Abstract
Significance: Skin tissue damage is a major challenge and a burden on healthcare systems, from burns and other trauma to diabetes and vascular disease. Although the biological complexities are relatively well understood, appropriate repair mechanisms are scarce. Three-dimensional bioprinting is a layer-based approach to regenerative medicine, whereby cells and cell-based materials can be dispensed in fine spatial arrangements to mimic native tissue.
Recent Advances: Various bioprinting techniques have been employed in wound repair-based skin tissue engineering, from laser-induced forward transfer to extrusion-based methods, and with the investigation of the benefits and shortcomings of each, with emphasis on biological compatibility and cell proliferation, migration, and vitality.
Critical issues: Development of appropriate biological inks and the vascularization of newly developed tissues remain a challenge within the field of skin tissue engineering.
Future Directions: Progress within bioprinting requires close interactions between material scientists, tissue engineers, and clinicians. Microvascularization, integration of multiple cell types, and skin appendages will be essential for creation of complex skin tissue constructs.
Keywords: : 3D printing, wound repair, skin tissue engineering, biofabrication

Zhilian Yue, PhD

Gordon G. Wallace, DSc, PhD
Scope and Significance
The application of tissue engineering has been beneficial in improving wound treatment and alleviating the skin donor problem.1 However, as the skin is a complex structure containing pigmentation, vessels, hair follicles, and different cell types, integrated into a dynamic structure with different properties distributed throughout, many challenges remain.2,3 Ideally, tissue-engineered constructs mimic both structural organization and biological function in native tissue.4 Bioprinting, an additive manufacturing technique used for combining biological components and biomaterials in a structured manner, shows promise in this imitation process due to the improved three-dimensional (3D) spatial control of the multiple components that can be introduced within a single construct.5 Therefore, significant improvements in the development of tissue-engineered skin grafts can be made with bioprinting. In addition, a better understanding of dermatological diseases can be achieved via a thorough understanding of the mechanisms involved in wound healing, using the continuously developed skin constructs.6 The knowledge accrued can further expedite the development of bioprinting platforms to deliver skin constructs with optimal biological functions. Further, it may provide economic advantages and improve reproducibility of the constructs.5
Translational Relevance
In small wounds, the stratum basale of the epidermis is capable of regenerating the epidermis of the skin. However, when the stratum basale is affected and the dermis is damaged, regeneration is delayed and scarring will occur. Bioprinting techniques may contribute to skin tissue engineering, by creating precisely controlled spatial cell arrangements within a construct, mimicking the multiple layers found in native skin, and replacing the regenerative elements of the skin. In this manner, damaged skin could be restored and the amount of scar formation decreased. This is of particular importance within chronic wounds, wounds beyond the epidermis, and large area wounds, as the natural regeneration process is restricted. By offering the potential to better simulate the anatomical structure of the skin in a controllable and reproducible manner, bioprinting represents an appealing approach to improve the efficacy of cell therapy for treatment in full-thickness and complex skin wounds.
Clinical Relevance
A range of tissue-engineered skin grafts have been developed, and some of them have been approved for clinical utilization. These include epidermal, dermal, and epidermal/dermal substitutes. However, for most applications, tissue-engineered skin has yet to supplant the current gold standard of a split-thickness autograft. Approaches that enable effective skin wound repair and regeneration of clinical relevance are required. Bioprinting provides a reproducible method for mass production of biological and mechanical appropriate constructs with a high spatial organization of cells. Clinically, the introduction of cell-laden biofabricated constructs may accelerate wound healing and reduce scarring at sites of injury, thereby increasing quality of life. The possibilities in increasing the control of spatial integration of biomaterials and cells in the 3D printed skin constructs may improve on the regeneration process, possibly decreasing necessary intervention, thereby increasing the efficiency of cell therapy and decreasing the strain on the medical system. Further, the development of new skin substitutes may also improve the manageability for the clinician. Lastly, the creation of skin substitutes may provide a valuable insight into skin diseases.6
Discussion of Findings and Relevant Literature
The skin provides a protective barrier with immunologic and sensorial functions. It prevents the invasion of harmful substances and pathogens, such as bacteria, and protects against loss of moisture and electrolytes.7–9 When damaged, a natural regeneration process is initiated, which consists of three distinct phases: the inflammatory, new tissue formation, and tissue remodeling phases. However, when the wound encompasses all skin layers and/or regenerative elements, healing is delayed or inhibited. Consequently, inadequately vascularized scar tissue can be formed.8,10 To enhance the healing process, dressings and (synthetic) skin grafts have been applied to the damaged area. However, current treatments are insufficient in extensive burns, chronic wounds, and other wounds that involve the loss of large amounts of skin. Hence, there is an overwhelming need for the development of new skin replacement strategies.
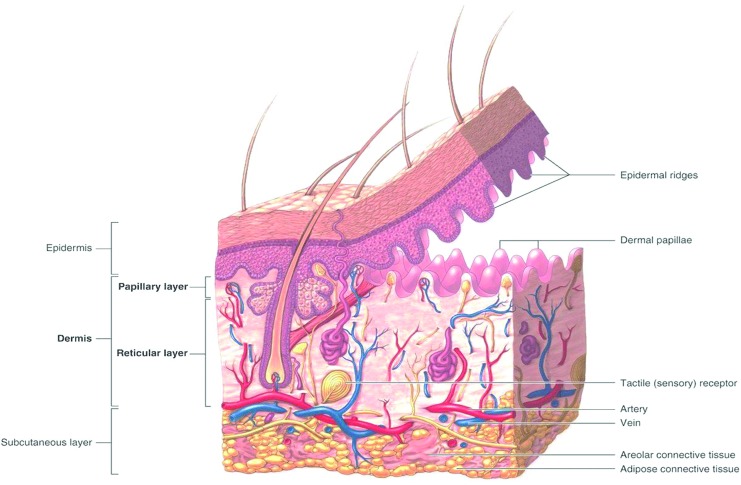
To develop a skin substitute, it is important to understand formation, mechanical properties, and cellular composition of the skin.11 Healthy skin consists of three main components: the epidermis, dermis, and hypodermis (Fig. 1).12 The epidermis, containing mainly keratinocytes, is primarily responsible for the barrier function of the skin, including the prevention of harmful pathogens from entering the body. It is structured from the outer layer to the inner layer of the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and the stratum basale. Underneath the stratum basale the connecting layer can be found between the epidermis and the dermis, known as the basement membrane zone. The dermis consists of fibroblasts and extracellular matrix (ECM) components (collagen and elastin), blood vessels, nerves, and appendageal structures. It is composed of two layers, the papillary dermis (the upper layer), which is responsible for the prevention of sliding between the epidermis and the dermis and the provision of oxygen and nutrients to the epidermis, and the reticular dermis, which is mainly responsible for the mechanical properties of the skin. The hypodermis consists mostly of adipose tissue.13
Figure 1.
Anatomy of the skin.12 Permission obtained from McGraw-Hill Education. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Wound treatment
Traditional wound dressings, such as bandages and gauze, are used regularly to provide protection against pathogens and other environmental hazards to stimulate appropriate wound healing. However, as they absorb fluid extrusions and adhere to the wound, and induce damage on removal, application of dressings may lead to impairment of proper healing.14 Modern wound dressings have, thus, been developed to overcome the limitations by promoting the healing process in a moist environment. Examples of modern dressings include hydrocolloids, alginate, and nonalginate dressings.15 Wound treatments have been adapted to incorporate bioactive molecules, such as growth factors and antimicrobial molecules.
For treatment of large area skin loss, including severe burns, wound dressings are not sufficient. Thus, skin grafts, where a piece of healthy skin from a donor area, either partial thickness (split skin) or full thickness or containing only epidermis, is harvested and applied to the wound, are often required to accelerate wound coverage and healing. For epithelial autografting, epidermal cultures of a patient's keratinocytes have been explored, to form confluent cell sheets. This has proved time-consuming and costly. An alternative approach has been developed, where autologous cells are used in suspension and applied directly to a wound surface by using a spray-on technique. This method shows increased applicability to clinical situations, and offers the potential to facilitate one-stage treatment for partial thickness burns, and full-thickness wounds when combined with a dermal regeneration template.16,17
Currently, autografts are a preferred method as they provide good adhesion and pain relief, with minimal rejection of the graft. This method is inherently limited by the availability of donor sites and also, it leads to further trauma, potentially resulting in additional complications. Allografts gained popularity owing to their higher availability. However, in most cases, they provide only temporary “biological dressings.” Concerns remain especially regarding immunological rejection, disease transmission, sensitization of the recipient, and ethical considerations. The limitations and developments mentioned earlier have prompted the advance of skin tissue engineering and synthetic skin grafts.10 For a comprehensive review on wound treatments, the readers are referred to Boateng et al.18
Skin tissue engineering
A contemporary and evolving process involves skin tissue engineering. These skin replacements are generally designed to remain within the skin and degrade over time to reduce damage induced on removal. By combining scaffolds with cells and biomolecules, attempts are made to replace the skin with a biological and mechanically sufficient replacement to restore tissue function. However, the synthetic skin grafts are often difficult to handle, with poor adhesion to the wound bed, poor vascularization, no promotion of regeneration of full-thickness wounds, and high manufacturing costs.14 One of the main causes of these complications is the relative simplicity of the current tissue-engineered constructs.19 To restore the skin to its natural healthy conditions, the tissue replacement should closely resemble the skin layering and composition. Due to the complexity of the skin, the development of a functional replacement tissue remains challenging. Generally speaking, to engineer skin substitutes, four key requirements must be met: (1) Engineered skin must be able to provide a moist environment; to promote healing and prevent attachment of the dressing to the surrounding tissues. (2) Engineered skin must be able to enhance natural wound-healing responses. (3) Replacement tissue must also be able to provide adequate oxygen exchange with the surrounding environment. (4) It must limit the infiltration of potential harmful bacteria and pathogens.14,20
Other considerations in the engineering of skin substitutes are patient safety, degradation time, duration of cover, shelf life, cost, mechanical stability, adequate vascularization, and number of stages for completion of treatment.21 Preferably, a replacement has the structural composition and mechanical properties that are similar to the ECM of the skin.22 Tissue-engineered skin substitutes currently applied are epidermal substitutes, dermal substitutes, and dermo-epidermal substitutes.23,24 Epidermal substitutes are often cultured (split thickness) epithelial autografts that can be used in extensive burns. However, they have the same disadvantages as with most of the autografts: high infection risk, high cost, limited collection site.22 Examples of dermal substitutes used in a clinical setting are Integra®, AlloDerm®, Dermagraft®, and Matriderm®. The application of Integra®, AlloDerm®, or Dermagraft® involves a two-step process.19 Integra®, a crosslinked bovine collagen-based matrix, has a good long-term aesthetic and functional outcome, but with high cost and a poor adhesion to wound site. AlloDerm®, human acellular dermis in a lyophilized form, provides a matrix closely resembling the patients' skin delivering good vascularization and regeneration. This method is restrained by high costs, disease transmission between donors. Dermagraft®, a synthetic material made from bioabsorbable polyglactin seeded with neonatal human foreskin fibrolasts, improves ease of handling for the surgeon, shows no rejection, and can be applied on chronic wounds; however, poor ECM structures, infections, and cellulitis have been reported. Matriderm®, a noncrosslinked lyophilized bovine material, is a one-stage process that promotes vascularization and improves stability and elasticity of regenerated tissue.22 This material, currently used for dermal grafts, has also been applied as an ink material in biofabrication. Lastly, examples of dermo-epidermografts are PermaDerm® and DenovoSkin®, which more closely resemble the native skin, but no clinical trials have been reported as of yet.23 For more comprehensive overviews of current skin substitutes, refer to Hrabchak et al., Metcalfe and Ferguson, or Chua et al.3,19,23
Tissue-engineered skin can be enhanced by the addition of growth factors and other biomolecules, regulating several wound-healing-related processes, as the ECM has been an important factor in growth factor regulation and delivery.25 Major growth factors and cytokines that can be added to modulate wound healing are epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α). These influence re-epithelialization, granulation tissue formation, matrix formation and remodeling, and inflammation levels.26,27 When added unaided, proteases at the wound site have shown to interfere with efficacy. Therefore, combining growth factors with wound dressings and/or tissue-engineered skin grafts improves healing by creating an appropriate wound environment. To improve the therapeutic efficacy of growth factors, a number of delivery strategies have been developed to enable controlled spatial/temporal presentation of growth factors at the site of injury. These include, for example: lipid nanoparticle systems, ECM-inspired growth factor delivery systems, and nanofibrous structures.27,28 Due to the limited scope of this article, readers are referred to a couple of excellent reviews by Gainza et al., Barrientos et al., and Pachuau.26–28
Improvements in tissue-engineered skin substitute healing can be made in regards to the high manufacturing costs, regeneration of the skin, immune rejection, ease of handling, and complexity of construct to closer resemble the anatomy of the skin. High manufacturing costs may be addressed by the use of bioprinting, which would reduce manual labor and also bring standardization to the fabrication process to produce reproducible skin substitutes.
Bioprinting
Bioprinting is an emerging additive manufacturing tool in tissue engineering and regenerative medicine. Using a computer-aided design, it provides an unprecedented ability to strategically assemble biomaterials and cells to build 3D structures. Bioprinting provides an economically viable and reproducible method for the creation of spatially organized, biologically compatible, and mechanically stable constructs, mimicking the natural organization of healthy skin. In addition, patient-specific wound dressings and skin substitutes could be fabricated with embedded ECM components, growth factors, and cell types, precisely tailored to each region and wound depth, facilitating the body's natural response to trauma while protecting the wound site.10
For bioprinting, core elements involve the type of printer and bioink formulation, which act in concert to determine the printing method and process, and subsequently the biological and mechanical characteristics of the 3D printed construct. Key modalities of printing that have been explored for cell printing are inkjet printing, laser-induced forward transfer (LIFT), and extrusion printing (discussed in the next section). The materials used to build the 3D construct are constituent of the bioinks; these materials comprise synthetic and/or naturally derived polymers, and they are employed to facilitate cell printing and to provide extracellular environments to support cell proliferation and/or differentiation as required.
Bioprinting modalities and bioink
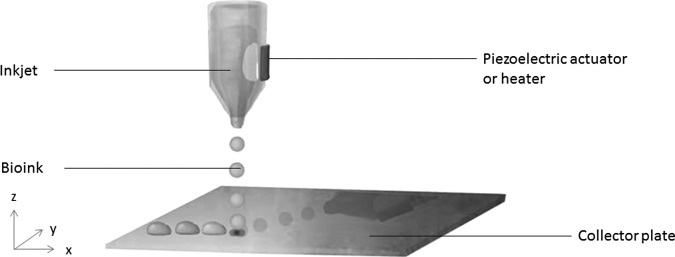
In inkjet printing, a cartridge is filled with a bioink, which is dispensed in droplets onto the collector plate via thermal or acoustic forces (Fig. 2).4 For thermal printing, pressure pulses are created by electrically heating the print head. Acoustic printing applies an acoustic wave to eject droplets.29–31 The size of the droplets can be controlled by regulating various parameters, such as temperature and viscosity of the bioink, and the amplitude and frequency of the printing parameters.30
Figure 2.
Schematic representation of an inkjet printer.
As with all bioprinting approaches, inkjet printing has its advantages and limitations. Regarding thermal approaches, inks are exposed to temperatures over 200°C for short bursts, and so the capacity of inks to recover from abrupt temperature change is critical. Interestingly, the impact on encapsulated cells appears to be negligible.32,33 Another significant limitation relates to the mechanical stresses imposed on encapsulated cells, with a considerably narrow viscosity tolerance range.34 Moreover, frequent nozzle clogging and inconsistent droplet formation impart further difficulties. Therefore, bioinks used within this technique should remain within a 3.5–12 mPa/s viscosity range and low cell densities (typically <106 cells/mL) are usually employed.35 Despite these limitations, however, printing resolution can be high, enabling precise positioning of cells.34 In addition, the availability of such printers is vast, generally resulting in low maintenance and service costs.
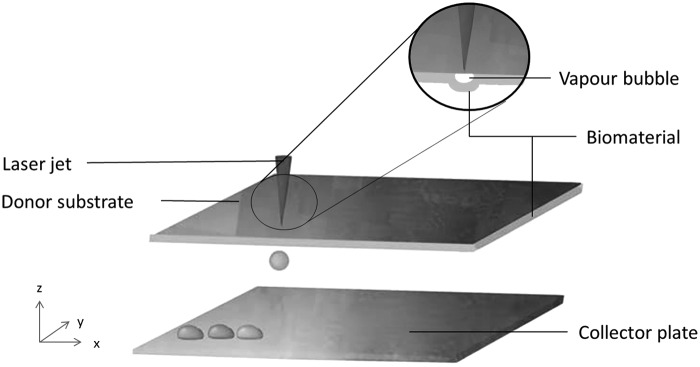
LIFT within the field of bioprinting is often referred to as LAB or LABP (Fig. 3).36 Unlike inkjet printing, LIFT does not require a printing nozzle, subjugating particular limitations in regards to clogging of the nozzle. Figure 3 illustrates a simple representation of the technology, where a donor layer, containing an energy-absorbing layer commonly made of gold or titanium but also polymers such as gelatine or triazine, is excited through laser pulses.37,38 These pulses penetrate and vaporize the donor layer, creating a vapor bubble. The formed hydrogel jet cascades onto the collector slide as a fine resolution droplet with high spatial control.39 Droplet size can be regulated by laser energy, hydrogel depth, and viscosity.40 The ink viscosity ranges for LIFT methods are not as narrow as inkjet methods, 1–300 mPa/s compared with 3.5–12 mPa/s. Correspondingly, nozzle, needle, and/or tip clogging are not an issue.36
Figure 3.
A schematic representation of LIFT. LIFT, laser-induced forward transfer.
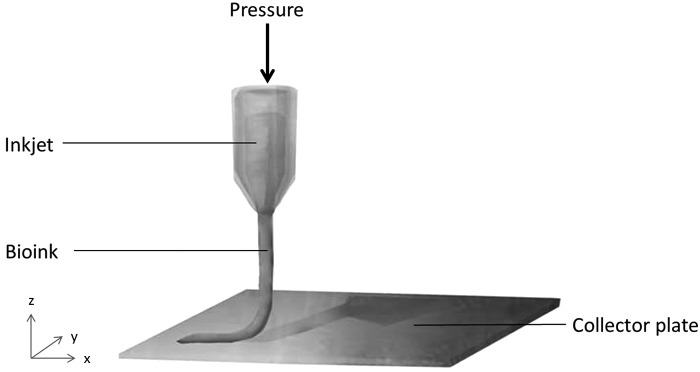
Arguably the most extensively explored bioprinting approach in tissue engineering so far involves microextrusion.41–43 Instead of droplets, extrusion bioprinting dispenses continuous cylindrical strands of hydrogel by using air or mechanical force (Fig. 4).44–46 The former system employs pneumatics to drive the filament through the tip, which is restricted only by pressure and the delay of its volume. Mechanical or robotic extrusion printers use either piston or screw mechanisms to project hydrogels, with modest spatial control and resolution.
Figure 4.
A schematic representation of an extrusion printer.
Due to relative simplicity, ink properties for extrusion printing are less restricted compared with other approaches, supporting a vast viscosity range of 30 mPa/s to at least 6 × 107 mPa/s.30 The cell density can also be increased significantly, therefore making it possible to achieve physiological relevant cell densities. In addition to cell suspensions, cell pellets, tissue spheroids, and tissue strands have also been successfully printed.46–49
A significant shortcoming of extrusion-based bioprinting, apart from the resolution (hundreds of microns), involves the shear stresses experienced by encapsulated cells during printing. High viscosities result in enhanced shape fidelity at the expense of high shear stress. Thus, to achieve both high cell viability and shape fidelity, a bioink should demonstrate shear-thinning behavior during printing and then rapid recovery or cessation after extrusion.43 Ink viscosity determines printability and cell viability, which are influenced by polymer concentration and crosslinking. Coupled with these material properties are the hardware restrictions bestowed on inks, ranging from, but not restricted to, pressure, flow rate, temperature, and substrate interactions.35,50
Skin printing
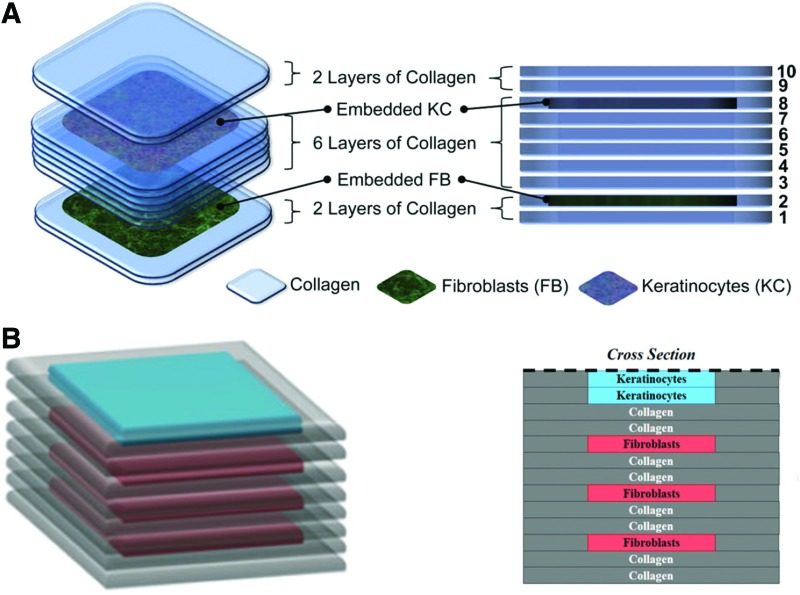
In recent years, skin printing has gained popularity in the field of skin tissue engineering. Several printing techniques have been applied in the attempt of skin tissue formation. In 2009, Lee et al. applied a stage-controlled inkjet printer to create a layer-by-layer construct containing both primary adult human dermal fibroblasts and primary adult human epidermal keratinocytes, which were printed in a polydimethylsiloxane mold simulating a nonplanar skin wound. The artificial skin was constructed by printing layers of collagen, onto which a layer of cells could be deposited. After each layer, the cell-containing gel was crosslinked by using nebulized NaHCO3 vapor. The design consisted of 10 layers of collagen, the second layer seeded with fibroblasts, and the eighth layer containing keratinocytes (Fig. 5A). Viability after printing was comparable to the control group for both fibroblasts (>95%) and keratinocytes (>80%), indicating minimal damage due to inkjet printing and shear stresses. However, an inhomogeneous organization of the printed cells was still present.13 An alternate method was described by Lee et al., where an alternate spatial organization of collagen layers was applied. To approach the structure and spatial cell organization of native skin, keratinocytes were printed with a high density in the two upper layers, whereas a lower density of fibroblasts was printed into three layers equally distributed throughout the remaining construct (Fig. 5B).13 The 3D printed constructs were better able to retain their shape after 7 days of culture, compared with manual deposition.51 Potential of inkjet printing in skin tissue engineering has been shown by the heightened control of cell density, organization, and combining multiple cell types.
Figure 5.
Construction of two designs of layer-by-layer printing of collagen, human dermal fibroblasts, and keratinocytes. (A) Illustrates the design containing 10 layers, with layer 2 consisting of collagen and embedded fibroblasts, and layer 8 containing keratinocytes. Obtained and modified from13 with permission from Elsevier. (B) Illustrates the method containing 2 top layers containing keratinocytes and 3 layers of fibroblasts distributed equally in the bottom 11 layers.6 Obtained and modified from Lee et al.6 with permission from Mary Ann Liebert. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
LIFT was applied in the development of 3D spatially controlled constructs resembling human skin and, in both instances, resulted in well-organized tissue constructs. Koch et al. created a bi-layered construct, containing murine fibroblasts and human immortalized keratinocytes in Matriderm. Ten days of culture demonstrated proliferation in all cells, which was used to verify the cell vitality. Also, a layer of laminin, the main component of the basal lamina, had formed in between the keratinocyte and fibroblast layer. The formation of this layer showed the increasing complexity that can be formed by the 3D spatial arrangement of the cells and the influence of multiple cell types in one structure. The intra-cellular communication was assessed by analysis of adherence and gap junctions. Adherence junctions were found in abundance between the keratinocytes, but to a lesser extent between the fibroblasts.36 Similarly, Michael et al. applied LIFT to fabricate a cellularized skin substitute, assembling a construct containing 20 layers of keratinocytes on 20 layers of fibroblasts in Matriderm®. These constructs were tested in vivo in full-thickness wounds in mice. All animals survived the surgery and surrounding tissue connected with the implanted skin substitute; no inflammatory or necrotic processes were detected. Proliferation in the epidermal and dermal layer was found in both healthy mouse skin and the skin constructs, but not in the negative control. In the skin substitute, a blood vessel was formed after 11 days, but complete vascularization was not achieved.52 Both studies show LIFT as a promising technique in skin tissue engineering, due to improved cellular spatial arrangement, angiogenesis stimulation, and integration with host tissues.
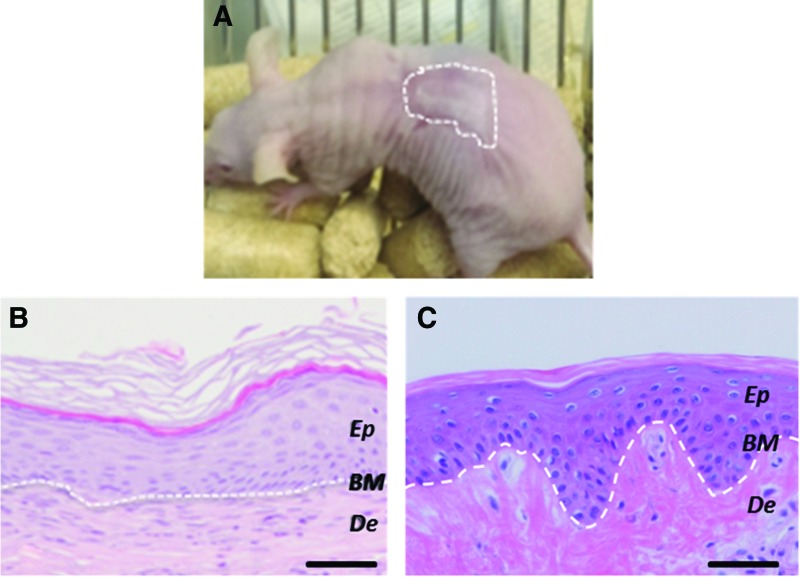
Recently, extrusion printing was applied for the development of skin substitutes. Kim et al. applied a combination of gelatin and agar with extrusion printing to create skin substitutes for the treatment of laser tattoo removal.53 Multiple skin types were emulated, with varying colors and light absorption, to accommodate multiple different skin types. For all types, constructs were determined to be of 138.5 ± 0.1 μm and 0.81 ± 0.04 mm thickness for the epidermis and dermis, respectively, which is relatively low, but within the range of native human skin.53 Cubo et al. printed a skin construct with a dermal layer consisting of human dermal fibroblasts, CaCl2, and human plasma.2 Interestingly, the authors applied a printing technique whereby the ink is mixed during the printing process. Directly afterward, human keratinocytes were printed over the initial construct. The constructs were left in incubation overnight, after which the constructs were implanted in immunodeficient mice. The in vivo skin showed a structure similar to human skin, contrasting with the surrounding native mouse skin (Fig. 6). Identified were the stratum basale, stratum spinosum, stratum granulosum, and stratum corneum, suggesting a normal functioning epidermal layer. In addition, neoangiogenesis formation was observed.2
Figure 6.
The visual appearance of a bioprinted graft in an immunodeficient mouse is shown in (A) and (B) shows a typical haematoxylin and eosin (H/E)-stained sample of the bioprinted human skin grafts in immunodeficient mice and (C) shows a typical H/E-stained sample of native human skin, with the white line indicating the dermo-epidermal junction (scale bar: 100 lm). Obtained and modified from Cubo et al.2 with permission from IOP Publishing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Similarly, attempts have been made in regard to the creation of an in situ bioprinting technique, where human dermal fibroblasts and human keratinocytes have been loaded into a printer and printed directly on the dorsa of athymic mice, resulting in complete closure of the wound after 3 weeks.54
Further optimization of bioprinting techniques and combination with other biofabrication techniques may lead to more complex tissue constructs, best mimicking the organization of the skin and the different ECM components in each layer.10
Future Directions
Bioprinting techniques show potential in expanding current knowledge, in developing tissues closer resembling native tissues, and in addressing current skin tissue engineering complications such as skin type differences, vascularization, and cell organization. During the printing process, the bioink formulation must be both stable and fluidic enough to be extruded. After extrusion, the material must be such that the printed structure retains shape and provides mechanical behavior similar to the specific tissue. The most common approach to bioprinting involves cell encapsulation in a hydrogel, as the mechanical properties of the resulted structure and water retention properties of most hydrogels closely resemble the ECM. Therefore, other biomolecules, such as growth factors, can be added to the bioprinted constructs to influence epithelialization, tissue formation, tissue remodeling, and inflammation. Examples of biomolecules incorporated into hydrogels are VEGF in fibrin gels for neural stem culture and the bioprinting of fibroblast growth factor-2 and bone morphogenetic protein-2 onto sub-micron fibrous scaffolds to increase spatial control of cells. Besides, the printing of growth factors onto a hydrogel in specific patterns can also influence cell organization.54–56 Incorporation of growth factors within micro particles, which are combined with a bioink, improves release control. Previously, VEGF-laden gelatin microparticles have been incorporated into 3D printed constructs, for a precise organization and sustained localized release, resulting in heightened vessel formation in mice compared with a fast release profile.57 More recently, growth factors have been incorporated into PLGA microparticles and printed in micrometer scale resolutions.58 This type of microparticle-based biomolecule inclusions could be customized and applicable for different types of tissue regeneration and can, therefore, also be explored in combination with wound healing, thereby increasing targeting precision, improving control of growth factor release, and creating the possibility of individualizing the process based on patient need.
Take-Home Messages.
Biofabrication holds promise in healing of large or chronic skin wounds.
Biofabrication may be applied in healing of deep wounds, encompassing several layers, and preventing scar formation in the dermal layer of the skin.
Reproducibility may be increased with the application of biofabrication.
Biofabrication may hold promise in tissue engineering; however, further developments in fabrication techniques and bioinks are a necessity.
Predominantly vascularization within the constructs remains an important challenge in skin tissue fabrication.3 The native dermis is a vascularized tissue and can range between 0.3 and 4 mm in thickness depending on the location. The epidermis depends on the vascularization within the dermis for its oxygen and nutrient exchange. Therefore, in thicker multiple-layered constructs, there is often necrosis of the epidermal layer. Significant advances in creating vascularized networks and 3D printed structures will have to be made before the development of large functional tissue constructs can be realized. The introduction of several other skin components, such as hair follicles, sweat glands, and melanocytes, has yet to be realized.
Summary
Bioprinting has been used to demonstrate the potential to expedite skin tissue engineering. By combining current knowledge on material science, engineering, and cell biology, this interdisciplinary approach can be applied to address current limitations, especially in vascularization and skin appendages, increasing reproducibility, and increasing patient specificity. However, progression can only be made through close collaboration between clinicians and researchers.
Abbreviations and Acronyms
- 3D
three-dimensional
- ECM
extracellular matrix
- IL
interleukin
- LAB/LABP
laser-assisted bioprinting
- LIFT
Laser-induced forward transfer
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
The authors wish to acknowledge funding from the Australian Research Council (ARC) Centre of Excellence Scheme (CE140100012), the use of facilities at the University of Wollongong Electron Microscopy Centre, and support of the Australian National Fabrication Facility (ANFF)—Materials Node. Professor Gordon G. Wallace acknowledges the support of the ARC through an ARC Laureate Fellowship (FL110100196). This research has been conducted with the support of the University of Utrecht Biofabrication mobility grant and the Australian Government Research Training Program Scholarship. Associate Professor Christopher Baker acknowledges the support through a research grant of the F & E Bauer Foundation.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Sylvia van Kogelenberg, MSc, received her degree from the Utrecht University and the University of Wollongong. She has explored melt electrospinning for support in tissue constructs, and more recently, she has been focusing on bioink development of seaweed-derived polysaccharides for wound-healing applications. Zhilian Yue, PhD, is a senior research fellow in the Intelligent Polymer Research Institute, University of Wollongong. Her research focuses on tissue engineering and regenerative medicine and drug delivery. More recently, she has been working on bioink development for cell printing. Jeremy N. Dinoro, MSc, received his degree from the University of Wollongong and Utrecht University. He has explored bioink development utilizing seaweed-derived polysaccharides for wound-healing applications. More recently, he has been working on optimizing bioprinting and bioreactor systems for in vitro toxicology testing. Christopher Baker, MBBS, FACD, is Associate Professor and Director of Dermatology Department at St Vincent's Hospital Melbourne. He is a clinical dermatologist, with research interests in 3D printing and biofabrication of skin, clinical trials of new therapies, and quality-of-life studies. Gordon G. Wallace, DSc, PhD, is Professor and Executive Research Director of the Australian Research Council Centre of Excellence for Electromaterials Science and Director of the Intelligent Polymer Research Institute, University of Wollongong. He is an Australian Research Council Laureate Fellow, and a Fellow of the Australian Academy of Science, Australian Academy of Technological Sciences and Engineering, Institute of Physics, and Royal Australian Chemical Institute. Professor Wallace is a materials scientist in the field of polymers and new fabrication technologies and the use of these in medical applications.
References
- 1.Long WL, Wang S, Yeong WY, Naing MW. Skin bioprinting: impending reality or fantasy? Trends Biotechnol 2016;34:689–699 [DOI] [PubMed] [Google Scholar]
- 2.Cubo N, Garcia M, del Cañizo JF, Velasco D, Jorcano JL. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 2016;9:015006. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007;4:413–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malda J, Visser J, Melchels FP, et al. . 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 2013;25:5011–5028 [DOI] [PubMed] [Google Scholar]
- 5.Mironov V, Trusk T, Kasyanov V, Little S, Swaja R, Markwald R. Biofabrication: a 21st century manufacturing paradigm. Biofabrication 2009;1:022001. [DOI] [PubMed] [Google Scholar]
- 6.Lee V, Singh G, Trasatti JP, et al. . Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 2013;20:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandika P, Ko SC, Jung WK. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int J Biol Macromol 2015;77:24–35 [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich HP, Krummel TM. Regulation of wound healing from a connective tissue perspective. Wound Repair Regen 1996;4:203–210 [DOI] [PubMed] [Google Scholar]
- 9.Madison KC. Barrier function of the skin: “la raison d'etre” of the epidermis. J Invest Dermatol 2003;121:231–241 [DOI] [PubMed] [Google Scholar]
- 10.Pereira RF, Barrias CC, Granja PL, Bartolo PJ. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013;8:603–621 [DOI] [PubMed] [Google Scholar]
- 11.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 2008;9:628–638 [DOI] [PubMed] [Google Scholar]
- 12.McKinley M, O'Loughlin V, Bidle T, eds. Anatomy & Physiology: An Integrative Approach, 2nd ed. Columbus, OH, 2016:191 [Google Scholar]
- 13.Lee W, Debasitis JC, Lee VK, et al. . Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009;30:1587–1595 [DOI] [PubMed] [Google Scholar]
- 14.Pereira GG, Dimer FA, Guterres SS, Kechinski CP, Granada JE, Cardozo NSM. Formulation and characterization of poloxamer 407®: thermoreversible gel containing polymeric microparticles and hyaluronic acid. Quím Nova 2013;36:1121–1125 [Google Scholar]
- 15.Bhatnagar M, Bhatnagar A. Wound Dressings from Algal Polymers. In: Kim SK, Chojnacka K, eds. Marine Algae Extracts: Processes, Products, and Applications. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co: 2015:523–556 [Google Scholar]
- 16.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn wounds: eleven years of clinical experience. Burns 2006;32:538–544 [DOI] [PubMed] [Google Scholar]
- 17.Wood FM, Stoner ML, Fowler BV, Fear MW. The use of a non-cultured autologous cell suspension and Integra® dermal regeneration template to repair full thickness wounds in a porcine model: a one step process. Burns 2007;33:693–700 [DOI] [PubMed] [Google Scholar]
- 18.Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2007;97:2892–2923 [DOI] [PubMed] [Google Scholar]
- 19.Hrabchak C, Flynn L, Woodhouse KA. Biological skin substitutes for wound cover and closure. Expert Rev Med Devices 2006;3:373–385 [DOI] [PubMed] [Google Scholar]
- 20.Groeber F, Holeiter M, Hampel M, Hinderer S, Schenke-Layland K. Skin tissue engineering—in vivo and in vitro applications. Adv Drug Deliv Rev 2011;63:352–366 [DOI] [PubMed] [Google Scholar]
- 21.Yildirimer L, Thanh NT, Seifalian AM. Skin regeneration scaffolds: a multimodal bottom-up approach. Trends Biotechnol 2012;30:638–648 [DOI] [PubMed] [Google Scholar]
- 22.Kirchmajer DM, Gorkin R., III An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J Mater Chem B 2015;3:4105–4117 [DOI] [PubMed] [Google Scholar]
- 23.Chua AWC, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 2016;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyame TT, Chiang HA, Orgill DP. Clinical applications of skin substitutes. Surg Clin North Am 2014;94:839–850 [DOI] [PubMed] [Google Scholar]
- 25.Briquez PS, Hubbell JA, Martino MM. Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv Wound Care 2015;4:479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 27.Gainza G, Villullas S, Pedraz JL, Hernandez RM, Igartua M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine 2015;11:1551–1573 [DOI] [PubMed] [Google Scholar]
- 28.Pachuau L. Recent developments in novel drug delivery systems for wound healing. Expert Opin Drug Deliv 2015;12:1895–1909 [DOI] [PubMed] [Google Scholar]
- 29.Martin GD, Hoath SD, Hutchings IM. Inkjet printing-the physics of manipulating liquid jets and drops. J Phys Conf Ser 2008;105:012001 [Google Scholar]
- 30.Fang Y, Frampton JP, Raghavan S, et al. . Rapid generation of multiplexed cell cocultures using acoustic droplet ejection followed by aqueous two-phase exclusion patterning. Tissue Eng Part C Methods 2012;18:647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demirci U. Acoustic picoliter droplets for emerging applications in semiconductor industry and biotechnology. J Microelectromechan Syst 2006;15:957–966 [Google Scholar]
- 32.Cui X, Boland T, D'Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul 2012;6:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu T, Gregory CA, Molnar P, et al. . Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006;27:3580–3588 [DOI] [PubMed] [Google Scholar]
- 34.Calvert P. Inkjet printing for materials and devices. Chem Mater 2001;13:3299–3305 [Google Scholar]
- 35.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–785 [DOI] [PubMed] [Google Scholar]
- 36.Koch L, Deiwick A, Schlie S, et al. . Skin tissue generation by laser cell printing. Biotechnol Bioeng 2012;109:1855–1863 [DOI] [PubMed] [Google Scholar]
- 37.Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB. Laser-based direct-write techniques for cell printing. Biofabrication 2010;2:032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiele NR, Chrisey DB, Corr DT. Gelatin-based laser direct-write technique for the precise spatial patterning of cells. Tissue Eng Part C Methods 2010;17;289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unger C, Gruene M, Koch L, Koch J, Chichkov BN. Time-resolved imaging of hydrogel printing via laser-induced forward transfer. Appl Phys A Mater Sci Process 2011;103:271–277 [Google Scholar]
- 40.Gruene M, Deiwick A, Koch L, et al. . Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C Methods 2010;17:79–87 [DOI] [PubMed] [Google Scholar]
- 41.Melchels FP, Dhert WJ, Hutmacher DW, Malda J. Development and characterisation of a new bioink for additive tissue manufacturing. J Mater Chem B 2014;2:2282–2289 [DOI] [PubMed] [Google Scholar]
- 42.Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res 2010;40:395–414 [Google Scholar]
- 43.Ferris CJ, Stevens LR, Gilmore KJ, et al. . Peptide modification of purified gellan gum. J Mater Chem B 2015;3:1106–1115 [DOI] [PubMed] [Google Scholar]
- 44.Khalil S, Sun W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater Sci Eng C 2007;27:469–478 [Google Scholar]
- 45.Jakab K, Damon B, Neagu A, Kachurin A, Forgacs G. Three-dimensional tissue constructs built by bioprinting. Biorheology 2006;43:509–513 [PubMed] [Google Scholar]
- 46.Owens CM, Marga F, Forgacs G, Heesch CM. Biofabrication and testing of a fully cellular nerve graft. Biofabrication 2013;5:045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mironov V, Visconti RP, Kasyanov V, et al. . Organ printing: tissue spheroids as building blocks. Biomaterials 2009;30:2164–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehesz AN, Brown J, Hajdu Z, et al. . Scalable robotic biofabrication of tissue spheroids. Biofabrication 2011;3:025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Y, Ozbolat IT. Tissue strands as “bioink” for scale-up organ printing. Eng Med Biol Soc 2014;2014:1428–1431 [DOI] [PubMed] [Google Scholar]
- 50.Nikkhah M, Eshak N, Zorlutuna P, et al. . Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012;33:9009–9018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JM, Yeong WY. Design and printing strategies in 3D bioprinting of cell-hydrogels: a review. Adv Healthc Mater 2016;5:2856–2865 [DOI] [PubMed] [Google Scholar]
- 52.Michael S, Sorg H, Peck CT, et al. . Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One 2013;8:57741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H, Hau NT, Chae YG, Lee BI, Kang HW. 3D printing-assisted fabrication of double-layered optical tissue phantoms for laser tattoo treatments. Lasers Surg Med 2016;48:392–399 [DOI] [PubMed] [Google Scholar]
- 54.Campbell PG, Miller ED, Fisher GW, Walker LM, Weiss LE. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials 2005;26:6762–6770 [DOI] [PubMed] [Google Scholar]
- 55.Ker ED, Nain AS, Weiss LE, et al. . Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011;32:8097–8107 [DOI] [PubMed] [Google Scholar]
- 56.Lee YB, Polio S, Lee W, et al. . Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol 2010;223:645–652 [DOI] [PubMed] [Google Scholar]
- 57.Poldevaart MT, Gremmels H, van Deventer K, et al. . Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J Control Release 2014;184:58–66 [DOI] [PubMed] [Google Scholar]
- 58.Tarafder MS, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication 2016;8:025003. [DOI] [PubMed] [Google Scholar]