories, as well as by imputing values in the normal range for missing serum albumin. We used complete case analysis when removing patients with missing data did not change the β estimates by > 10% or the model c-statistic by > 0.05. Fit of the model was assessed by the Hosmer-Lemeshow goodness-of-fit test. Discrimination was assessed by the area under the receiver operating characteristic curve (c-statistic). Analyses were performed using SAS version 9.4. (SAS Institute, Cary, NC).
Prediction Model
Score.
A score for each variable in the model was derived by dividing the parameter estimates by the lowest value and then rounding off to the next 0.5. This readily usable method was applied successfully in a previous study.23
External validation.
We chose the DFCI as our validation cohort (VC) despite its being the larger of the two, given that a greater number of values were missing for albumin (approximately 30%), a predictor of importance in our a priori hypothesis. Baseline characteristics of the DC and the VC were comparable. External validation was performed by calculating a score for each individual patient in the DFCI dataset and evaluating various model performance measures.
RESULTS
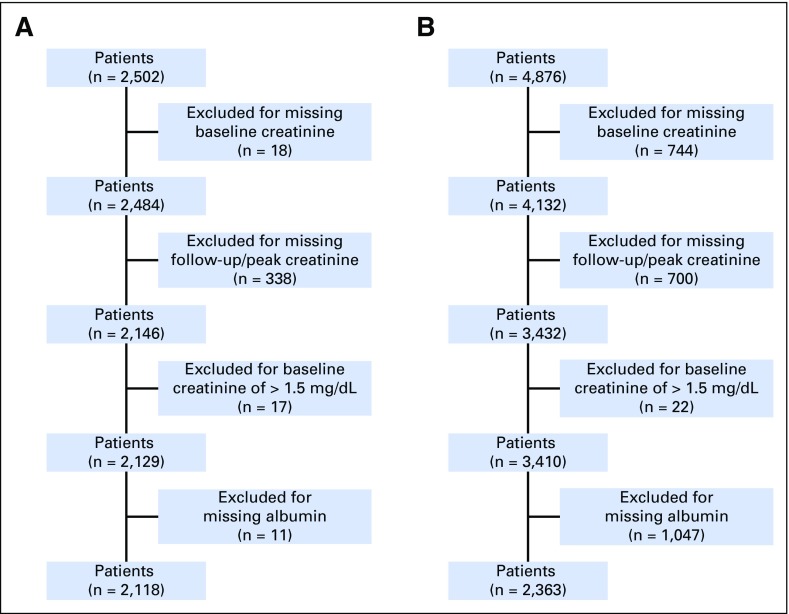
We identified 2,502 and 4,876 adult patients in the DC and the VC, respectively, who received cisplatin. After application of inclusion and exclusion criteria, the final sample sizes in the analysis were 2,118 patients in the DC and 2,363 patients in the VC (Figs 1A and 1B).
Fig 1.
(A) Development cohort. (B) Validation cohort.
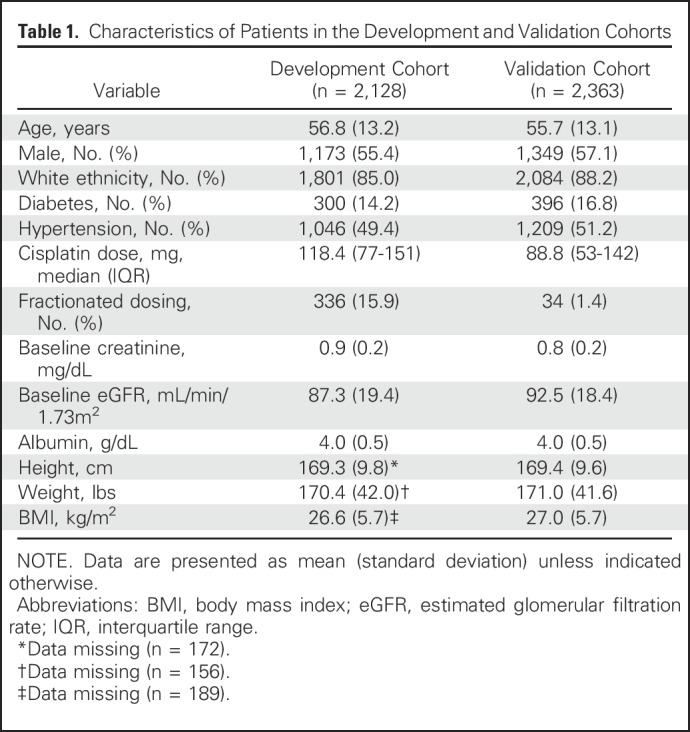
The clinical characteristics of patients included in the final analysis are listed in Table 1. Patients in the DC and the VC, respectively, tended to be middle aged (56.8 years [95% CI, 56.3 to 57.4 years]; range, 18 to 89 years; and 55.7 years [95% CI, 55.2 to 56.2 years]; range, 18 to 90 years); overweight (BMI, 26.6 kg/m2 [95% CI, 26.4 to 26.9 kg/m2] and 27.0 kg/m2 [95% CI, 26.7 to 27.1 kg/m2]); white (85.0% [95% CI, 84% to 87%] and 88.2% [95% CI, 87% to 89%]); and male (55.4% [95% CI, 53% to 58%] and 57.1% [95% CI, 55% to 59%]). Approximately one half of the patients (49.4% [95% CI, 47% to 52%] and 51.2% [95% CI, 49% to 53%]) had hypertension; 14.2% (95% CI, 13% to 16%) and 16.8% (95% CI, 15% to 18%) had diabetes; and the baseline creatinine was 0.88 mg/dL (95% CI, 0.87 to 0.89 mg/dL) and 0.83 mg/dL (95% CI, 0.82 to 0.84 mg/dL). The median cisplatin dose was higher (118.0 mg [IQR, 77-151 mg] v 88.7 mg [IQR, 53-142 mg]) and was more frequently fractionated over multiple days (15.9% [95% CI, 14% to 17%] v 1.4% [95% CI, 1% to2%]) in the DC compared with the VC.
Table 1.
Characteristics of Patients in the Development and Validation Cohorts
C-AKI occurred in 13.6% and 11.6% patients in the DC and the VC, respectively, with the majority being Acute Kidney Injury Network–stage I (11% and 9.8%, respectively), and less frequently, stage II (1.9% and 1.4%, respectively) and stage III (0.7% and 0.4%, respectively). Using the alternative definitions of a 1.5 times rise in creatinine and a doubling of creatinine from baseline to peak, the frequency rates of AKI were 7.8% and 2.6%, respectively, in the DC and 5.9% and 1.8%, respectively, in the VC. Baseline eGFR on univariate analysis did have a significant association with C-AKI (odds ratio [OR], 1.8 [95% CI, 1.2 to 2.8]; P = .005). This relation was no longer significant after age was added to the model (OR, 1.2 [95% CI, 0.8 to 1.2]; P = .25). Hence, it seems that the relation between eGFR and C-AKI was driven mostly by age.
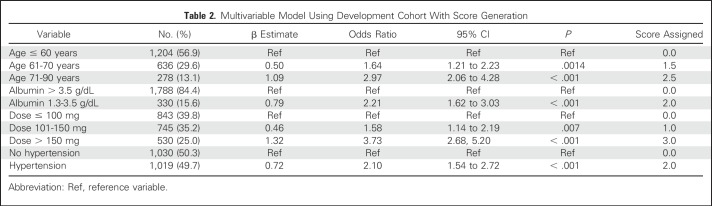
The multivariable logistic regression model considered age, sex, ethnicity, BMI, history of diabetes, history of hypertension, cisplatin dose, fractionated dosing, eGFR, use of diuretics, use of an angiotensin-converting enzyme inhibitor and/or an angiotensin-receptor blocker, use of mannitol, and serum albumin. An angiotensin-converting enzyme inhibitor and/or angiotensin-receptor blocker, diuretics, mannitol, and amifostine were used in 7%, 5%, 54.2%, and < 5%, respectively, of our DC and were not associated with C-AKI on multivariable analysis. Variables statistically significantly associated with C-AKI included a history of hypertension, serum albumin 2.0 to 3.5 g/dL, age older than 60 years, and dose > 100 mg (Table 2). There was no change in the model parameters when eGFR was forced in the model as a continuous variable or as a categorical one. Specifically, there were no variables with a > 10% change in the parameter estimate, and the c-statistic remained identical at 0.72 with or without eGFR in the model. Given this result and the fact that no significant association between eGFR and C-AKI was indicated on multivariable analysis, and because it did not improve the predictive ability of our model, eGFR was omitted. The scores generated for the significant variables are listed in Table 2. The total score for each patient was computed by summing the individual risk factor scores.
Table 2.
Multivariable Model Using Development Cohort With Score Generation
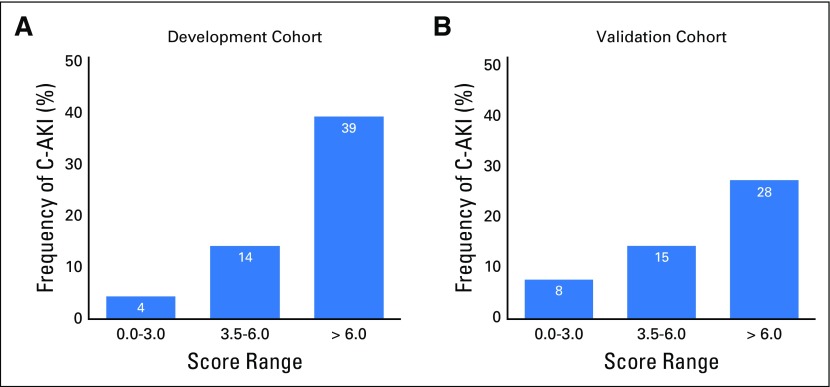
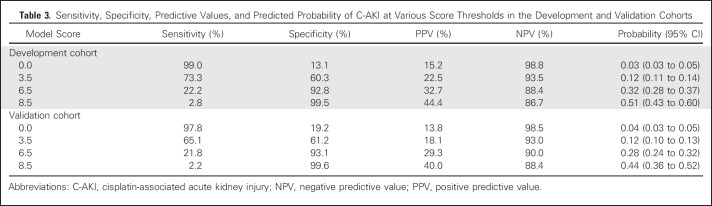
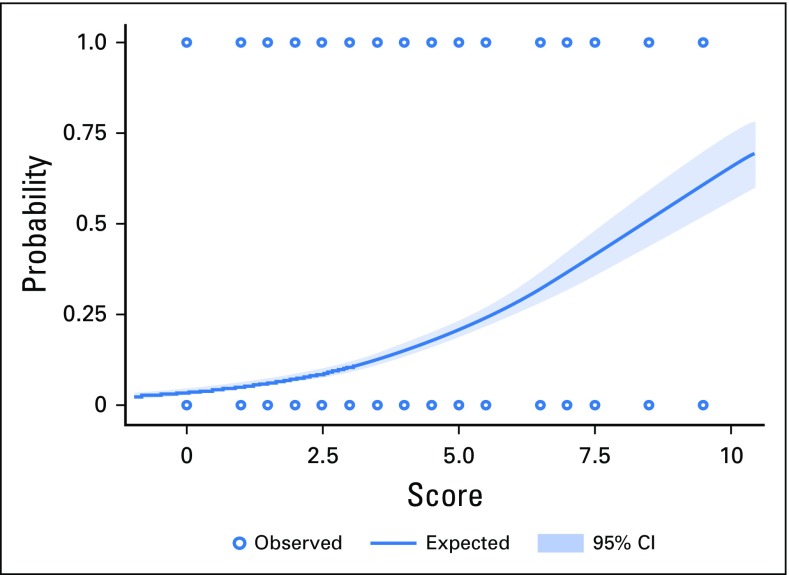
The Hosmer-Lemeshow goodness-of-fit test that is based on deciles of risk indicated that the model was a good fit (P = .16). The c-statistics of the variable-based and score-based models in the DC were identical at 0.72 (95% CI, 0.70 to 0.76). The adjusted OR for C-AKI for a one-unit increase in the score was 1.49 (95% CI, 1.39 to 1.59). In the DC and the VC, an increasing event rate of C-AKI in both cohorts was seen with higher scores—4% and 8% for a score range of 0 to 3; 14% and 15% for a score range of 3.5 to 6; and 39% and 28% for scores > 6 (Fig 2). Simple statistics of the score distributions are listed in Appendix Table A1 (online only). The score range in each cohort was 0 to 9.5, with a median score of 3.0 (IQR, 2.5-4.5) and 3.0 (IQR, 1.5-4.5) in the DC and the VC, respectively. The predicted probability of C-AKI at scores of 3, 5.5, and 8.5 were 0.10 (95% CI, 0.09 to 0.12), 0.24 (95% CI, 0.21 to 0.26), and 0.51 (95% CI, 0.43 to 0.60) in the DC and 0.12 (95% CI, 0.10 to 0.13), 0.28 (95% CI, 0.24 to 0.32), and 0.44 (95% CI, 0.36 to 0.52) in the VC (Table 3 and Fig 3). After applying the score-based model to the VC, the c-statistic was 0.70 (95% CI, 0.67 to 0.73).
Fig 2.
Frequency of cisplatin-associated acute kidney injury (C-AKI) in the development and validation cohorts across score ranges.
Table 3.
Sensitivity, Specificity, Predictive Values, and Predicted Probability of C-AKI at Various Score Thresholds in the Development and Validation Cohorts
Fig 3.
Predicted probability of cisplatin-associated acute kidney injury (C-AKI) across scores, with 95% CIs.
DISCUSSION
This study of > 4,400 patients from two tertiary care cancer centers demonstrates that C-AKI is a relatively common problem, occurring in approximately 13% of patients after the first course of cisplatin. Risk factors predictive of C-AKI included age, history of hypertension, cisplatin dose, and hypoalbuminemia. The predictive model demonstrated reasonably good discrimination and calibration. To our knowledge, this is the largest study of cisplatin nephrotoxicity involving all types of cancer, and the first prediction model for C-AKI to use readily available clinical and demographic data.
After a single course of cisplatin, C-AKI has been reported to occur in 7% to 32% of patients, using various definitions for AKI.9,14,24,25 Risk factors for C-AKI reported in previous studies include older age, female sex, smoking, hypoalbuminemia,26,27 hypokalemia, single dose (v fractionated dose),24 black ethnicity,11 cumulative dose (greater number of chemotherapy cycles), and lower systolic blood pressure.28 However, the findings from some of these studies are limited by a lack of information on reproducibility and by small sample sizes.
Older age and hypertension have been identified as risk factors in some studies.14,27,29 Our study confirmed these associations, albeit with lower magnitudes of association and narrower CIs. Some studies, but not all, have found female sex14,26 and black ethnicity11,14 to be risk factors for C-AKI. However, although we had adequate representation of the two sexes, we did not find a significant association with sex. Our sample consisted predominantly of white patients, limiting our power to examine the association with ethnicity. Some studies24,30,31 have found that C-AKI was less common when the cisplatin dose was divided over 2 to 5 days rather than when administered in a single infusion. However, our study did not replicate that association. Mannitol was administered in 54% of patients in the DC but was not associated with a lower risk of C-AKI in univariate or multivariable analysis, a finding similar to some prior results.32 Despite the widely held assumption that patients with a lower eGFR have a higher risk of C-AKI, this has not been studied systematically and is not supported by evidence-based studies. Within the range of our study, baseline renal function before cisplatin administration was not significantly associated with C-AKI.
Hypoalbuminemia was found to be a risk factor not only for C-AKI,24 but also for AKI in other settings.33-36 In a phase I/II clinical trial of 400 patients treated with cisplatin, de Jongh et al26 found hypoalbuminemia to be an independent risk factor for nephrotoxicity. Our study supports this association and identifies a range of albumin values (2.0 to 3.5 g/dL) at which patients have a higher risk. Hypoalbuminemia may be a surrogate for sicker patients who might have an elevated risk of AKI of any kind, including C-AKI. It is postulated that, because cisplatin is largely protein bound, low serum albumin makes more circulating cisplatin available to be sequestered by the kidney, leading to nephrotoxicity. This mechanism has been implicated in the enhanced toxicity of several other protein-bound drugs.37-41
Our model was developed from routinely collected clinical data. We chose AKI at the end of the first course as our primary outcome to reach a simpler understanding of risk factors involved without influence of recurrent cisplatin exposure from multiple courses, as well as influence of dose adjustments, interruptions, and delays that might result from various toxicities (renal and non-renal) in the setting of cisplatin use. Moreover, we aimed to identify additional factors that should be considered over current standard of care, which is focused heavily on renal function alone. Importantly, Figure 2 suggests that even for patients with a SCr ≤ 1.5 g/dL, there are clear categories of risk, with an increasing frequency of AKI with higher prediction model scores, emphasizing that solely using a renal function cutoff may not be sufficient to predict the risk of C-AKI. Our study included patients with a wide variety of types of cancers, in contrast to the majority of studies, which focused on single cancer types. We validated our model externally in a large population of patients at a different institution and demonstrated good performance.
Our study has limitations. We did not include other chemotherapeutic agents concurrently administered with cisplatin, which may alter the incidence of AKI. Our model was developed using a population that was predominantly white. The model was developed in a tertiary care referral center; hence, its validity in community practice populations remains to be tested. However, it is notable that the variables included in our model are part of the routine care of patients with cancer. We excluded patients with creatinine > 1.5 mg/dL, which constituted only 0.8% and 0.6% of our DC and VC, respectively. The excluded numbers were not large enough to meaningfully study this subgroup. It is possible that there are as yet unidentified predictors of AKI that could be added to improve the model. It is worth noting that the method used for deriving the score depended on the parameter estimates, which may vary on the basis of the population studied.
In conclusion, C-AKI remains a frequent complication of cisplatin use. Our study presents a validated prediction model for C-AKI, developed using readily available patient data. This model will empower providers and patients with more accurate, patient-specific information regarding the risk of kidney injury. Identification of high-risk individuals may facilitate appropriate preventative options such as more frequent laboratory monitoring, avoidance of concurrent use of other renal tubular toxins, and careful evaluation of dosing and administration of additional intravenous fluids. Our study also suggests that baseline kidney function measured by SCr may not be a good predictor of the risk of C-AKI after the first course. Future studies are needed to test the application of this model in clinical practice to determine whether C-AKI in this setting can be predicted and mitigated.
ACKNOWLEDGMENT
We thank Stacey Duey of the Research Patient Data Registry group and Caitlen Fontes and John Orechia of the Oncology Data Retrieval Systems group for aggregation, management, and facilitation of the clinical research data used from the respective databases in this study. We also acknowledge the statistical consultation provided by the Harvard Catalyst and Jiani Hu from Dana-Farber Cancer Institute.
Appendix
Supplementary Methods
The types of tumors treated with cisplatin were lung, testis, ovary, bladder, cervix, vulva, prostate, various head and neck cancers, breast, bone, esophagus, stomach, gall bladder, intestines, skin, thymus, brain, and adrenal gland, and tumors with unknown primary, among others.
In the development and validation cohorts, Lasix was used in 107 patients (5%) and 12 patients (0.5%), hydrochlorothiazide in zero patients (0%) and four patients (0.1%), and ACEI/ARB in 151 patients (7%) and 90 patients (4%), respectively. Among these, only the use of ACEI/ARB was significantly related to C-AKI on univariate analysis, but this relationship was no longer significant in the multivariable model. The other antihypertensives were not associated with C-AKI in either cohort. Mannitol was administered in 1,148 patients (54.2%) in the development cohort and was not significantly associated with C-AKI. Amifostine was used in < 5% of patients in each of cohort and was not associated with C-AKI. We considered including serum magnesium in the model, but because of the substantial amount of missing data (28% in the development cohort and 53% in the validation cohort), it could not be evaluated.
Case-Based Examples
Consider the following two cases in which risk estimation may be helpful for in-office counseling.
Case 1.
A 55-year old man with no significant medical history is diagnosed with bladder cancer. His oncologist would like to prescribe a cisplatin-based regimen with a dose of 50 mg/m2 intravenous every 3 weeks. On the basis of a body surface area of 1.7 m2, his cisplatin dose would be 85 mg. His baseline creatinine is 1.0 mg/dL and his serum albumin is 4.2 g/dL.
His risk scores for C-AKI after the first course would be as follows: age < 60 years = 0; dose < 100 mg = 0; no hypertension = 0; and albumin > 3.5 g/dL = 0. Therefore, his total risk score would be 0 and his predicted probability of developing C-AKI would be 3% (95% CI, 3% to 5%).
Case 2.
A 66-year old woman with a medical history of type 2 diabetes mellitus, hypertension, hyperlipidemia, and morbid obesity with gastric cancer status after radical gastrectomy is prescribed cisplatin-based adjuvant chemotherapy with a dose of 100 mg/m2 every 3 weeks. On the basis of a body surface area of 2.1 m2, her dose would be 210 mg. She has suffered some complications from her gastric surgery and has recently been on total parenteral nutrition. Her baseline creatinine is 1.0 mg/dL and her serum albumin is 2.8 g/dL.
Her risk scores for C-AKI after the first course would be as follows: age 61 to 70 years = 1.5; hypertension = 2; dose > 150 mg = 3; albumin < 3.5 g/dL = 2. Therefore, her total risk score would be 8.5 and her predicted probability of developing C-AKI would be 51% (95% CI, 43% to 60%).
Table A1.
Score Distribution in the Development and Validation Cohorts
AUTHOR CONTRIBUTIONS
Conception and design: Shveta S. Motwani, Benjamin D. Humphreys, Sushrut S. Waikar, Gary C. Curhan
Financial support: Ann H. Partridge, Gary C. Curhan
Administrative support: Ann H. Partridge
Collection and assembly of data: Shveta S. Motwani, Sushrut S. Waikar, Gary C. Curhan
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Development and Validation of a Risk Prediction Model for Acute Kidney Injury After the First Course of Cisplatin
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Shveta S. Motwani
No relationship to disclose
Gearoid M. McMahon
Research Funding: GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Benjamin D. Humphreys
Honoraria: Janssen Pharmaceuticals, Roche, Merck
Research Funding: Biogen Idec
Patents, Royalties, Other Intellectual Property: Evotec AG
Travel, Accommodations, Expenses: Janssen Pharmaceuticals, Roche, Merck
Ann H. Partridge
No relationship to disclose
Sushrut S. Waikar
Consulting or Advisory Role: Cerus, GlaxoSmithKline, MediBeacon, Astellas Pharma, Takeda Pharmaceuticals
Research Funding: Allena Pharmaceuticals, Pfizer, Satellite Healthcare
Expert Testimony: Pfizer, GE Health Care, Janssen Pharmaceuticals
Gary C. Curhan
Stock or Other Ownership: Allena Pharmaceuticals
Consulting or Advisory Role: Allena Pharmaceuticals, AstraZeneca, Decibel Therapeutics
Research Funding: Allena Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Uptodate
REFERENCES
- 1.Surveillance, Epidemiology, and End Results (SEER) : SEER Cancer Statistics Review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/
- 2.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23:460-464, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73:994-1007, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Miller RP, Tadagavadi RK, Ramesh G, et al. : Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2:2490-2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahal A, Bellows BK, Sonpavde G, et al. : Incidence of severe nephrotoxicity with cisplatin based on renal function eligibility criteria: Indirect comparison meta-analysis. Am J Clin Oncol 39:497-506, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Bennis Y, Savry A, Rocca M, et al. : Cisplatin dose adjustment in patients with renal impairment, which recommendations should we follow? Int J Clin Pharm 36:420-429, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Tsao C-K, Moshier E, Seng SM, et al. : Impact of the CKD-EPI equation for estimating renal function on eligibility for cisplatin-based chemotherapy in patients with urothelial cancer. Clin Genitourin Cancer 10:15-20, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Pal SK, Ruel N, Villegas S, et al. : CKD-EPI and cockcroft-gault equations identify similar candidates for neoadjuvant chemotherapy in muscle-invasive bladder cancer. PLoS One 9(4): http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3983198&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidera Y, Kawakami H, Sakiyama T, et al. : Risk factors for cisplatin-induced nephrotoxicity and potential of magnesium supplementation for renal protection. PLoS One 9(7): http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4096506&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagrange JL, Médecin B, Etienne MC, et al. : Cisplatin nephrotoxicity: A multivariate analysis of potential predisposing factors. Pharmacotherapy 17:1246-1253 [PubMed] [Google Scholar]
- 11.Bhat ZY, Cadnapaphornchai P, Ginsburg K, et al. : Understanding the risk factors and long-term consequences of cisplatin-associated acute kidney injury: An observational cohort study. PLoS One 10(11): http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4640577&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu HE, Bai K-J, Hsieh Y-C, et al. : Multiple analytical approaches demonstrate a complex relationship of genetic and nongenetic factors with cisplatin- and carboplatin-induced nephrotoxicity in lung cancer patients. Biomed Res Int 2014: https://www.hindawi.com/journals/bmri/2014/937429/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato K, Watanabe S, Ohtsubo A, et al. : Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer 16: http://www.biomedcentral.com/1471-2407/16/222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faig J, Haughton M, Taylor RC, et al. : Retrospective analysis of cisplatin nephrotoxicity in patients with head and neck cancer receiving outpatient treatment with concurrent high-dose cisplatin and radiotherapy. Am J Clin Oncol [epub ahead of print on June 8, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steyerberg EW, Moons KGM, van der Windt DA, et al. : Prognosis Research Strategy (PROGRESS) 3: Prognostic model research. PLoS Med: http://dx.plos.org/10.1371/journal.pmed.1001381 [DOI] [PMC free article] [PubMed]
- 16.Reilly BM, Evans AT: Translating clinical research into clinical practice: Impact of using prediction rules to make decisions. Ann Intern Med 144:201-209, 2006 [DOI] [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III ): Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report: http://www.ncbi.nlm.nih.gov/pubmed/12485966 [PubMed]
- 18.Lackland DT, Elkind MS V, D’Agostino R, et al. : Inclusion of stroke in cardiovascular risk prediction instruments: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43:1998-2027, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Collins GS, Reitsma JB, Altman DG, et al. : Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Eur Urol 67:1142-1151, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Waikar SS: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol 17:1688-1694, 2006 [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services : Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 22.Ravani P, Parfrey P, Gadag V, et al. : Clinical research of kidney diseases III: Principles of regression and modelling. Nephrol Dial Transplant 22:3422-3430, 2007 [DOI] [PubMed] [Google Scholar]
- 23.McMahon GM, Zeng X, Waikar SS: A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med 173:1821-1827, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart DJ, Dulberg CS, Mikhael NZ, et al. : Association of cisplatin nephrotoxicity with patient characteristics and cisplatin administration methods. Cancer Chemother Pharmacol 40:293-308, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Baek SH, Kim SH, Kim JW, et al. : Effects of a DPP4 inhibitor on cisplatin-induced acute kidney injury: study protocol for a randomized controlled trial. Trials 16: http://www.ncbi.nlm.nih.gov/pubmed/26021829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jongh FE, van Veen RN, Veltman SJ, et al. : Weekly high-dose cisplatin is a feasible treatment option: Analysis on prognostic factors for toxicity in 400 patients. Br J Cancer 88:1199-1206, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasaja Y, Sutandyo N, Andrajati R: Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pac J Cancer Prev 16:1117-1122, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Komaki K, Kusaba T, Tanaka M, et al. : Lower blood pressure and risk of cisplatin nephrotoxicity: A retrospective cohort study. BMC Cancer 17(1): http://www.ncbi.nlm.nih.gov/pubmed/28219368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan KP, Snavely AC, Wind LS, et al. : Rates of renal toxicity in cancer patients receiving cisplatin with and without mannitol. Ann Pharmacother 48:863-869, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Rades D, Seidl D, Janssen S, et al. : Chemoradiation of locally advanced squamous cell carcinoma of the head-and-neck (LASCCHN): Is 20mg/m2 cisplatin on five days every four weeks an alternative to 100mg/m2 cisplatin every three weeks? Oral Oncol 59:67-72, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Rades D, Fehlauer F, Sheikh-Sarraf M, et al. : Toxicity of two cisplatin-based radiochemotherapy regimens for the treatment of patients with stage III/IV head and neck cancer. Head Neck 30:235-241, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Santoso JT, Lucci JA, Coleman RL, et al. : Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: A randomized trial. Cancer Chemother Pharmacol 52:13-18, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Chawla LS, Amdur RL, Amodeo S, et al. : The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79:1361-1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiedermann CJ, Wiedermann W, Joannidis M: Hypoalbuminemia and acute kidney injury: A meta-analysis of observational clinical studies. Intensive Care Med 36:1657-1665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahoti A, Kantarjian H, Salahudeen AK, et al. : Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer 116:4063-4068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee E-H, Baek S-H, Chin J-H, et al. : Preoperative hypoalbuminemia is a major risk factor for acute kidney injury following off-pump coronary artery bypass surgery. Intensive Care Med 38:1478-1486, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Reiss SN, Buie LW, Adel N, et al. : Hypoalbuminemia is significantly associated with increased clearance time of high dose methotrexate in patients being treated for lymphoma or leukemia. Ann Hematol 95:2009-2015, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Maat MM, van Leeuwen HJ, Edelbroek PM: High unbound fraction of valproic acid in a hypoalbuminemic critically ill patient on renal replacement therapy. Ann Pharmacother 45: http://www.ncbi.nlm.nih.gov/pubmed/21325101 [DOI] [PubMed] [Google Scholar]
- 39.Van Berkel MA, Hurdle AC, Twilla JD: Phenytoin-rifampin drug interaction in a hypoalbuminemic, renal failure patient: A complex clinical case. Pharmacother J Hum Pharmacol Drug Ther 33:e96-e100, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Zusman O, Farbman L, Tredler Z, et al. : Association between hypoalbuminemia and mortality among subjects treated with ertapenem versus other carbapenems: Prospective cohort study. Clin Microbiol Infect 21:54-58, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Ulldemolins M, Roberts JA, Rello J, et al. : The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 50:99-110, 2011 [DOI] [PubMed] [Google Scholar]