Abstract
Introduction:
In the United States, opioid regulations have become increasingly stringent in recent years. Increased regulatory scrutiny, in part, is related to heightened awareness through literature and a recent media blitz on the opioid prescription epidemic. These regulations have the potential to impact prescription trends by health care providers. Our objective was to evaluate changes in the type and dose of opioid prescriptions among patients who are referred by oncologists to an outpatient palliative care clinic.
Materials and Methods:
We reviewed the electronic health records of 750 patients who were seen as new consultations at MD Anderson Cancer Center’s outpatient palliative care clinic between January 1 and April 30 each year from 2010 through 2015. Data collected included demographics, cancer type and stage, symptom assessment, performance status, opioid type, and opioid dose defined as the morphine equivalent daily dose (MEDD).
Results:
Median age was 59 years (interquartile range [IQR], 51 to 67), 383 (51%) were female, 529 (70%) were white, and 654 (87%)of patients had advanced cancer. In 2010, median MEDD before referral was 78 mg/d (IQR, 30 to 150); however, by 2015, the MEDD had progressively decreased to 40 mg/d (IQR, 19 to 80; P = .001). Hydrocodone was the most common opioid prescribed between 2010 and 2015; however, after its reclassification as a schedule II opioid in October 2014, the use of tramadol, a schedule IV opioid, increased (P < .001).
Conclusion:
During the past several years, the MEDD prescribed by referring oncologists has decreased. After hydrocodone reclassification, the use of tramadol with less stringent prescription limits has increased.
INTRODUCTION
Pain is a common symptom experienced by patients with cancer throughout the trajectory of their illness. A recent meta-analysis found that cancer pain is reported by 39% of survivors of cancer, by 55% of patients who undergo active cancer treatment, and by 66% of patients with advanced, metastatic, or terminal-stage disease.1 Undertreated cancer pain is associated with several other physical and psychosocial symptoms and can impact quality of life.2-5 The effectiveness of opioids in treating cancer-related pain and dyspnea has been well established.6-8 Guidelines published by various organizations, such as the WHO,9,10 National Comprehensive Cancer Network,11 European Association for Palliative Care,12 European Society of Medical Oncology,13 and, most recently, the ASCO Adult Cancer Survival Guidelines,14 have clarified and highlighted the importance of the appropriate assessment and management of pain, including the use of opioids.11-14
In the United States, opioids are highly regulated at both the federal and state level.15 Opioids are considered controlled substances under the Controlled Substances Act and are divided in distinct schedules or categories depending on their abuse or dependency potential.16 Schedule II opioids have the most stringent prescription limits. To control the opioid prescription epidemic and related misuse, abuse, and deaths, several regulatory changes have recently been implemented. Some examples include the risk evaluation and mitigation strategy (REMS) for extended-release and long-acting opioids issued by the US Food and Drug Administration in July 2012,17 mandatory sharing of prescription data with state-run prescription drug monitoring programs (PDMPs; in Texas, this program was launched in full scale in June 2012),18 and, most recently, the reclassification of hydrocodone from schedule III to schedule II by the US Drug Enforcement Administration in October 2014.19 These increasingly stringent regulations might be a result, in part, of heightened awareness through literature and the recent media blitz on the detrimental consequences of the opioid prescription epidemic.
Changes in regulations have been shown to affect prescription trends, which can influence the availability of opioids to the population of patients with cancer.20 To our knowledge, no study has evaluated the impact of recent regulatory changes on the type and dose of opioids that are prescribed by oncologists and their inclination to consult palliative care services for pain management. The primary objective of this study was to evaluate the changes in the type and dose of opioids among patients who were referred by oncologists to an outpatient palliative care clinic during the past 6 years. We hypothesized that, as stricter regulations were implemented, patients who were referred to MD Anderson Cancer Center’s palliative care clinic for pain management would present with a decreased morphine equivalent daily dose (MEDD) and increased use of non–schedule II—schedule III, schedule IV, or nonscheduled—opioids.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center. The requirement for informed consent was waived by the institutional review board. We reviewed the electronic health records of 750 patients who were seen as new consultations at MD Anderson Cancer Center’s outpatient palliative care clinic between January 1 and April 30 each year from 2010 through 2015—75 randomly selected patients in each of the years from 2010 to 2014 and 375 in 2015. These sample sizes were based on the workload—least number of provider’s vacation time, holidays, and appointment cancellations on the basis of our observations—and on the possibility of comparing the average MEDD used in 2015 with the ones used in 2010 to 2014 because of the reclassification of hydrocodone as a schedule II opioid in October 2014.
The study included patients older than 18 years, with a diagnosis of either early-stage cancer or advanced-stage cancer—defined as locally advanced, recurrent, or metastatic disease—who had been treated with opioids. We classified opioids on the basis of their distinct schedules as outlined in the Controlled Substances Act.16 Schedule II opioids include hydromorphone, methadone, oxycodone, fentanyl, morphine, and hydrocodone—after its reclassification as a schedule II opioid in October 2014. Schedule III opioids include codeine in an amount that does not exceed 90 mg per dose unit, buprenorphine, and hydrocodone—before its reclassification as a schedule II opioid. Schedule IV opioids include tramadol—after its classification as a schedule IV opioid in August 2014—and propoxyphene.16 We classified tramadol as a nonscheduled opioid before August 2014. Patients who had not received any opioids before the consultation were excluded from the study. After patients fulfilled the initial criteria, we used Statistical Package for the Social Sciences for Windows version 24 (SPSS, Chicago, IL) was used to generate a random sample of 75 patients who were treated between January 1 and April 30 each year from 2010 through 2014 and 375 patients who were treated between January 1 and April 30 in 2015.
Data were collected on patient demographics, such as age, sex, and race. Clinical information was collected on patient cancer type, disease stage, Eastern Cooperative Oncology Group (ECOG) performance status,21 the CAGE (Cut-down, Annoyed, Guilty, Eye-opener) questionnaire for alcoholism,22 the Memorial Delirium Assessment Scale,23 the Edmonton Symptom Assessment Scale,24 current or prior history of tobacco use, and current or prior history of illicit drug use. Data on the opioid daily dose, defined as the MEDD, were collected by using standard conversion ratios.25 MEDD was inclusive of scheduled and as-needed opioids required by patients. Data on opioid type included both long-acting and short-acting opioids. When patients were using the same opioid for both long-acting and short-acting needs, it was reported once. When patients were using different long-acting and short-acting opioids, only the long-acting opioid was reported. We collected opioid data by accessing individual patient charts without using any filters; all of the collected information was matched with the prescribers’ paper and electronic documentation.
Statistical Considerations
Descriptive statistics were obtained for demographic and clinical variables. Statistics for continuous variables, such as the MEDD, provided the mean, standard deviation, and a 95% CI, as well as the median and interquartile range (IQR). MEDD was also summarized by patient characteristics, such as sex, race, CAGE history, tobacco use, illicit drug use, and cancer type, and was compared between or among patient characteristics groups by using the Wilcoxon rank-sum test or Kruskal-Wallis test. Statistics for categorical variables, such as opioid type, were reported in terms of frequency (proportion) over the year and were compared by using the χ2 test or Fisher’s exact test when appropriate. With 375 patients in 2010 to 2014 and 375 in 2015, we had 90% power to detect an effect size of 0.237 in the average MEDD by using a two-sided t test with a significance level of .05. All tests were two-sided, and P ≤ .5 was considered statistically significant. All computations were carried out in SAS (SAS/STAT User’s Guide, Version 9.3; SAS Institute, Cary, NC).
RESULTS
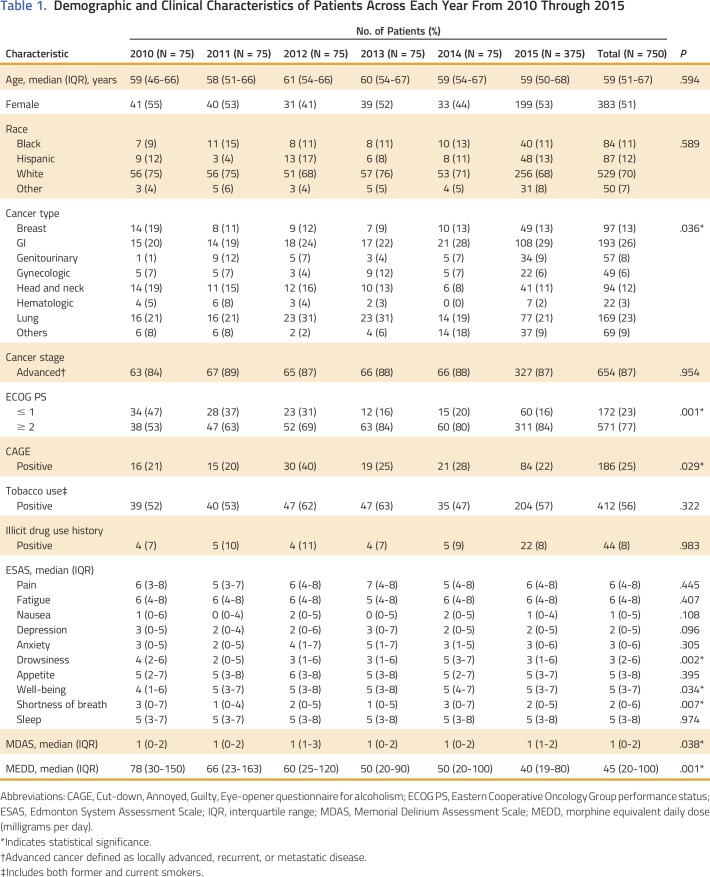
Table 1 summarizes the demographic and clinical characteristics of the patients annually, from 2010 through 2015. Median age was 59 years (IQR, 51 to 67). Of 750 patients in this study, 383 (51%) were female, 529 (70%) were white, and the most common cancers were GI (26%), lung (22%), breast (13%), and head and neck (12%). Most patients (654; 87%) had advanced cancer. Patients with poor performance status (ECOG ≥ 2) were more common in later years (P < .001).
Table 1.
Demographic and Clinical Characteristics of Patients Across Each Year From 2010 Through 2015
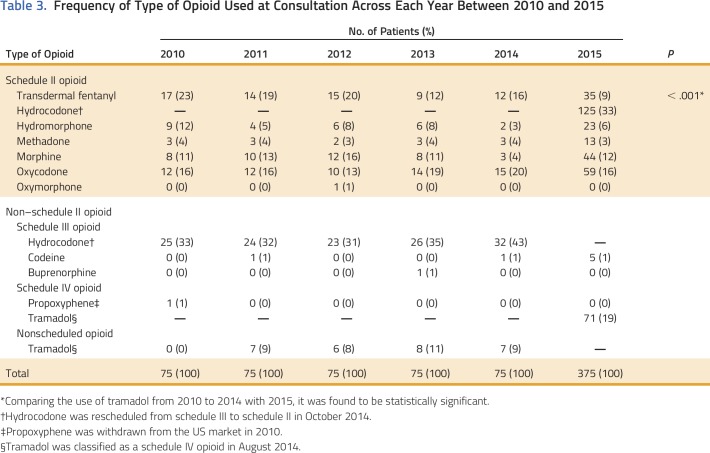
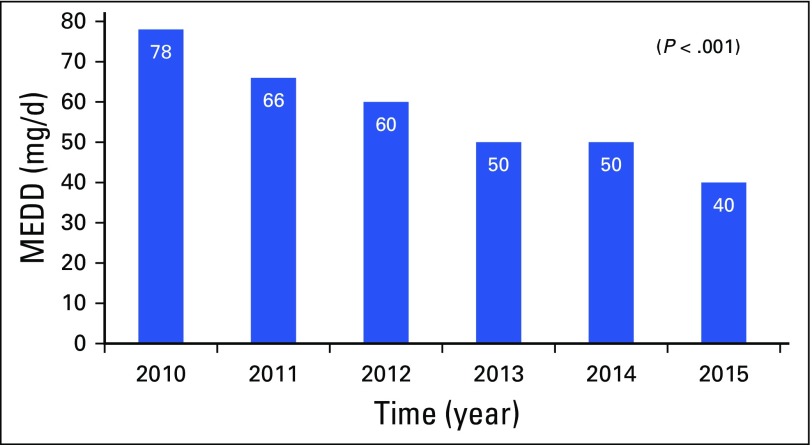
In 2010, median MEDD was 78 mg/d (IQR, 30 to 150); however, by 2015, the MEDD had progressively decreased to 40 mg/d (IQR, 19 to 80; P = .001) as shown in Table 1 and Appendix Figure A1 (online only). Median MEDD was statistically higher for males (P = .042), white race (P = .029), and positive CAGE (P = .053), as shown in Table 2. Overall, the most commonly prescribed opioid was hydrocodone, followed by oxycodone and transdermal fentanyl; however, use of transdermal fentanyl and hydromorphone has declined in recent years (Table 3). A sharp increase—to 43%—in hydrocodone use was observed before its reclassification in 2014; however, its use declined to 33% in 2015, which is consistent with its use in earlier years, from 2010 to 2013 (Table 3). Overall, tramadol, a schedule IV opioid, was prescribed significantly more frequently in 2015—up 19%—than in previous years, from 2010 to 2014 (P < .001; Table 3).
Table 2.
Morphine Equivalent Daily Dose (milligrams per day) by Patient Characteristic
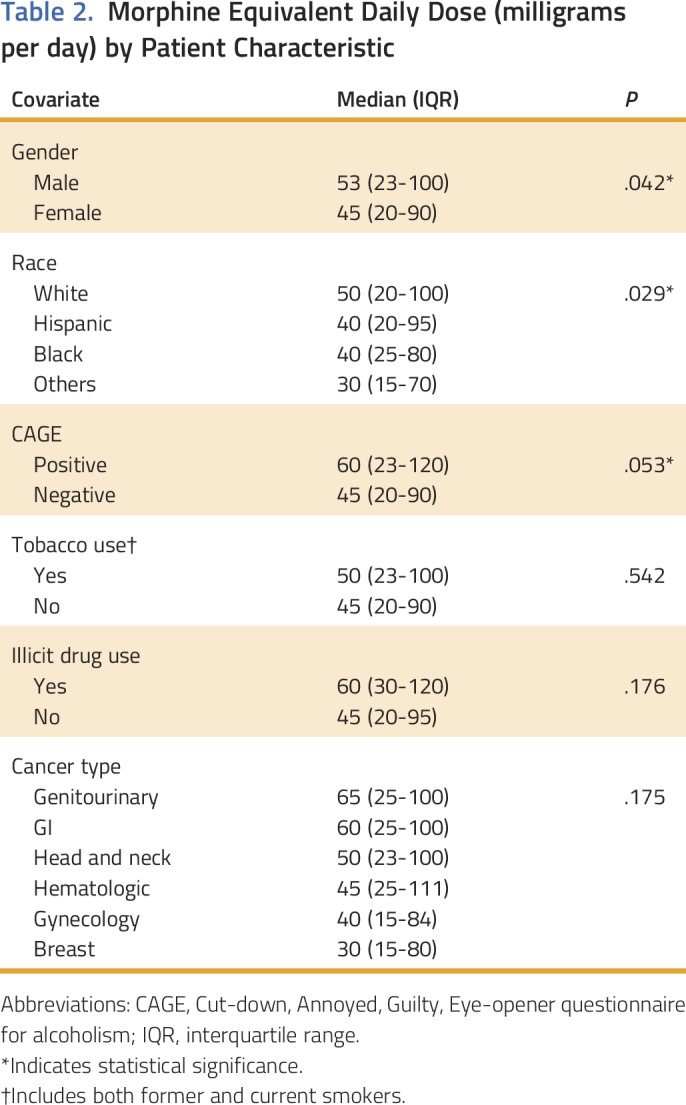
Table 3.
Frequency of Type of Opioid Used at Consultation Across Each Year Between 2010 and 2015
DISCUSSION
Our findings indicate that, in recent years, patients who were referred to MD Anderson Cancer Center’s outpatient palliative care clinic have received lower MEDDs upon referral. Tramadol, a schedule IV opioid with less stringent prescription limits, was prescribed more often after hydrocodone was reclassified. In contrast, use of schedule II opioids, such as transdermal fentanyl and hydromorphone, has declined. Some factors that may have contributed to this observed trend include increased regulatory scrutiny related to the prescription opioid epidemic and opioid-related deaths in the United States26; therefore, it is possible that oncologists are less comfortable prescribing and titrating opioids to treat cancer pain.27 We analyzed potential confounders that could have contributed to reduced MEDDs, such as earlier referral to palliative care, type and stage of cancer upon referral, and patients’ performance status (ECOG), and other dominant uncontrolled symptoms, such as fatigue, anxiety, nausea, and loss of appetite; however, we were unable to find any correlation with such factors. For example, the referral pattern and the percentage of patients with advanced cancer have been consistent across all study years (Table 1). In fact, patients with poor performance status (ECOG ≥ 2) were referred more often in recent years (Table 1). Poor ECOG performance status is often associated with high symptom burden, especially pain,28 which can lead to higher opioid use. Likewise, we have not observed any surge in non–pain-dominant symptoms, such as fatigue, anxiety, nausea, or loss of appetite, that could have led to early referral to palliative care (Table 1).
These findings are observational in nature, and causation cannot be established; therefore, we emphasize that more research is needed to better understand the reasons for these trends. For example, we suggest the use of prospective anonymous surveys of the attitudes and beliefs of prescribing physicians and advance practice providers regarding the use of opioids in patients with cancer as well as regular monitoring of patterns of opioid prescription, with special emphasis on associating those prescriptions with patient-reported pain scores.
Our findings demonstrate that hydrocodone remained the most prescribed opioid from 2010 to 2015, despite its reclassification as a schedule II opioid, which suggests that both providers and patients are comfortable with its use. However, it is important to recognize that hydrocodone is mostly available as a combination product with acetaminophen; therefore, its effectiveness in treating cancer pain can be limited in patients with liver dysfunction and/or liver metastatic disease and in patients with breakthrough pain that requires frequent dose administration. In contrast, morphine has the most evidence to support its use in cancer settings and has multiple low-cost immediate and extended-release formulations, which make it potentially ideal for these patients; however, it was used less frequently than hydrocodone. Tramadol was prescribed more often after hydrocodone reclassification. Tramadol is an opioid μ-receptor agonist, and it also inhibits the reuptake of norepinephrine and serotonin,29 which modulates the descending inhibitory pathways that are responsible for pain relief. It can lower the threshold for seizure activity, even at usual or lower doses, and therefore warrants close monitoring in patients with cancer.30 Tramadol can also cause serotonin syndrome by interacting with drugs that are frequently used in patients with advanced cancer, such as antiemetics, antidepressants, and neuroleptics.31 Although the WHO three-step ladder for cancer pain suggests the use of codeine and tramadol in combination with or without nonopioid analgesics and adjuvants for mild-to-moderate pain,9,10 it is important to recognize that these combinations may potentiate adverse effects and require close monitoring. Uncontrolled pain can be a potential concern in patients with mild-to-moderate pain who are treated with opioids, such as codeine or tramadol. In a recent multicenter randomized controlled trial that included adults with mild-to-moderate cancer pain who were opioid naïve, low-dose morphine provided better pain relief and tolerability than did codeine or tramadol32; therefore, low doses of strong opioids can be used to control mild-to-moderate cancer pain, especially in patients with advanced cancer, among whom continued pain is a concern.
In this cohort of patients with advanced cancer, 186 (25%) were CAGE positive. CAGE-positive patients were observed to have higher MEDDs than CAGE-negative patients (65 mg/d v 45 mg/d). Although this finding does not directly support our hypothesis, it does highlight the importance of screening patients for alcohol abuse. Alcoholism is strongly associated with tobacco and illicit drug use.33,34 Patients with these risk factors often have higher pain expression34,35 that can lead to higher MEDDs. Unfortunately, only a small percentage of CAGE-positive patients have documented alcohol abuse before a palliative care consultation34,35; therefore, it is important to screen patients for such risk factors before prescribing opioids and additional dose titrations by referring health care providers.
Opioid regulatory programs, such as REMS, for extended-release and long-acting opioids and PDMPs are implemented to enhance the safe use of opioid prescriptions. REMS for extended-release and long-acting opioids was approved by the US Food and Drug Administration to control opioid abuse, addiction, and related deaths.17 This strategy strongly encourages prescribers to complete continuing education courses; to provide counseling to their patients; to discuss safety, risks, storage, and safe disposal of opioids; and to encourage patients and caregivers to read medication guides provided by dispensing pharmacies. It also recommends the use of tools, such as patient-prescriber agreements and risk assessment tools, to improve patient safety. Likewise, PDMPs are state-run electronic databases that collect, monitor, and analyze prescriptions that are transmitted by dispensing pharmacies and practitioners.18 These databases are effective tools that can be used by health care providers to monitor opioid prescription abuse and diversion.
The palliative care clinic at MD Anderson Cancer Center is fully staffed by 22 board-certified palliative care physicians, registered nurses, medical assistants, psychologists, counselors, social workers, and pharmacists. It serves patients at two different locations within the institution and provides full-day service from Monday through Friday. On average, providers see 8 to 10 new referrals and 35 to 40 follow-ups on a given day. The clinic accepts referrals that originate internally within the institution from a variety of primary services, most frequently from thoracic, breast, GI, head and neck, and gynecology.
In recent years, we have implemented several opioid safety initiatives, such as universal screening of patients with such tools as CAGE and substance abuse history questionnaires and Screener and Opioid Assessment for Patients with Pain-Revised,36 complemented by information on the basis of patient-reported medical histories. Patients who demonstrate aberrant opioid use are monitored by a special interdisciplinary team.37 To enhance the safe use of opioids and safe storage and disposal, we provide written and video instructions to all patients who receive opioid prescriptions from these clinics.38
Despite a robust data set, there are several limitations to this study. First, patients were treated at a comprehensive cancer center where dedicated palliative care services are available; hence, data from this single institution cannot be generalized to other clinical settings, such as community-based programs. Second, as a result of the retrospective nature of this study, we are unable to observe the trends of opioid prescriptions by the referring health care providers in subsequent years. Third, as regulatory changes, such as the implementation of REMS and checking PDPMs for opioid prescriptions, are not mandatory in the state of Texas, their relative association with the observed reduction in MEDDs and changes in prescription trends cannot be established. Although these might be contributing factors, this association should be further researched. Fourth, in this retrospective review, we were unable to evaluate the response to cancer treatment on the basis of patients’ overall disease status that could have led to less need for stronger opioids. Fifth, opioid-related adverse effects and their impact on prescription trends were not studied in this retrospective chart review. Sixth, because referrals to palliative care were made by different clinicians within the institution with varying levels of understanding and knowledge of opioids, we want to acknowledge that this should be considered a potential confounder when interpreting results. Last, the increase use of tramadol, as observed in 2015, could be a result of the recognition of its role as a possible weak opioid after its classification as a schedule IV opioid in 2014.
The literature has demonstrated an increase in referral volume on the basis of changes to the name of the palliative care department to the Supportive Care Center at MD Anderson Cancer Center.39 We want to acknowledge that the name, supportive care, is used for internal referrals to the palliative care department. Use of this department name has been consistent from 2008 to present, and our data were obtained between 2010 and 2015; therefore, we do not anticipate any potential confounding effects as a result of changes in departmental name. However, this should be considered a limiting factor when interpreting the results. We want to emphasize that because of the observational nature of this study, causation between regulatory changes and prescription trends could not be established. To make definitive conclusions about the impact of opioid regulations as the cause of observed prescription trends, we need additional research in other clinical settings.
In conclusion, over the past several years, there has been a decrease in MEDDs prescribed by referring oncologists along with a trend toward prescribing less-regulated opioids, such as tramadol. Additional research is required to confirm these findings in other clinical settings.
ACKNOWLEDGMENT
We thank the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editorial assistance.
Appendix
Fig A1.
Morphine equivalent daily dose (MEDD; milligrams per day) across each year from 2010 to 2015 at referral to palliative care.
AUTHOR CONTRIBUTIONS
Conception and design: Ali Haider, Yee Choon Meng, Eduardo Bruera
Administrative support: Ali Haider, Janet L. Williams, Eduardo Bruera
Collection and assembly of data: Ali Haider, Donna S. Zhukovsky, Yee Choon Meng, Joseph Baidoo, Kimberson C. Tanco, Holly A. Stewart, Tonya Edwards, Manju P. Joy, Leela Kuriakose, Zhanni Lu, Eduardo Bruera
Data analysis and interpretation: Ali Haider, Donna S. Zhukovsky, Janet L. Williams, Diane D. Liu, Eduardo Bruera
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Opioid Prescription Trends Among Patients With Cancer Referred to Outpatient Palliative Care Over a 6-Year Period
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Ali Haider
No relationship to disclose
Donna S. Zhukovsky
No relationship to disclose
Yee Choon Meng
No relationship to disclose
Joseph Baidoo
No relationship to disclose
Kimberson C. Tanco
No relationship to disclose
Holly A. Stewart
No relationship to disclose
Tonya Edwards
No relationship to disclose
Manju P. Joy
No relationship to disclose
Leela Kuriakose
No relationship to disclose
Zhanni Lu
No relationship to disclose
Janet L. Williams
No relationship to disclose
Diane D. Liu
No relationship to disclose
Eduardo Bruera
No relationship to disclose
REFERENCES
- 1.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, et al. : Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J Pain Symptom Manage 51:1070-1090.e9, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Guay M, Yennurajalingam S, Parsons H, et al. : Association between self-reported sleep disturbance and other symptoms in patients with advanced cancer. J Pain Symptom Manage 41:819-827, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Kroenke K, Theobald D, Wu J, et al. : The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J Pain Symptom Manage 40:327-341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado-Guay M, Parsons HA, Li Z, et al. : Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer 17:573-579, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Zaza C, Baine N: Cancer pain and psychosocial factors: A critical review of the literature. J Pain Symptom Manage 24:526-542, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Koyyalagunta D, Bruera E, Solanki DR, et al. : A systematic review of randomized trials on the effectiveness of opioids for cancer pain. Pain Physician 15:ES39-ES58, 2012. (suppl 3) [PubMed] [Google Scholar]
- 7.Jennings AL, Davies AN, Higgins JP, et al. : A systematic review of the use of opioids in the management of dyspnoea. Thorax 57:939-944, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruera E, Macmillan K, Pither J, et al. : Effects of morphine on the dyspnea of terminal cancer patients. J Pain Symptom Manage 5:341-344, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Stjernswärd J: WHO cancer pain relief programme. Cancer Surv 7:195-208, 1988 [PubMed] [Google Scholar]
- 10.Stjernswärd J, Colleau SM, Ventafridda V: The World Health Organization cancer pain and palliative care program. Past, present, and future. J Pain Symptom Manage 12:65-72, 1996 [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology, Adult Cancer Pain. https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf
- 12.Caraceni A, Hanks G, Kaasa S, et al. : Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol 13:e58-e68, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Ripamonti CI, Santini D, Maranzano E, et al. : Management of cancer pain: ESMO clinical practice guidelines. Ann Oncol 23:vii139-vii154, 2012. (suppl 7) [DOI] [PubMed] [Google Scholar]
- 14.Paice JA, Lacchetti C, Bruera E: Management of chronic pain in survivors of adult cancers: ASCO clinical practice guideline summary. J Oncol Pract 12:757-762, 2016. 27460497 [Google Scholar]
- 15.Webster LR, Grabois M: Current regulations related to opioid prescribing. PM R 7:S236-S247, 2015. (suppl 11) [DOI] [PubMed] [Google Scholar]
- 16.US Department of Justice Drug Enforcement Administration : Controlled substance schedules. https://www.deadiversion.usdoj.gov/schedules/
- 17.US Food and Drug Administration : Risk evaluation and mitigation strategy (REMS) for extended-release and long-acting opioid analgesics. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm163647.htm
- 18.US Department of Justice Drug Enforcement Administration : State prescription drug monitoring programs. https://www.deadiversion.usdoj.gov/faq/rx_monitor.htm#1
- 19.Drug Enforcement Administration. Department of Justice : Schedules of controlled substances: Rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed Regist 79:49661-49682, 2014 [PubMed] [Google Scholar]
- 20.IMS Institute for Health Informatics : Medicine use and spending shift, A review of the use of medicines in U.S in 2014. http://www.fdanews.com/ext/resources/files/04-15/IIHI_Use_of_Medicines_Report_2015.pdf?1429048559
- 21.Oken MM, Creech RH, Tormey DC, et al. : Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-655, 1982 [PubMed] [Google Scholar]
- 22.Mayfield D, McLeod G, Hall P: The CAGE questionnaire: Validation of a new alcoholism screening instrument. Am J Psychiatry 131:1121-1123, 1974 [DOI] [PubMed] [Google Scholar]
- 23.Breitbart W, Rosenfeld B, Roth A, et al. : The Memorial Delirium Assessment Scale. J Pain Symptom Manage 13:128-137, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Bruera E, Kuehn N, Miller MJ, et al. : The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 7:6-9, 1991 [PubMed] [Google Scholar]
- 25.Bruera E, Elsayem A: The MD Anderson Symptom Control and Palliative Care Handbook. Houston, TX, University of Health Science Center at Houston, 2015 [Google Scholar]
- 26.Centers for Disease Control and Prevention : Prescription opioid overdose data. https://www.cdc.gov/drugoverdose/data/overdose.html
- 27.Breuer B, Fleishman SB, Cruciani RA, et al. : Medical oncologists’ attitudes and practice in cancer pain management: A national survey. J Clin Oncol 29:4769-4775, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Kamal AH, Nipp RD, Bull J, et al. : Symptom burden and performance status among community-dwelling patients with serious illness. J Palliat Med 18:542-544, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Grond S, Sablotzki A: Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879-923, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Beyaz SG, Sonbahar T, Bayar F, et al. : Seizures associated with low-dose tramadol for chronic pain treatment. Anesth Essays Res 10:376-378, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beakley BD, Kaye AM, Kaye AD: Tramadol, pharmacology, side effects, and serotonin syndrome: A review. Pain Physician 18:395-400, 2015 [PubMed] [Google Scholar]
- 32.Bandieri E, Romero M, Ripamonti CI, et al. : Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol 34:436-442, 2016 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization : WHO expert committee on problems related to alcohol consumption. Second report. http://www.who.int/substance_abuse/expert_committee_alcohol/en/ [PubMed]
- 34.Dev R, Parsons HA, Palla S, et al. : Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer 117:4551-4556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons HA, Delgado-Guay MO, El Osta B, et al. : Alcoholism screening in patients with advanced cancer: Impact on symptom burden and opioid use. J Palliat Med 11:964-968, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barclay JS, Owens JE, Blackhall LJ: Screening for substance abuse risk in cancer patients using the Opioid Risk Tool and urine drug screen. Support Care Cancer 22:1883-1888, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Arthur JA, Edwards T, Lu Z, et al. : Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer 122:3732-3739, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Reddy A, de la Cruz M, Rodriguez EM, et al. : Patterns of storage, use, and disposal of opioids among cancer outpatients. Oncologist 19:780-785, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalal S, Bruera S, Hui D, et al. : Use of palliative care services in a tertiary cancer center. Oncologist 21:110-118, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]