Abstract
Purpose
There are no approved treatments for recurrent/metastatic head and neck squamous cell carcinoma refractory to platinum and cetuximab. In the single-arm, phase II KEYNOTE-055 study, we evaluated pembrolizumab, an anti–programmed death 1 receptor antibody, in this platinum- and cetuximab-pretreated population with poor prognosis.
Methods
Eligibility stipulated disease progression within 6 months of platinum and cetuximab treatment. Patients received pembrolizumab 200 mg every 3 weeks. Imaging was performed every 6 to 9 weeks. Primary end points: overall response rate (Response Evaluation Criteria in Solid Tumors v1.1, central review) and safety. Efficacy was assessed in all dosed patients and in subgroups on the basis of programmed death ligand 1 (PD-L1) expression and human papillomavirus (HPV) status.
Results
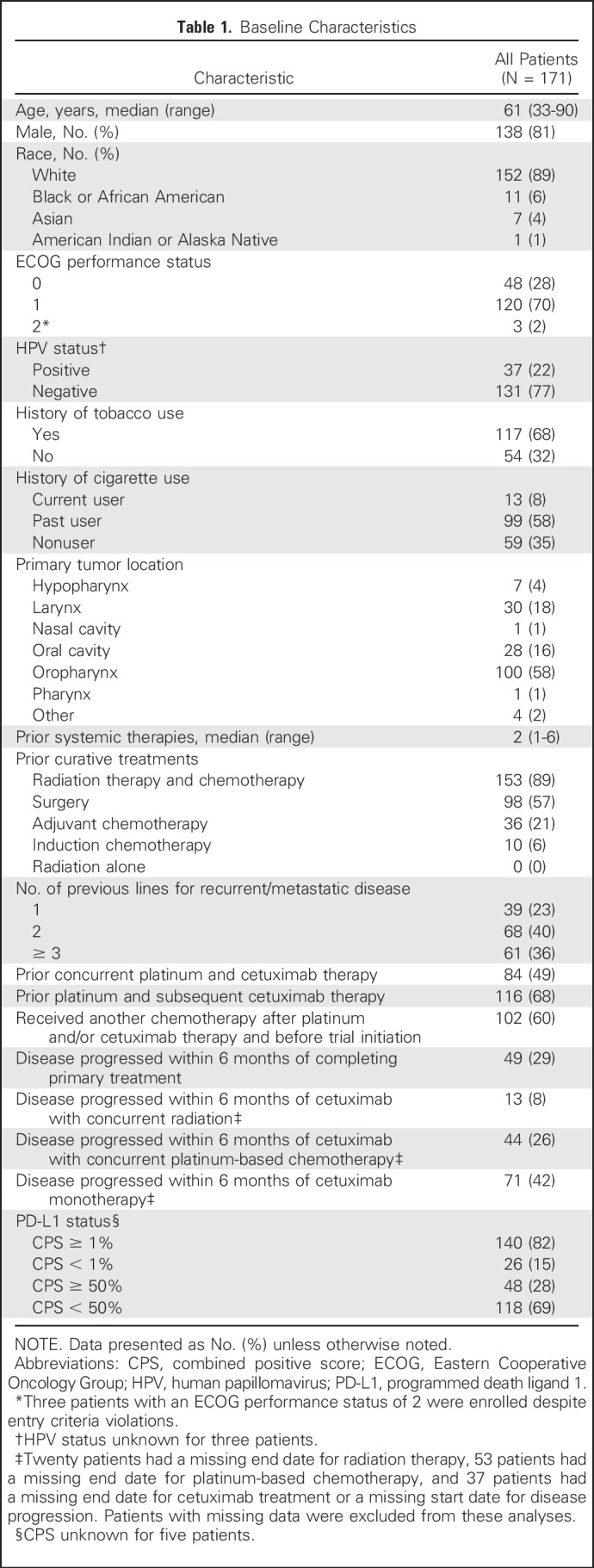
Among 171 patients treated, 75% received two or more prior lines of therapy for metastatic disease, 82% were PD-L1 positive, and 22% were HPV positive. At the time of analysis, 109 patients (64%) experienced a treatment-related adverse event; 26 patients (15%) experienced a grade ≥ 3 event. Seven patients (4%) discontinued treatment, and one died of treatment-related adverse events. Overall response rate was 16% (95% CI, 11% to 23%), with a median duration of response of 8 months (range, 2+ to 12+ months); 75% of responses were ongoing at the time of analysis. Response rates were similar in all HPV and PD-L1 subgroups. Median progression-free survival was 2.1 months, and median overall survival was 8 months.
Conclusion
Pembrolizumab exhibited clinically meaningful antitumor activity and an acceptable safety profile in recurrent/metastatic head and neck squamous cell carcinoma previously treated with platinum and cetuximab.
INTRODUCTION
In locally advanced head and neck squamous cell carcinoma (HNSCC), an aggressive approach that combines chemotherapy, surgery, and/or radiotherapy improves survival and reduces risk of recurrence.1 For recurrent/metastatic (R/M) HNSCC not amenable to curative-intent treatment, palliative chemotherapy is the mainstay of therapy, but efficacy of such treatments is limited.2 The best median overall survival for treatment in the first-line setting of R/M HNSCC (10 months) used a combination of cetuximab, platinum, and fluorouracil.3 After progression on or after platinum and cetuximab, there are no approved treatment options.4 Methotrexate, which is commonly prescribed in this setting, yields an overall response rate of 6% and median overall survival of 6 months.5
Immunotherapy targeting the programmed death 1 (PD-1) pathway is effective for a wide range of tumors.6-9 The PD-1–programmed death ligand 1 (PD-L1) interaction is implicated in immune escape in HNSCC, with evidence of overexpression of the PD-1 ligands, PD-L1 and PD-L2, both on tumor cells and within the tumor microenvironment.10-12 Upregulation of this pathway may allow the tumor to evade immune surveillance.10,13,14
Pembrolizumab (MK-3475) is an anti–PD-1 antibody that disrupts the interaction between PD-1 and its ligands. It has demonstrated robust antitumor activity and a favorable safety profile in multiple tumor types and is currently approved for R/M HNSCC with disease progression on or after platinum-containing chemotherapy in the United States.9,15-18 In HNSCC, pembrolizumab was well tolerated and exhibited durable antitumor activity in patients with R/M HNSCC during the multicohort, phase Ib KEYNOTE-012 study (ClinicalTrials.gov identifier, NCT01848834).7,19 KEYNOTE-012 had no prior therapy requirements.
KEYNOTE-055 (ClinicalTrials.gov identifier, NCT02255097) is the first study, to our knowledge, designed to evaluate efficacy and safety of pembrolizumab in patients with R/M HNSCC resistant to both platinum and cetuximab. We report here the results observed in this single-arm, phase II study.
METHODS
Patients
Eligible patients were ≥ 18 years old with confirmed R/M HNSCC of the oral cavity, oropharynx, hypopharynx, or larynx resistant to both platinum and cetuximab. Concurrent platinum and cetuximab treatment was not required, but patients were required to have had progressive disease or recurrence within 6 months of the last dose of each therapy. Additional eligibility criteria included measurable disease, provision of newly obtained core or excisional biopsy for PD-L1 expression analysis, Eastern Cooperative Oncology Group performance status of 0 to 1,20 and adequate organ function. There was no limit to the number of prior systemic therapies for R/M disease. Patients were excluded if they had active CNS metastases, carcinomatous meningitis, autoimmune disorders requiring systemic treatment, noninfectious pneumonitis, known hepatitis B or C infection, additional malignancies requiring active treatment, or history of HIV infection. Previous treatment with drugs specifically targeting the PD-1 pathway was not allowed. Systemic immunosuppressive therapy had to be concluded within 7 days; chemotherapy, targeted small molecule therapy, or radiation therapy within 2 weeks; and anticancer monoclonal antibody therapy within 4 weeks before first dose of pembrolizumab.
Study Oversight
The study protocol was approved by regulatory boards or ethics review committees at each study center. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent before study entry. Study investigators participating in the trial are listed in Appendix Table A1 (online only).
Study Design and Treatment
KEYNOTE-055 is a multicenter, phase II, single-arm study. Patients received pembrolizumab 200 mg intravenously every 3 weeks until documented progressive disease, intolerable toxicity, intercurrent illness preventing further treatment, patient or physician decision to withdraw, or completion of 24 months of treatment. In the case of radiographic progressive disease, progression was to be confirmed by repeat imaging performed ≥ 4 weeks later. If the repeat imaging assessment showed a < 20% tumor burden compared with nadir or stabilization or improvement of the lesion, progressive disease was not confirmed and the patient was allowed to remain on treatment. Patients achieving a complete response who received ≥ 24 weeks of pembrolizumab could discontinue therapy. Patients who stopped pembrolizumab after achieving a confirmed complete response or after completion of 2 years of treatment could receive up to an additional year of treatment on subsequent progression.
Study Assessments
Tumor assessments were performed by computed tomography or magnetic resonance imaging at baseline, 9 weeks after the first dose, every 6 weeks thereafter for the first year, and every 9 weeks thereafter. Primary efficacy end point of overall response rate, defined as the proportion of patients with complete or partial response, was assessed by central imaging vendor review using Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1). Key secondary end points included progression-free survival, overall survival, and duration of response. Progression-free survival was defined as time from allocation to progressive disease according to RECIST v1.1 or death due to any cause, whichever occurred first. Overall survival was defined as time from allocation to death due to any cause. Duration of response was the interval from first RECIST v1.1–recorded response to progressive disease in patients who achieved at least a partial response.
Overall response rates in human papillomavirus (HPV) –positive and PD-L1–positive patients were prespecified secondary end points. HPV status for patients with oropharyngeal tumors was determined by local institution. Although the majority of institutions used p16 immunohistochemistry, this methodology was not protocol mandated. Patients with nonoropharyngeal disease were considered to be HPV negative. PD-L1 expression was retrospectively evaluated using an investigational version of the PD-L1 IHC 22C3 pharmDx assay (Dako North America, Carpinteria CA) that has been approved as a companion diagnostic for use in non–small-cell lung cancer in the United States.21 The staining protocol used in this study was as described in the instructions for the commercial assay. Expression was scored using a combined positive score (CPS) and defined as the percentage of tumor and mononuclear inflammatory cells within the tumor nests and adjacent supporting stroma expressing PD-L1 at any intensity. CPS was chosen as an exploratory biomarker because of the association between PD-L1 expression on tumor and inflammatory cells and response to pembrolizumab observed during the KEYNOTE-012 trial.19 CPS was measured on a scale from 0 to 100%. PD-L1 positivity was defined using a CPS ≥ 1% cutoff. Data were analyzed based on raw scores from the initial read for CPS ≥ 50%.
Adverse events were monitored throughout the study and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Patients were followed for an additional 30 days for adverse events and 90 days for serious adverse events and events of interest after discontinuing treatment. Certain events of interest were preselected based on potential immune-related etiology; these events are referred to as immune-mediated adverse events and are reported regardless of causality.
Statistical Analyses
Efficacy and safety end points were assessed in all patients who received one or more doses of pembrolizumab. Target enrollment size was 150 patients. It was assumed that at least 135 of these patients would be evaluable for the primary efficacy analysis. On the basis of this assumption, the study was designed to have approximately 85% power to demonstrate that the overall response rate is > 5%, with a type I error rate of 1.25% if true overall response rate was 13%. Success for this hypothesis required responses in at least 14 of 135 patients.
Response rates, 95% CIs, and one-sided P values for testing the null hypotheses were estimated using the exact binomial method. Patients with missing baseline or postbaseline data were considered nonresponders. The study was considered to have achieved its efficacy objective if the P value for the primary hypothesis was < .0125.
Kaplan-Meier statistics were applied for estimates of progression-free survival, overall survival, and duration of response. Patients without a postbaseline efficacy analysis or without survival data were censored at day 1. Patients with missing progression-free survival or overall survival data were censored at the date of their last assessment. Additional statistical details are provided in the Data Supplement.
RESULTS
Patients
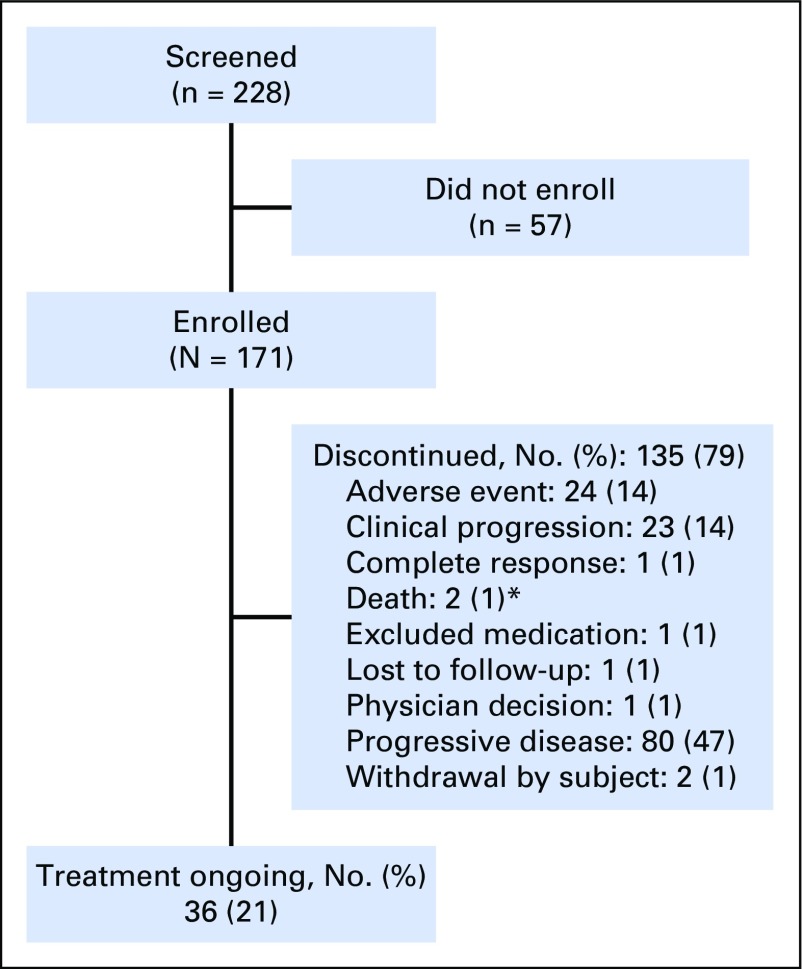
From October 24, 2014, through September 23, 2015, 228 patients were screened; of these, 171 were enrolled and received one or more doses of pembrolizumab (Fig 1). Most common reasons for screen failure were decline in Eastern Cooperative Oncology Group performance status to > 1 (24 patients), CNS metastases and/or carcinomatous meningitis (10 patients), inadequate organ function (seven patients), and unwilling and/or unable to provide consent (seven patients). Median age was 61 years; 81% were male, 68% reported previous tobacco use, and 75% received two or more lines of prior systemic therapy (Table 1). The majority of patients (140 of 171; 82%) were PD-L1 positive using a CPS of ≥ 1%; 48 (28%) had a CPS of ≥ 50%. Thirty-seven patients (22%) were HPV positive; 131 (77%) were HPV negative. The data-cutoff date for these analyses was April 22, 2016. At that time, the median (range) follow-up duration was 7 (0 to 17) months, and 36 patients (21%) were still receiving pembrolizumab.
Fig 1.
Patient disposition. *One patient died because of cardiac arrest (not treatment related) and one died because of pneumonitis (treatment related).
Table 1.
Baseline Characteristics
Adverse Events
At the time of data cutoff, patients had received pembrolizumab for a median (range) of 90 (1 to 401) days. Treatment-related adverse events of any grade were reported in 109 (64%) patients; most common were fatigue, hypothyroidism, nausea, AST increase, and diarrhea (Table 2). The majority of treatment-related adverse events were of grade 1 or 2; 26 patients (15%) experienced an event of grade 3 or higher. The only immune-mediated adverse events (any grade or treatment attribution) reported in ≥ 2% of patients were hypothyroidism (27 of 171; 16%), pneumonitis (seven of 171; 4%), and hyperthyroidism (four of 171; 2%). Seven patients (4%) discontinued because of treatment-related adverse events (Appendix Table A2, online only). One patient died of treatment-related pneumonitis.
Table 2.
Treatment-Related Adverse Events in All Treated Patients (N = 171)

Clinical Activity
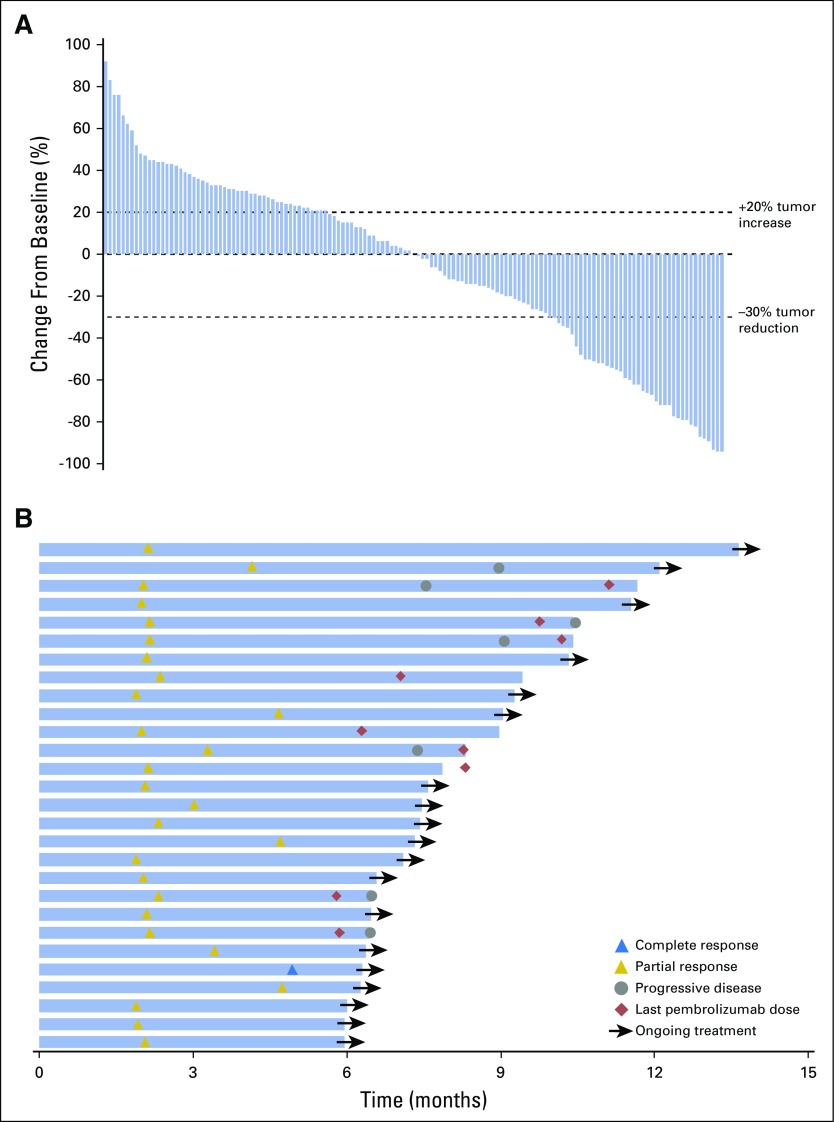
Overall response rate was 16% (95% CI, 11% to 23%; P < .001; Table 3). One patient achieved complete response, 27 patients (16%) achieved partial response, and 33 patients (19%) and 87 patients (51%) experienced stable and progressive disease, respectively. Response rates were similar regardless of HPV status, with rates of 16% in HPV-positive patients and 15% in HPV-negative patients (Table 3). Overall, 50% (70 of 141) of evaluable patients experienced reduction from baseline in target lesion size (Fig 2A).
Table 3.
Antitumor Activity of Pembrolizumab
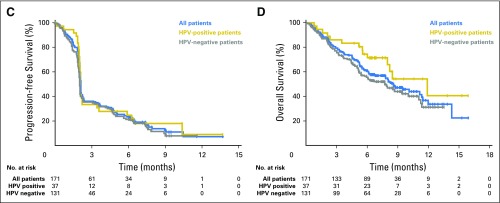
Fig 2.
Efficacy of pembrolizumab. (A) The best percentage change from baseline in target lesions (ie, difference in size of target lesions between baseline and postbaseline assessments divided by baseline target lesion size times 100) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) by central imaging vendor review (n = 141). (B) Treatment exposure and response duration in patients with a confirmed complete or partial response per RECIST v1.1 by central imaging vendor review (n = 28). (C) Kaplan-Meier estimate of progression-free survival per RECIST v1.1 by central imaging vendor review. (D) Kaplan-Meier estimate of overall survival. HPV, human papillomavirus.
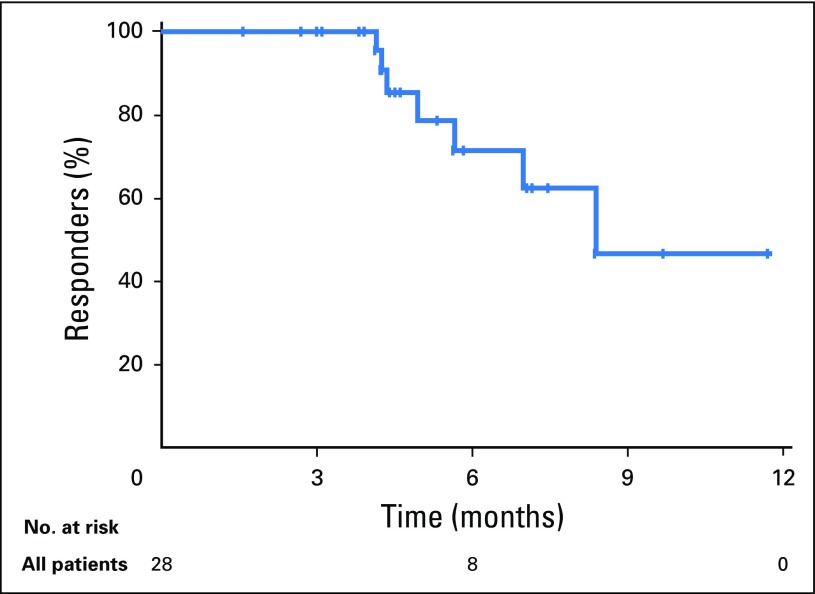
At data cutoff, median (range) time to response was 2 (2 to 5) months. Median (range) follow-up time for responders was 9 (7 to 17) months. Median (range) response durations were 8 (2+ to 12+) months in all responders (Appendix Fig A1, online only), not reached (3+ to 12+ months) in HPV-positive responders, and 7 (2+ to 10+) months in HPV-negative responders. At the data cutoff, 21 patients (75% of responders) had an ongoing response, and eight responses had lasted ≥ 6 months (Fig 2B).
Median progression-free survival was 2.1 months (95% CI, 2.1 to 2.1) in all patients and did not differ based on HPV status (Fig 2C). The 6-month progression-free survival rate was 23% in all patients, 25% in HPV-positive patients, and 21% in HPV-negative patients. Median overall survival was 8 months (95% CI, 6 to 11 months) in all patients, with similar survival observed regardless of HPV status (Fig 2D). Overall survival at 6 months was 59% in all patients, 72% in HPV-positive subgroups, and 55% in HPV-negative subgroups.
Clinical Activity by PD-L1 Status
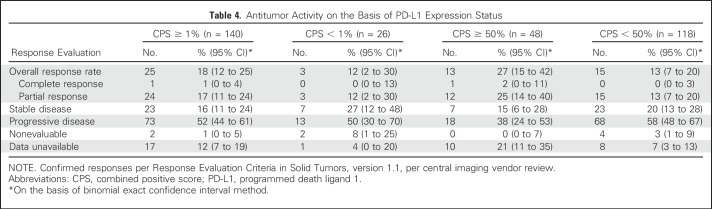
Overall response rates (95% CI) by PD-L1 expression status using a CPS cutoff of 1% were 18% (12% to 25%) in PD-L1–positive patients (CPS ≥ 1%) and 12% (2% to 30%) in PD-L1–negative patients (CPS < 1%; Table 4). When the CPS was analyzed to 50% on the basis of raw scores, rates (95% CI) were 27% (15% to 42%) in patients with CPS ≥ 50% and 13% (7% to 20%) in patients with CPS < 50%. The one complete response achieved during the study was observed in a patient with a CPS of ≥ 50%. Six-month progression-free survival rates in PD-L1–positive patients were 24% (CPS ≥ 1%) and 31% (CPS ≥ 50%); rates were 20% and 20% in patients with CPS < 1% and CPS < 50%, respectively. Overall survival at 6 months in PD-L1–positive patients was 59% (CPS ≥ 1%) and 60% (CPS ≥ 50%); rates were 56% and 58% in patients with CPS < 1% and CPS < 50%, respectively. Kaplan-Meier estimates of progression-free survival and overall survival are outlined in Appendix Figure A2 (online only).
Table 4.
Antitumor Activity on the Basis of PD-L1 Expression Status
DISCUSSION
We have demonstrated that pembrolizumab exhibited clinically significant antitumor activity and a manageable safety profile in patients with HNSCC whose disease progressed on both platinum and cetuximab. The primary objective of this study was met; 16% of patients achieved confirmed response by central review, and responses were durable, with some > 12 months at the time of this publication. These results are consistent with those reported previously for the KEYNOTE-012 study, in which 18% of patients with R/M HNSCC responded to pembrolizumab. No specific prior treatments were mandated in KEYNOTE-012.7,19 In both studies, patients responded to pembrolizumab regardless of HPV status. In addition, given the median overall survival of 8 months reported in the current study and in the R/M HNSCC cohorts of KEYNOTE-012,7,19 survival with pembrolizumab in R/M HNSCC is encouraging, especially when considered in conjunction with the toxicity profile and in comparison with historical reference populations.3-5 It is particularly notable that patients in KEYNOTE-055 had prolonged responses, given that increasing lines of therapy are generally associated with worse outcomes in oncology. Pembrolizumab may therefore lead to significant improvements in outcomes for some patients regardless of prior treatment with platinum and cetuximab.
To our knowledge, our study is the first and largest report of data from a phase II trial investigating PD-1 inhibition in patients with R/M HNSCC refractory to both platinum and cetuximab, a patient population with poor outcome and no approved treatment options. Patients in this study were heavily pretreated, with 75% receiving two or more prior lines of systemic therapy. There is a paucity of efficacy data in the literature for patients with R/M HNSCC refractory to both platinum and cetuximab with which to compare these results. Nonetheless, overall response and survival rates reported with pembrolizumab seem favorable even when compared with other treatments in patients with fewer prior therapies. For example, response rates to single-agent cetuximab, afatinib, and methotrexate in patients with R/M HNSCC who progressed after treatment with platinum were 10%, 10%, and 6%, respectively.5,22 In addition, median overall survival seen with pembrolizumab in the current study was encouraging compared with that seen with afatinib (7 months) and methotrexate (6 months) in previously treated R/M disease.5 Results were similar to those from the phase III CheckMate-141 trial in which nivolumab, another anti–PD-1 antibody, resulted in improvement in overall survival (8 months) compared with investigator’s choice standard of care (5 months) in patients with platinum-refractory R/M HNSCC.23 Consistent with prior studies of immunotherapy in cancer, overall survival in KEYNOTE-055 was encouraging despite no apparent improvement in progression-free survival. This consistent finding across multiple studies implies that progression-free survival may not be the best outcome of interest for immunotherapy trials.
Importantly, pembrolizumab was well tolerated in a patient population that has already endured aggressive, toxic chemotherapy regimens; 64% of patients experienced a treatment-related adverse event, the majority of which were grade 1 to 2. Few patients, however, were removed from treatment because of toxicity. The safety profile of pembrolizumab seen in this study was consistent with the profile previously reported in R/M HNSCC and similar to that observed with PD-1 inhibition using nivolumab.7,9,15-18,23
Results reported here add to a growing body of evidence that patients whose tumors express PD-L1 may be more likely to respond to PD-1 pathway inhibition.7,19,23 In CheckMate-141, 17% of patients with PD-L1 expression of ≥ 1% of tumor cells responded to treatment with nivolumab.23 Similarly, in the current study, 18% of patients with ≥ 1% PD-L1 expression responded to pembrolizumab compared with 12% of patients with < 1% expression. It should be noted that our study included a small number of patients with CPS < 1% (n = 26); these findings should be interpreted with this limitation in mind. In both CheckMate-141 and KEYNOTE-055, higher response rates were noted in patients with higher PD-L1 expression. One difference between these two analyses is consideration of PD-L1 expression, not only on tumor cells but also on tumor-associated inflammatory cells. Findings from KEYNOTE-012 demonstrated the importance of PD-L1 expression on inflammatory cells, because a difference in response rate was not seen when measuring expression on tumor cells alone.19 Thus, inflammatory cells were incorporated into the scoring system used in the current study. Nonetheless, even PD-L1–negative patients responded to pembrolizumab at a rate that is clinically meaningful; 6- and 12-month progression-free survival and overall survival rates were relatively similar between PD-L1–negative and PD-L1–positive patients. These data suggest that therapeutic benefit of pembrolizumab is not limited to patients with PD-L1–positive tumors. In light of these results, we are not currently advocating for the use of CPS as a clinical decision method for whether patients should receive pembrolizumab. The investigation of additional biomarkers to aid in appropriate patient selection for PD-1 inhibitors, which have shown activity in a wide range of malignancies, continues to be an important area of study. Identifying a biomarker with adequate negative predictive value would have significant value for this patient population.
In conclusion, pembrolizumab exhibited clinically significant antitumor activity and an acceptable safety profile in heavily pretreated R/M HNSCC regardless of HPV status. Results from this study indicate that pembrolizumab is an active agent for a patient population with limited options. The robust clinical activity demonstrated in this trial confirms the activity of this class of agents and supports ongoing immunotherapy studies in head and neck cancer.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in the study. We also thank Deepti Aurora-Garg, Cecilia Thomas, Allison Moore, Tim Sausser, Robin Mogg, and Lingkang Huang (employee of Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Kenilworth, NJ) for their contributions. Medical writing assistance was provided by Matthew Grzywacz, PhD, and Dana Francis, PhD, of the oncology team at ApotheCom, Yardley, PA. This assistance was funded by Merck & Co., Inc., Kenilworth, NJ.
Appendix
Fig A1.
Duration of response in patients with confirmed response per Response Evaluation Criteria in Solid Tumors, version 1.1, by central imaging vendor review (n = 28).
Fig A2.
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival by programmed death ligand 1 (PD-L1) status.
Table A1.
Investigators Participating in the Trial
Table A2.
Discontinuations Because of Treatment-Related Adverse Events
Footnotes
Supported by Merck & Co., Inc., Kenilworth, NJ.
Presented in part at the 2016 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016, and the European Society for Medical Oncology 2016 Congress, Copenhagen, Denmark, October 7-11, 2016.
Clinical trial information: NCT02255097.
Listen to the podcast by Dr Psyrri at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan D. Cheng
Administrative support: Amy Meister
Provision of study materials or patients: Joshua Bauml, Tanguy Y. Seiwert, Stephen V. Liu, Jared Weiss, Robert Haddad
Collection and assembly of data: Joshua Bauml, Tanguy Y. Seiwert, David G. Pfister, Stephen V. Liu, Nabil F. Saba, Jared Weiss, Lori Wirth, Ammar Sukari, Hyunseok Kang, Michael K. Gibson, Steven Powell, Amy Meister, Xinxin Shu, Robert Haddad
Data analysis and interpretation: Joshua Bauml, Tanguy Y. Seiwert, David G. Pfister, Francis Worden, Stephen V. Liu, Jill Gilbert, Nabil F. Saba, Jared Weiss, Lori Wirth, Hyunseok Kang, Erminia Massarelli, Steven Powell, Amy Meister, Xinxin Shu, Jonathan D. Cheng, Robert Haddad
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Joshua Bauml
Consulting or Advisory Role: Clovis Oncology, Bristol-Myers Squibb, Merck, AstraZeneca, Genentech, Celgene, Guardant Health, Boehringer Ingelheim
Research Funding: Merck (Inst), Carevive Systems (Inst), Novartis (Inst), Incyte (Inst)
Tanguy Y. Seiwert
Consulting or Advisory Role: Merck, Amgen, Bristol-Myers Squibb, AstraZeneca, Innate Pharma, Eli Lilly
Honoraria: Merck, Amgen, Bristol-Myers Squibb, AstraZeneca, Innate Pharma, Eli Lilly
Travel, Accomodations, Expenses: Merck, Amgen, Bristol-Myers Squibb, AstraZeneca, Innate Pharma, Eli Lilly
David G. Pfister
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: AstraZeneca, Bayer AG, MedImmune, Merck
Francis Worden
Consulting or Advisory Role: Merck
Research Funding: Bristol-Myers Squibb, Merck, AstraZeneca, Galera Therapeutics
Travel, Accommodations, Expenses: Merck
Stephen V. Liu
Consulting or Advisory Role: Genentech, Boehringer Ingelheim, Pfizer, ARIAD Pharmaceuticals, Eli Lilly, Celgene
Research Funding: Genentech/Roche, Pfizer, Threshold Pharmaceuticals, Clovis Oncology, Corvus Pharmaceuticals, Esanex, Bayer AG, OncoMed, Ignyta, Merck, MedImmune
Jill Gilbert
Leadership: American Board of Internal Medicine
Honoraria: Sanofi
Consulting or Advisory Role: TRM Oncology
Research Funding: AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Karyopharm Therapeutics (Inst), Pfizer (Inst), Threshold Pharmaceuticals (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Nabil F. Saba
Consulting or Advisory Role: Merck, Bristol-Myers Squibb, Pfizer, Eli Lilly
Jared Weiss
Consulting or Advisory Role: Biodesix, AstraZeneca, OncoPlex Diagnostics, Eli Lilly, EMD Serono, Genentech
Research Funding: Astellas Pharma (Inst), Celgene (Inst), Pfizer (Inst), Novartis (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Lori Wirth
Consulting or Advisory Role: Merck, Amgen, Eisai, Blueprint, Loxo
Ammar Sukari
Stock or Other Ownership: Bristol-Myers Squibb
Consulting or Advisory Role: Eisai
Speakers' Bureau: Novartis Oncology, Merck
Hyunseok Kang
Honoraria: AstraZeneca
Research Funding: VentiRx Pharmaceuticals (Inst), Merck (Inst), AstraZeneca (Inst), Plexxikon (Inst), Bristol-Myers Squibb (Inst), Advaxis (Inst), Novartis (Inst)
Michael K. Gibson
Honoraria: MedImmune
Research Funding: AstraZeneca/MedImmune
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Erminia Massarelli
Consulting or Advisory Role: Nektar
Research Funding: Merck (Inst), MedImmune (Inst), Bristol-Myers Squibb (Inst), AstraZeneca, (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Steven Powell
Research Funding: Merck, Bristol-Myers Squibb, Incyte, Genentech, Novartis, Pfizer (Inst)
Amy Meister
Employment: Merck
Stock or Other Ownership: Merck
Xinxin Shu
Employment: Merck
Jonathan D. Cheng
Employment: Merck
Stock or Other Ownership: Merck
Robert Haddad
Consulting or Advisory Role: Celgene, Merck, Eisai, Bristol-Myers Squibb, Pfizer, AstraZeneca
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), AstraZeneca (Inst), Eisai
REFERENCES
- 1.Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4:156–171. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- 2.Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13:35–46. doi: 10.1007/s11864-011-0176-y. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Echarri MJ, Lopez-Martin A, Hitt R. Targeted therapy in locally advanced and recurrent/metastatic head and neck squamous cell carcinoma (LA-R/M HNSCC) Cancers (Basel) 2016;8:E27. doi: 10.3390/cancers8030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machiels JP, Haddad RI, Fayette J, et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:583–594. doi: 10.1016/S1470-2045(15)70124-5. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 8.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 10.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 11.Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yearley J, Gibson G, Yu N, et al. 18LBA PD-L2 expression in human tumors: Relevance to anti-PD-1 therapy in cancer. Eur J Biochem. 2016;51:S718. doi: 10.1158/1078-0432.CCR-16-1761. (abstr) [DOI] [PubMed] [Google Scholar]
- 13.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sui X, Ma J, Han W, et al. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget. 2015;6:19393–19404. doi: 10.18632/oncotarget.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 17.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 18.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. doi: 10.1200/JCO.2016.68.1478. Chow LQM, Haddad R, Gupta S, et al: Antitumor activity of pembrolizuamb in biomarker-unselected patients with recurrent/metastatic head and neck squamous cell carcinoma: Results from the phase 1b KEYNOTE-012 expansion cohort. J Clin Oncol 34:3838-3845, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 21. Dako: Dako, an Agilent technologies company, announces FDA approval of new companion diagnostic for lung cancer. http://www.agilent.com/about/newsroom/presrel/2016/24oct-ca16033.html.
- 22.Seiwert TY, Fayette J, Cupissol D, et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann Oncol. 2014;25:1813–1820. doi: 10.1093/annonc/mdu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]