Abstract
Purpose
The clinical activity observed in a phase I dose-escalation study of concurrent therapy with nivolumab (NIVO) and ipilimumab (IPI) in patients with previously treated or untreated advanced melanoma led to subsequent clinical development, including randomized trials. Here, we report long-term follow-up data from study CA209-004, including 3-year overall survival (OS).
Patients and Methods
Concurrent cohorts 1, 2, 2a, and 3 received escalating doses of NIVO plus IPI once every 3 weeks for four doses, followed by NIVO once every 3 weeks for four doses, then NIVO plus IPI once every 12 weeks for eight doses. An expansion cohort (cohort 8) received concurrent NIVO 1 mg/kg plus IPI 3 mg/kg once every 3 weeks for four doses, followed by NIVO 3 mg/kg once every 2 weeks, which is the dose and schedule used in phase II and III studies and now approved for patients with unresectable or metastatic melanoma.
Results
Among all concurrent cohorts (N = 94) at a follow-up of 30.3 to 55.0 months, the 3-year OS rate was 63% and median OS had not been reached. Objective response rate by modified WHO criteria was 42%, and median duration of response was 22.3 months. Incidence of grade 3 and 4 treatment-related adverse events was 59%. The most common grade 3 and 4 treatment-related adverse events were increases in lipase (15%), alanine aminotransferase (12%), and aspartate aminotransferase (11%). One treatment-related death (1.1%) occurred in a patient who had multiorgan failure 70 days after the last dose of NIVO plus IPI.
Conclusion
This is the longest follow-up for NIVO plus IPI combination therapy in patients with advanced melanoma. The 3-year OS rate of 63% is the highest observed for this patient population and provides additional evidence for the durable clinical activity of immune checkpoint inhibitors in the treatment of advanced melanoma.
INTRODUCTION
Antibodies that inhibit cytotoxic T-lymphocyte antigen-4 (CTLA-4) or programmed death-1 (PD-1) have become a mainstay in the treatment of advanced melanoma. Ipilimumab (anti–CTLA-4) was the first drug to show an overall survival (OS) benefit in a randomized trial of patients with unresectable or metastatic melanoma,1 and its approval in 2011 marked the first new treatment option for this disease in more than a decade. More recently, nivolumab and pembrolizumab (anti–PD-1) have each demonstrated superior efficacy compared with ipilimumab alone in phase III studies.2,3
Dual blockade of CTLA-4 and PD-1 was initially evaluated in a phase I dose-escalation study (CA209-004).4 This study was subsequently amended to include the dose and schedule for nivolumab plus ipilimumab that was later evaluated in phase II (CheckMate 069) and phase III (CheckMate 067) randomized studies. The quality and kinetics of antitumor response in CA209-004 suggests that dual blockade of CTLA-4 and PD-1 improves antitumor response more than either agent alone. The objective response rate (ORR) with nivolumab plus ipilimumab in study CA209-004 was 40%, which was higher than that reported at the time for single-agent ipilimumab (11%) or nivolumab (28%).1,5 In CheckMate 069, nivolumab plus ipilimumab improved ORR compared with ipilimumab (59% v 11%).6,7 Two-year OS rates in CheckMate 069 were 64% for the combination versus 54% for ipilimumab, although 62% of patients in the ipilimumab arm received anti–PD-1 treatment upon experiencing progression.7 CheckMate 067 also demonstrated significantly improved progression-free survival (PFS) and ORR for nivolumab plus ipilimumab as well as nivolumab alone versus ipilimumab alone in patients with previously untreated advanced melanoma.2 At a minimum follow-up of 36 months in CheckMate 067, 3-year OS rates for nivolumab plus ipilimumab, nivolumab alone, and ipilimumab alone were 58%, 52%, and 34%, respectively.8
Here, we present long-term follow-up data, including 3-year OS, in patients who were treated with nivolumab plus ipilimumab in the phase I study, CA209-004. This analysis represents the longest follow-up so far for nivolumab plus ipilimumab in patients with advanced melanoma.
PATIENTS AND METHODS
Patients
Eligibility criteria have been described previously.4 In brief, patients had measurable, unresectable stage III or IV melanoma and an Eastern Cooperative Oncology Group performance status of 0 or 1. Previous treatment with T cell–modulating antibodies was not permitted for concurrent cohorts.
Study Design and Treatment
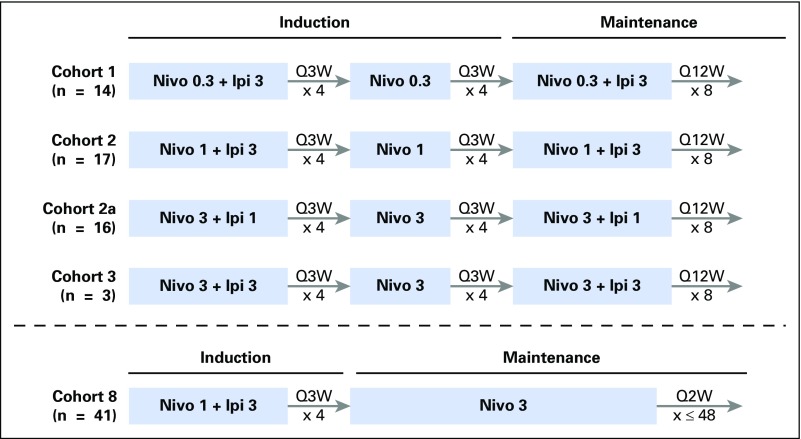
Study CA209-004 evaluated nivolumab and ipilimumab administered as concurrent or sequenced therapy in patients with previously treated or untreated advanced melanoma; data for concurrent cohorts are reported here. Concurrent cohorts 1 (n = 14), 2 (n = 17), 2a (n = 16), and 3 (n = 6) received induction therapy with nivolumab plus ipilimumab once every 3 weeks for four doses, followed by nivolumab alone once every 3 weeks for four doses, as follows: cohort 1: nivolumab 0.3 mg/kg plus ipilimumab 3 mg/kg; cohort 2: nivolumab 1 mg/kg plus ipilimumab 3 mg/kg; cohort 2a: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg; and cohort 3: nivolumab 3 mg/kg plus ipilimumab 3 mg/kg. Patients then received nivolumab plus ipilimumab (maintenance) at the initial dosage once every 12 weeks for 8 doses (see summary of dosing regimen in Appendix Fig A1, online only). In cohorts 1 to 3 (n = 53), patients were eligible for reinduction if they experienced investigator-assessed clinical benefit without unacceptable toxicity. Reinduction followed the dosage and schedule of the initial four doses of nivolumab and ipilimumab, followed by four doses of nivolumab. In cohort 8 (n = 41), patients received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg once every 3 weeks for four doses (induction), followed by nivolumab 3 mg/kg once every 2 weeks for up to 48 doses (maintenance). This is the regimen later used in phase II and III melanoma studies2,6 and was approved by the US Food and Drug Administration. Patients in cohort 8 were ineligible for reinduction.
Assessments
Primary end point was safety, including standard laboratory evaluations and adverse events (AEs) reported between the first dose and 100 days after the last dose of study therapy. AEs were coded according to the Medical Dictionary for Regulatory Activities (version 19.0).9 AE severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). Treatment-related AEs of potentially immunologic etiology—select AEs—were identified using a predefined list of Medical Dictionary for Regulatory Activities terms.
Secondary end points included tumor response and PFS. Tumor response was assessed by investigators with computed tomography or magnetic resonance imaging using modified WHO criteria at weeks 12, 18, 24, 30, and 36, then every 12 weeks thereafter. PFS—defined as the time from first dose to disease progression or death—was evaluated up to 2.5 years. OS—defined as the time from first dose to death—was an exploratory end point per the statistical analysis plan and is the primary focus of this report. Patients were observed for OS every 12 weeks for up to 3 years.
Tumor expression of programmed death-ligand 1 (PD-L1) was an exploratory end point assessed as described previously.4 A sample was defined as having high PD-L1 expression if ≥ 5% of tumor cells exhibited PD-L1 staining of any intensity in a section that contained ≥ 100 evaluable cells. The Dako immunohistochemical assay that was used in study CA209-004 is a prototype of the validated Dako assay that is now available (Dako, Carpinteria, CA).
Statistical Analysis
OS and PFS were estimated by using the Kaplan-Meier (KM) method with 95% CIs. Best overall response was summarized by using descriptive statistics with an estimation of exact 95% CIs. Duration of response was calculated for confirmed responders. All safety parameters were summarized by using descriptive statistics. A vector analysis method was used to determine the incidence rate of treatment-related AEs over time. A vector was defined for each study day to obtain the cumulative number of unique patients who experienced AEs that started at or before that study day and ended at or after that study day. Probability of AE burden—calculated as the cumulative number of unique patients with AEs/total patients—at each study day was plotted against study day to show the plots of AE rate over time.
RESULTS
Patient Characteristics and Drug Exposure
Most patients in all concurrent cohorts had an Eastern Cooperative Oncology Group performance status of 0 (77%) and no prior treatment with systemic cancer therapy (55%; Table 1). Forty-eight percent of patients had stage M1c disease without brain metastases, whereas 7% had prior brain metastases. Patients with brain metastases must have had treated brain metastases that were stable before study enrollment. Lactate dehydrogenase (LDH) levels were elevated above the upper limit of normal (ULN) in approximately 38% of patients. PD-L1 tumor expression was assessed in 62% of patients, whereas approximately 15% had PD-L1 tumor expression of ≥ 5%.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients From All Concurrent Cohorts, Cohorts 1 to 3, and Cohort 8
In cohorts 1 to 3 (Appendix Table A1, online only), the median number of nivolumab plus ipilimumab doses received during induction was 4.0 (range, 1 to 4), with 33 patients (62%) receiving all four doses. In cohort 8 (Appendix Table A2, online only), the median number of nivolumab plus ipilimumab doses received was 4.0 (range, 1 to 4), with 21 patients (51%) receiving all four doses. The median number of nivolumab doses received during maintenance in cohort 8 was 14.0 (range, 1 to 48). The median duration of treatment of all concurrent cohorts was 5.6 months (95% CI, 4.4 to 8.3 months).
OS and PFS
Among all concurrent cohorts (N = 94) at a follow-up of 30.3 to 55.0 months, median OS had not been reached (95% CI, 36.7 months to not reached [NR]; Fig 1). OS rates at 1, 2, and 3 years were 81% (95% CI, 71% to 87%), 72% (95% CI, 62% to 80%), and 63% (95% CI, 52% to 72%), respectively. The study was not designed to compare OS rates between cohorts, but the OS rates in cohorts 1 to 3 and cohort 8 were as follows: 85% (95% CI, 72% to 92%) and 75% (95% CI, 59% to 86%) at 1 year, and 79% (95% CI, 65% to 88%) and 63% (95% CI, 46% to 76%) at 2 years. The 3-year OS rate was 69% (95% CI, 55% to 80%) for cohorts 1 to 3 and was not calculated for cohort 8 because of a shorter follow-up period.
Fig 1.
(A and B) Kaplan-Meier curves of (A) overall survival (OS) and (B) progression-free survival (PFS) for all concurrent cohorts (N = 94). Symbols indicate censored observations.
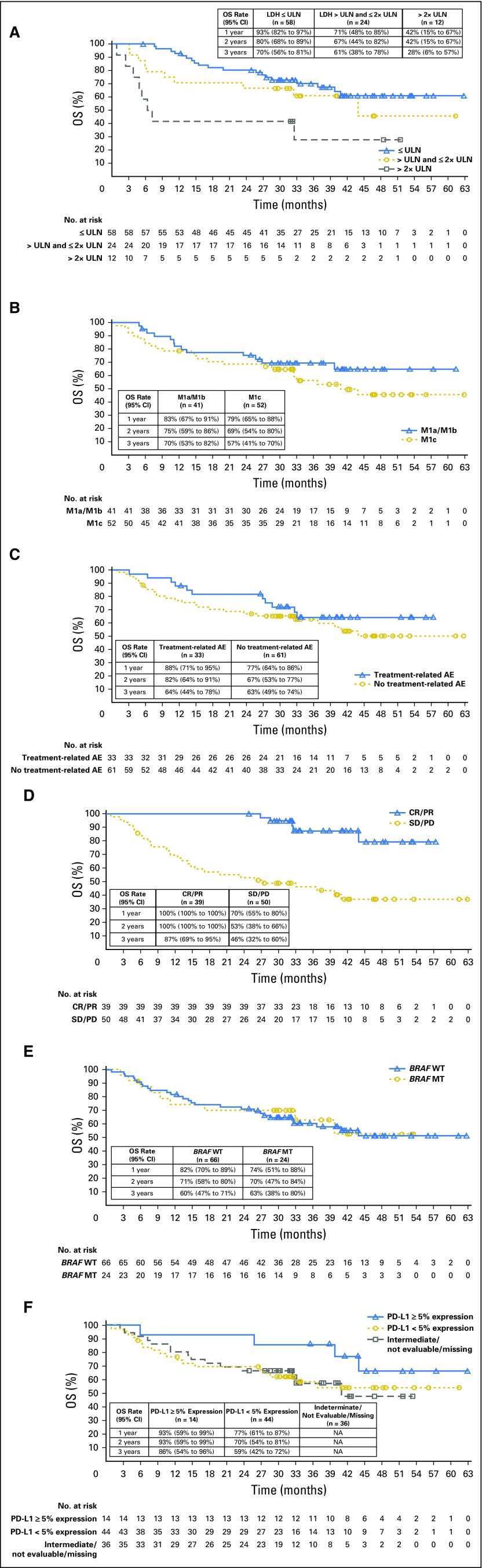
Median OS had not been reached (95% CI, 39.8 months to NR) in patients with normal LDH (≤ ULN), and was 43.9 months (95% CI, 11.1 months to NR) in those with elevated LDH (> ULN and ≤ 2× ULN; Appendix Fig A2, online only). Patients with highly elevated LDH (> 2× ULN) had a median OS of 6.8 months (95% CI, 2.5 months to NR). One-, 2-, and 3-year OS rates were higher in patients with normal versus elevated baseline LDH—93% and 71% at 1 year, 80% and 67% at 2 years, and 70% and 61% at 3 years. For patients with highly elevated LDH, 1-, 2-, and 3-year OS rates were 42%, 42%, and 28%, respectively. No clear separation in OS KM curves was observed between M1a/M1b and M1c subgroups or between BRAF wild-type and BRAF mutated melanoma subgroups, whereas a clear separation was observed between patients with ≥ 5% and those with < 5% PD-L1 tumor expression (Appendix Fig A2).
Median PFS in all concurrent cohorts was 6.2 months (95% CI, 3.2 months to 11.0 months) with a 12-, 24-, and 30-month PFS rate of 37% (95% CI, 27% to 47%), 28% (95% CI, 19% to 38%), and 17% (95% CI, 8% to 29%), respectively (Fig 1B).
Clinical Activity
In all concurrent cohorts, ORR was 41.5% (95% CI, 31.4% to 52.1%), with 18 patients (19.1%) achieving a complete response (Table 2); median duration of response was 22.3 months (95% CI, 13.8 months to 25.8 months; Table 2 and Fig 2). Median reduction in tumor burden in the target lesions was −64.7% for all concurrent cohorts. In cohorts 1 to 3, no clinically meaningful differences were observed in ORR in patients with PD-L1 expression levels of < 5% (eight of 23; 34.8% [95% CI, 16.4% to 57.3%]) and those with PD-L1 expression levels of ≥ 5% (eight of 14; 57.1% [95% CI, 28.9% to 82.3%]).
Table 2.
Response to Treatment in All Concurrent Cohorts

Fig 2.
Swimmer plots showing time to first response and duration of response in accordance with modified WHO criteria for responders who received concurrent nivolumab plus ipilimumab in each individual cohort.
Safety
Any-grade treatment-related AEs were reported in 96.8% of patients and were grade 3 and 4 in 58.5% of patients (Table 3). Grade 3 and 4 AEs most commonly occurred in the first 12 weeks of treatment. Late toxicity was uncommon (Fig 3). Treatment-related grade 3 and 4 AEs led to discontinuation in 24.5% of patients. Any-grade treatment-related AEs of potential immunologic etiology—select AEs—were reported in 92.6% of patients and were grade 3 and 4 in 40.4% of patients (Appendix Table A3, online only). The highest peaks in grade 3 and 4 select AEs comprised hepatotoxicity in cohorts 1 to 3 (Fig 3B) and GI events in cohort 8 (Fig 3C). In the primary analysis for study CA209-004, no treatment-related deaths were reported in all concurrent cohorts.4 In this updated report, one treatment-related death (1.1%) occurred in a patient who had multiorgan failure subsequent to enterocolitis, sepsis, and pancreatitis, 70 days after the last dose of nivolumab plus ipilimumab treatment in cohort 8.
Table 3.
Treatment-Related AEs That Occurred in at Least 5% of Patients in All Concurrent Cohorts

Fig 3.
(A) Proportion of patients with treatment-related grade 3 and 4 adverse events (AEs) over time for cohorts 1 to 3 and cohort 8*. (B and C) Proportion of patients with treatment-related select grade 3 and 4 AEs over time for (B) cohorts 1 to 3 and (C) cohort 8*. The percentage at each time point shows the number of patients experiencing at least one grade 3 and 4 AE divided by all treated patients (n = 53 for cohorts 1 to 3; n = 41 for cohort 8). *One treatment-related death (1.1%) occurred in a patient who had multiorgan failure subsequent to enterocolitis, sepsis, and pancreatitis, 70 days after the last dose of nivolumab plus ipilimumab treatment in cohort 8.
DISCUSSION
At a follow-up of 30.3 to 55.0 months, this analysis represents the longest follow-up thus far for nivolumab plus ipilimumab in advanced melanoma. Of note, our analysis included cohort 8, which received nivolumab plus ipilimumab at the dose and schedule used in phase II and III studies. Nivolumab plus ipilimumab resulted in an ORR of 42% and a 3-year OS rate of 63%, which is the highest OS rate reported to date for this patient population. Although the regimen produced a high rate of grade 3 and 4 AEs, toxicity was manageable with no new safety signals reported with long-term follow-up.
OS rates with nivolumab plus ipilimumab in our study are higher than those reported with the combination in subsequent phase II and III studies. Indeed, at 63%, the 3-year OS rate report here is similar to the 2-year OS rate (64%) reported with the combination in CheckMate 0697 and the 3-year OS rate (58%) reported with the combination in CheckMate 067.8 Reasons for differences in survival rates between this phase I trial and subsequent trials are unclear, but there are inherent limitations in cross-study comparisons, especially when comparing a small phase I study with larger, stratified, randomized phase II and III studies. One limitation of our study is that data were not collected on subsequent therapies; therefore, we were unable to evaluate their potential effect on OS. In contrast to CheckMate 069/067, prior systemic treatment of stage IV disease was allowed in study CA209-004. In addition, approximately 15% of all concurrent cohorts in this study had PD-L1 tumor expression of ≥ 5%, which is lower than expected on the basis of subsequent studies (CheckMate 069, 25%; CheckMate 067, 22%)2,7; however, we note that in study CA209-004, a large proportion of patients had indeterminate, not evaluable, or missing PD-L1 status. Tumor tissue requirements also differed between study CA209-004 and CheckMate 069/067—that is, study CA209-004 permitted any archival, pretreatment tissue sample, whereas CheckMate 069/067 did not. In addition, the proportion of patients with LDH > ULN or LDH > 2× ULN, respectively, was 38% and 13% in the current study compared with 25% and 6% in CheckMate 0696 and 36% and 12% in CheckMate 067.2
Although the interpretation of OS differences by subgroups is limited by small patient numbers, a key finding of our trial was the high rates of survival in patients with elevated LDH. The 3-year OS rate in patients with elevated LDH (> ULN and ≤ 2× ULN; n = 24) was 61%, and even among the small subgroup with highly elevated baseline LDH (> 2× ULN; n = 12), the 3-year OS rate was 28%. The 3-year OS rate with dabrafenib plus trametinib was 25% for patients with elevated LDH.10 In a pooled analysis of dabrafenib plus trametinib in patients with metastatic BRAF-mutated melanoma, those with highly elevated baseline LDH (≥ 2× ULN; n = 70) had a 2-year OS rate of 7%.11 OS KM curves for patients with M1a/M1b versus M1c disease largely overlapped. This may reflect the heterogeneity in the M1c classification and the need for more refined measures of tumor burden.11,12 There was a trend toward improved OS for cohorts 1 to 3 versus cohort 8; however, small sample size, staggered enrollment, differences in PD-L1 expression, and other factors preclude any definitive conclusions. The main difference in treatment between cohorts 1 to 3 and cohort 8 was in maintenance therapy: cohorts 1 to 3 received nivolumab plus ipilimumab, whereas cohort 8 received nivolumab. In addition, cohorts 1 to 3 were eligible for reinduction, whereas cohort 8 was not.
The 2- and 3-year OS rates for the combination in our trial were 72% and 63%, respectively. In CheckMate 067, 3-year OS rate was 58% for the combination and 52% for nivolumab alone; 3-year OS rate was 65% for the combination and 61% for nivolumab in patients with PD-L1 expression of ≥ 5%, but was 56% and 50%, respectively, in patients with PD-L1 expression of < 5%.8 The 3-year OS rate reported with pembrolizumab was 41% in both ipilimumab-treated and ipilimumab-naive patients, and 45% in treatment-naive patients.13 In the phase III KEYNOTE-006 study, OS rate at a median follow-up of 33.9 months was 50% with pembrolizumab.14 In total, the data suggest a small advantage for the combination over anti-PD-1 alone. Moreover, the greatest effect for the addition of ipilimumab to nivolumab may be in the PD-L1–negative population.8 Other populations may benefit more from the combination, including patients with elevated LDH8 or active brain metastases15; however, additional prospective studies are needed for confirmation.
Patients with BRAF mutations can be treated with front-line targeted agents or immunotherapy. For dabrafenib plus trametinib versus dabrafenib alone, 3-year OS rates were 44% and 32%, respectively.10 Of importance, the COMBI-d study was restricted to patients with BRAF V600E/K mutation–positive melanoma, whereas our study included patients with BRAF wild-type and BRAF mutated melanoma. We observed similar 3-year OS rates in patients with BRAF wild-type (60%) and BRAF mutated (63%) melanoma, although subsequent therapy with a BRAF/MEK inhibitor may have contributed to survival outcomes in the BRAF mutation–positive subgroup. In CheckMate 067, 3-year OS rate with the combination was 53% in patients with BRAF wild-type melanoma and 68% in those with BRAF mutated melanoma.8 In CheckMate 067, 42% of patients in the nivolumab plus ipilimumab group received any subsequent therapy and 13% were treated with BRAF inhibitors.8 Although randomized trials are warranted, data from our trial and CheckMate 067 may suggest an advantage for front-line immunotherapy over targeted therapy in patients with BRAF-mutant advanced melanoma.
PFS rates were 37%, 28%, and 17% at 12, 24, and 30 months, respectively. One limitation of our study is that per protocol, tumor assessments were only performed up to a maximum of 2.5 years; therefore, we were unable to evaluate the PFS rate at 3 years. PFS may underestimate the percentage of patients who were disease free as a result of the local treatment of isolated progression or residual disease. The difference between PFS and OS is consistent with the notion that clinical benefit with immunotherapies can be obtained with nonconventional responses or without radiographic response.16 Furthermore, a proportion of patients may have received subsequent therapy with agents that have known clinical activity and/or survival benefit. A more detailed analysis of responses to subsequent therapies as well as the natural history of melanoma after treatment with immunotherapy is needed.
The overall safety profile reported here for nivolumab plus ipilimumab is similar to that reported in previous phase II and III studies,2,6-8,17 with no new safety signals emerging with long-term follow-up. The incidence of grade 3 and 4 treatment-related AEs was 59% in this phase I study, 54% in CheckMate 069,7 and 59% in CheckMate 067.8 Although late toxicity was uncommon, patients may be at low risk for grade 3 and 4 treatment-related AEs beyond the initial several months of therapy and after the completion of nivolumab plus ipilimumab treatment; thus, continued vigilance is warranted. Of note, there was no apparent difference in the incidence of late-occurring grade 3 and 4 treatment-related AEs between cohorts 1 to 3 and cohort 8.
In summary, this updated analysis from the phase I dose-escalation study CA209-004 provides the longest follow-up thus far for nivolumab plus ipilimumab. Data from the phase III study CheckMate 511 (ClinicalTrials.gov: NCT02714218) that is evaluating nivolumab 3 mg/kg plus ipilimumab 1 mg/kg versus nivolumab 1 mg/kg plus ipilimumab 3 mg/kg will provide insight into the optimal dose for this combination strategy. Although survival outcomes from this study and others are encouraging, further long-term follow-up across multiple studies, a refined understanding of potential biomarkers, and a more in-depth analysis of how treatment with immunotherapy influences the course of the disease will shed additional light on the clinical activity of the combination of nivolumab and ipilimumab.
ACKNOWLEDGMENT
We thank the patients who participated in this study and the clinical study teams. We thank Dako for collaborative development of the PD-L1 immunohistochemical 28-8 pharmDx assay. We also thank Jasmine Rizzo, the study medical monitor, for critical review of the manuscript. Professional medical writing and editorial assistance were provided by Jennifer DiNieri, PhD, and Cara Hunsberger, MS, of StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Appendix
Fig A1.
Dosing regimen for Study CA209-004 concurrent cohorts. In cohorts 1-3, patients were eligible for reinduction if they experienced investigator-assessed clinical benefit without unacceptable toxicity. Reinduction followed the dose and schedule of the initial 4 doses of nivolumab and ipilimumab, followed by 4 doses of nivolumab. Patients in cohort 8 were ineligible for reinduction.
Fig A2.
Overall survival (OS) by patient subgroups. Overall survival by (A) baseline LDH, (B) M-stage at study entry, (C) treatment-related AEs leading to discontinuation, (D) tumor response, (E) BRAF status, and (F) PD-L1 status in all concurrent cohorts. One-, 2-, and 3-year OS rates are shown in table insets. AE, adverse event; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Table A1.
Drug Exposure by Treatment Phase for Cohorts 1 to 3
Table A2.
Drug Exposure by Treatment Phase for Cohort 8

Table A3.
Treatment-Related AEs of Potential Immunologic Etiology (Select AEs) That Occurred in All Concurrent Cohorts

Footnotes
Supported by Bristol-Myers Squibb.
Presented in part at the 12th International Congress of the Society for Melanoma Research, San Francisco, CA, November 18-21, 2015, and at Melanoma Bridge, Naples, Italy, December 1-4, 2015.
Clinical trial information: NCT01024231.
AUTHOR CONTRIBUTIONS
Conception and design: Margaret K. Callahan, Harriet Kluger, Suba Krishnan, Rafia Bhore, Jedd D. Wolchok, Mario Sznol
Collection and assembly of data: Margaret K. Callahan, Harriet Kluger, Neil H. Segal, Alexander Lesokhin, Michael B. Atkins, John M. Kirkwood, Christine Horak, Jedd D. Wolchok, Mario Sznol
Data analysis and interpretation: Margaret K. Callahan, Harriet Kluger, Michael A. Postow, Alexander Lesokhin, Michael B. Atkins, John M. Kirkwood, Suba Krishnan, Rafia Bhore, Christine Horak, Jedd D. Wolchok, Mario Sznol
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Plus Ipilimumab in Patients With Advanced Melanoma: Updated Survival, Response, and Safety Data in a Phase I Dose-Escalation Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I), Celgene (I)
Consulting or Advisory Role: AstraZeneca, Moderna, Incyte
Research Funding: Bristol-Myers Squibb (Inst)
Harriet Kluger
Honoraria: Merck
Consulting or Advisory Role: Prometheus, Regeneron, Alexion Pharmaceuticals, Genentech/Roche, Nektar, Corvus Pharmaceuticals
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Michael A. Postow
Honoraria: Bristol-Myers Squibb, Merck
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Merck, Array BioPharma
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), Array BioPharma (Inst), Infinity Pharmaceuticals (Inst), Rgenix
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Neil H. Segal
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, AstraZeneca, MedImmune, Genentech/Roche, Merck
Research Funding: MedImmune, Bristol-Myers Squibb, Pfizer, Genentech/Roche, Merck
Alexander Lesokhin
Honoraria: Bristol-Myers Squibb, Janssen Oncology, Novartis, Juno Therapeutics
Consulting or Advisory Role: Bristol-Myers Squibb, Foundation Medicine (Inst), Janssen Oncology, Novartis, Aduro Biotech
Research Funding: Bristol-Myers Squibb (Inst), Janssen Oncology (Inst), Celgene (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: Serametrix (Inst)
Michael B. Atkins
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Genentech, Pfizer, Novartis, X4 Pharma, Genoptix, Bristol-Myers Squibb, Nektar, Merck, Exelixis, Acceleron Pharma, Peloton Therapeutics, Eisai, Celldex, Alexion Pharmaceuticals, AstraZeneca, MedImmune, Glactone Pharma, Agenus, Idera, Argos Therapeutics, Array BioPharma
John M. Kirkwood
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Novartis, Roche, Genentech, EMD Serono, Array BioPharma
Research Funding: Prometheus (Inst), Merck (Inst)
Suba Krishnan
Employment: Bristol-Myers Squibb, AstraZeneca, MedImmune
Stock or Other Ownership: Bristol-Myers Squibb
Rafia Bhore
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Christine Horak
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Jedd D. Wolchok
Stock or Other Ownership: Potenza Therapeutics, Tizona Therapeutics, Trieza Therapeutics
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, MedImmune, ZIOPHARM Oncology, Polynoma, Polaris, Genentech, F-star, BeiGene, Sellas Life Sciences, Eli Lilly, Potenza Therapeutics, Tizona Therapeutics, Amgen, AstraZeneca, Chugai Pharma
Research Funding: Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Coinventor on an issued patent for DNA vaccines for treatment of cancer in companion animals, coinventor on a patent for use of oncolytic Newcastle Disease virus
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Chugai Pharma, Roche, Janssen Pharmaceuticals, Kadmon
Mario Sznol
Stock or Other Ownership: Amphivena, Intensity Therapeutics, Adaptive Biotechnologies
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech/Roche, AstraZeneca, MedImmune, Symphogen, Kyowa Hakko Kirin, Lion Biotechnologies, Nektar, Novartis, Eli Lilly, Pfizer, Janssen Oncology, Vaccinex, Immune Design, Merck Sharp & Dohme, Biodesix, Alexion Pharmaceuticals, Adaptimmune, Lycera, Theravance, Modulate, Omniox, Seattle Genetics, Inovio Pharmaceuticals, AgonOx, Pierre Fabre, Baxalta, Shire Pharmaceuticals, Ignyta
Other Relationship: Haymarket Media, Research to Practice, TRM Oncology, Physician Education Resource, Imedex, AcademicCME, DAVA Oncology, Clinical Care Options, Vindico, Prime Oncology
REFERENCES
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010. [Erratum: N Engl J Med 363: 1290, 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Wolchok JD, Kluger H, Callahan MK, et al. : Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122-133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Chesney J, Pavlick AC, et al. : Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006-2017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, Chesney J, Pavlick AC, et al. : Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558-1568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 3771345–1356.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use : Medical Dictionary for Regulatory Activities, version 19.0. https://www.meddra.org/
- 10.Long GV, Flaherty KT, Stroyakovskiy D, et al. : Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long GV, Grob JJ, Nathan P, et al. : Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: A pooled analysis of individual patient data from randomised trials. Lancet Oncol 17:1743-1754, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Ribas A, Hamid O, Daud A, et al. : Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315:1600-1609, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Ribas A, Hamid O, et al. : Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol 34, 2016. (suppl 15; abstr 9503) [Google Scholar]
- 14.Robert C, Long GV, Schachter J, et al. : Long-term outcomes in patients with ipilimumab-naïve advanced melanoma in phase 3 KEYNOTE-006 study who completed pembrolizumab treatment. American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-6, 2016 (abstr 9504) [Google Scholar]
- 15.Long GV, Atkinson V, Menzies AM, et al. : A randomized phase II study of nivolumab or nivolumab combined with ipilimumab in patients (pts) with melanoma brain metastases (mets): The Anti-PD1 Brain Collaboration (ABC). American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-6, 2016 (abstr 9508) [Google Scholar]
- 16.Chiou VL, Burotto M: Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 33:3541-3543, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Updated results from a phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (CheckMate 067). American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016 (abstr 9505) [Google Scholar]