Summary
The study of burn flora is helpful in determining current antibiotic susceptibilities and locating development of multidrug resistant bacterial strains among the unit’s usual flora. In this study, we aimed to determine the bacteriological pattern of blood, urine and sputum infections and their correlation with burn wound infections. We used data from our burn registry program. All data on demographics, burn wounds and burn wound infection, bacteria isolated, sensitivity to different antibiotics, burn wound culture, sputum culture, urine culture and catheter tip culture were recorded. We had 1721 hospitalized burn patients. Mean age was 26.3+/-20.25 years old. Mean hospital stay was 14.41 days (range 0-64 days). Mean (SD) TBSA was 16.48 (20.67) years. Mortality rate was 5.9%. Burn wound infection was present in 38.54%. The most frequent species was Staphylococcus spp. (55.1%), followed by Pseudomonas (14.29%), Enterococcus (12.24%), E. coli (4%), Klebsiella and Proteus (both 2%). Urine culture was positive in 27.9%, sputum culture was positive in 1.14%, catheter tip culture was positive in 12.3% and blood culture was positive in 7.6% of the cases. There were correlations between positive wound culture and blood and urine culture, most of them with one bacteria species. The most frequent disseminated bacteria was Pseudomonas aeruginosa and the most sensitive antibiotic was Amikacin. More than 39.2% of our positive culture patients had 3 or more positive cultures, and 36.5% had similar culture results for one bacteria, which was a sign of disseminated infection
Keywords: burns, bacteriology, antibiotic, resistance, early excision, complication
Abstract
L’étude de la flore de la zone brûlée est utile pour connaître les possibilités d’antibiothérapie et la colonisation par des bactéries multi-résistantes. Le but de cette étude était de colliger les bactéries responsables d’infections sanguines, urinaires, respiratoires et d’étudier leur corrélation avec les bactéries retrouvées dans les zones brûlées, relevées dans les dossiers des patients. Les données démographiques et bactériologiques (avec antibiogrammes) au niveau de la brûlures et dans les sites infectés ont été relevées, chez 1 721 patients hospitalisés, d’âge moyen 26,3 +/- 20,25 ans. La durée moyenne d’hospitalisation était de 14,41 jours (0-64). Une infection de la brûlure a été diagnostiquée chez 38,54% des patients. Le genre le plus souvent retrouvé était Staphylococcus (55,1%). Par ordre décroissant, nous avons isolé Pseudomonas æruginosa (14.29%), Enterococcus sp. (12,24%), E. coli (4%), Klebsiella et Proteus (tous deux 2%). Les infections urinaires représentaient 27,9% du total, les examens de crachats ont été positifs dans 1,14%, les cultures de cathéters dans 12,3% et une septicémie était en cause dans 7,6% des cas. Les isolements dans le sang et l’urine (monobactériens le plus souvent) corrélaient bien avec la flore cutanée. La bactérie le plus souvent retrouvée en sites multiples était Pseudomonas æruginosa, l’amikacine étant l’antibiotique le plus régulièrement efficace. Plus de 39% des patients ayant eu des cultures positives en avaient 3 ou plus positives, dont 36,5% au même germe, ce qui témoigne d’une infection disséminée.
Introduction
Burns are one of the main traumas in our country, and every year we face new presentation of cases with burn injury. Burn wounds lack epidermis and circulation, so they are the best culture media with a centigrade temperature of 37 degrees. Therefore, they are the best media for bacterial growth. A few hours after the burn (normally 4-5 hours after) the wound surface becomes contaminated with many bacterial flora, which will start to grow and multiply.1
Some of these bacteria are more virulent than others, and have enzymes to dissolve their way into the deeper normal tissue. Some of them have flagella and good motility to pass through necrotic tissue and reach the deep normal soft tissue. Through motility and enzyme dissolution, they will reach the vascular and lymphatic vessels and start to disperse. At this point, bacteraemia and sepsis start.1,2
Due to the release of several cell mediators, burn patients have deficiencies in their immune system and cannot tolerate this invasion. Therefore, bacteria will seed and grow in other soft tissues or organs like the lungs, kidneys and blood. After this stage, positive cultures can be found in these organs. Soon afterwards, if not treated properly, septic shock, multiple organ failure and most probably death will follow.
The key sign of disseminated infection in the body is positive culture of different tissues with similar bacteria. This sign prompts aggressive treatment, debridement, empiric antibiotic treatment and probably immune system modulator treatment. Moreover, in order to prevent this scenario, physicians have to know which flora most frequently invade the burn wounds, and the antibiotic sensitivity of these bacteria. One of the major concerns in treating these cases is the time of treatment and aggressive debridement.
In this study we examined and compared the most frequent culture positive sites in the body, the bacteria that most frequently grow in these soft tissues and their sensitivity to antibiotics. In order to do this, we used data from our burn registry program.
Materials and methods
We prospectively gathered the data of burn patients in our country’s burn registry program, and inserted these data in a special questionnaire on age, sex, demographic data, anatomic distribution of burn, seasonal variation, cause of burn, extent of 3rd and 4th degree burn, previous clinical conditions, any treatment for burn at home, length of hospital stay, mode of therapy and surgical intervention, infection, burn wound culture, urine culture, sputum culture, blood and catheter tip culture, results of antibiotic sensitivity tests for each bacteria, antibiotics used, results of treatment, lab tests, percentage of burn surface area (TBSA), complications and their outcome.
Sepsis was defined as systemic inflammation response to infection and positive blood culture. Wound cultures with more than 100,000 bacteria in each gram of tissue were considered positive.
SIRS was defined as body temperature >38 or <36 C, heart rate>90/minute, respiratory rate >20/minute and white blood cell >12000 or <4000. Wound cultures were done by tissue culture. About 1 cubic centimetre of tissue including normal tissue was removed and sent for two examinations: 1. wound culture, colony count and antibiogram; 2. microscopic examination with hematoxylin and eosin, for examination of both normal tissue and burn tissue. Catheter cultures were done by cutting (10 millimetres of) the tip of the catheter and culturing it in the special media. Blood cultures were done by taking 3 samples of blood, each 10 cc and half an hour apart. Urine cultures were done by taking a urine sample mid-stream.
Follow up continued for more than 3 years. The correlation of the results of different cultures were also examined and an odds ratio for each one was calculated. Antibiotic susceptibility was determined with the agar disc diffusion method.
The results were analysed with SPSS 21 software and p values less than 0.05 were considered significant.
This study was approved by the ethics committee of our hospital. Consent was obtained from patients when their data was being recorded in the registry program. The study was conducted with a grant from Iran University of Medical Sciences, Research Vice-President’s Office.
Results
A total of 1721 burn patients were admitted to the hospital over a period of more than 2 years. Burns caused by open flame were the most frequent (49.8%), followed by scald (35.7%). Among flame burns, propane gas was the most frequent cause (59.7%), followed by gasoline (24.8%). The most frequent age group affected was the 25-34 group (20%). Sixty-three percent of our patients were male and 37% female. Male to female ratio was 1.7:1. Table I shows mode of burns, whether intentional or accidental.
Table I.
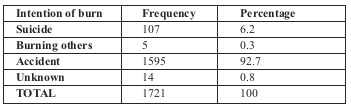
Mean (SD) age was 26.3 (20.25) years old. Mean (SD) TBSA was 16.48 (20.67). Mean hospital stay was 14.41+/- 10.91 days (range 0-64 days). Median hospital stay (LOS) was 11 days (SD = 10.91, mean = 14.41). Length of stay increased in relation to area burned (p<0.02).
Amniotic membranes were used as a temporary cover for 539 patients. Skin graft surgery was carried out for 978 (67.26%) patients.
A total of 47.7% of our cases developed signs of infection in their burn wound so burn wound biopsy and tissue culture were performed, with 481 (38.54%) of them receiving positive culture results.
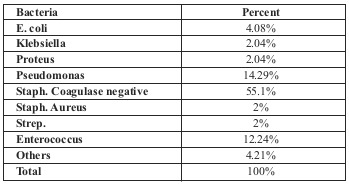
The most frequent bacteria found in burn wound culture was Staphylococcus spp. (55.1%), followed by Pseudomonas aeruginosa (14.29%), Enterococcus (12.24%), E. coli (4%), Klebsiella and Proteus (both 2%) (Table II).
Table II.
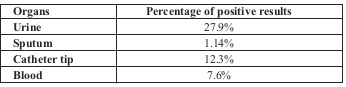
Urine culture was positive for 27.9%. The most frequent bacteria found was Pseudomonas aeruginosa (44%), followed by Staphylococcus spp. (33.1%), Enterococcus (20%), Klebsiella (1%) and Proteus (less than 1%).
Sputum culture was positive for 1.14%. The most frequent bacteria found were Pseudomonas aeruginosa and Staphylococcus spp. Catheter tip culture was positive for 12.3%. The most frequent bacteria found was Staphylococcus spp. (35%), followed by Pseudomonas aeruginosa (29%), Klebsiella (5%) and Proteus (4%).
Blood culture was positive for 7.6% of the cases (Table III). The most frequent bacteria found was Pseudomonas aeruginosa (51.6%), followed by Staphylococcus spp. (40%), Klebsiella (27%), Proteus (10%), Enterococcus (9.2%) and E. coli (3%).
Table III.
There were correlations between positive wound culture with blood and urine cultures. The odds ratio for burn wound positive culture and positive blood culture was [OR=1.33 (1.25-1.63)] and odds ratio for positive wound culture and positive urine culture was [OR=1.25 (1.15-1.46)].
The most common bacteria found in all cultures were Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella.
The most frequent antibiotics prescribed according to sensitivity tests were: Amikacin (91.9%), Ceftazidim (60.5%), Meropenem (37.7%), Imipenem (23.3%), Tazobactam (21%), Ciprofluxacin (38.5%), Cefixim (17%), Cefopime (22.6%) and Vancomycin (19%).
About 5.9% of the patients died, 3.9% discharged themselves (against their physician’s advice), 82.5% were discharged with partial recovery (needing further treatments) and 7.4% with complete recovery. Among those patients who had positive culture in 3 sites or more, more than 44.9% died due to disseminated infection and septic shock. More than 36.5% of them had similar bacteria. Most of them had infection with one bacteria and only 3 patients had positive cultures for multiple bacteria. Seventy-two percent of burn-related deaths were in patients suffering from a burn area of 40% and above.
Discussion
Annually, we have more than 100,000 burn patients in our country. About 6-8% of them are admitted to specialized burn centres and/ or general hospitals with burn unit facilities. Burn wounds are good media for bacterial growth. Soon after burn injury, colonization happens and bacteria grow more and more. Some of the more virulent ones will go deep into the burn tissue and will produce small abscesses underneath it. In this area there is no host defence, and after increasing in number, the bacteria will go to the normal surrounding soft tissue and then invade lymphatic tissue and blood vessels, especially venous vessels.1
Then bacteraemia will ensue, followed by sepsis and septic shock. Seeding of other tissues and organs will occur, for example, in lung tissue, the kidney and bladder, heart and its valves, brain and so on.1
It is obvious that the most frequent site of infection in burn patients is the burn wound, followed by lungs, kidneys and cardiac valves.
Controlling bacterial flora in the burn wound and preventing burn wound infection will result in the prevention of many infections in the body and septic shock. Failing to do so will result in disseminated infection in many soft tissues. Deterioration of the immune system aggravates this process. The same culture results in different tissues indicates disseminated infection that needs urgent and prompt medical and surgical action, such as aggressive debridement and starting IV antibiotics.
Therefore it is important to know which flora are normal in burn wounds and which flora and bacteria most frequently produce wound infections. We also have to know which bacteria most frequently invade the blood stream and produce infection in some of the tissues and organs in the body. Moreover, in each centre it is important to know the antibiotic susceptibility of these bacteria in order to rapidly start empiric antibiotics to treat these critical situations. In this way physicians can control the infection in the body and prevent unwanted complications and mortality in burn patients.
There are some reports that the most prevalent bacteria in burn wounds is Pseudomonas aeruginosa, 1,3,4,5,6,7,8,9,10,11,12,13,14 while others highlight the prevalence of Staph. aureus.2,15,16,17,18
There are some reports that other bacteria such as Acinetobacter Baumanii were more prevalent.19,20 However in our study the most frequent bacteria was Staph. Coagulase negative. It is accepted that the wound is sterile after a burn injury, but a few hours later it will be contaminated and colonized with bacteria in 33% of cases. This rises to 94% after 7 days and finally, after 2 weeks, 100% of wounds will have positive cultures. 21 In another report from Albania in 2013, colonization was seen in 43% of cases, and the most prevalent bacteria were Staph. 67% and Pseudomonas 24%.18
In Turkey, it was reported that 48.1% of cases were colonized and cultures were positive.14 In a report from Gaza in 2013, 45.8% of cases were culture positive.13 In 2013, a report from Romania asserted that a first sign of infection was seen in the first 2 weeks after injury in 97% of cases. 58% of them were Gram positive and 26% Gram negative bacteria.17 In these patients, 32% of cultures were positive for Staph. and 21% for Pseudomonas aeruginosa.
In 2014, Teckin et al. examined risk factors for infection in burn wounds. The risk factors they mentioned were: day of first excision, using invasive devices, a more than 24-hour delay in hospital admission, delay in dressing and local treatment of the burn wound and previous use of broad-spectrum antibiotics. TBSA more than 15% is also a risk factor for wound infection.15,2,20 Delay in local irrigation with chlorhexidine (antibiotic and detergent) is also mentioned as a factor for burn wound infection.21,22 Some infections are the result of bacterial translocation from the small intestine, and some are airborne.23 A number of measures have been proposed for the prevention of infection in burn wounds, such as: early debridement, frequent local irrigation, using H2O2, geranium oil, honey, topical antibiotics, biofilm disrupting agents and plastic wrap, acetic acid, and early excision and skin grafting. 11,20,24,25,26,27,28,29,30 Moreover, it is mentioned that prophylactic antibiotics are not recommended.31 In unfortunate situations when infection occurs, it may find its way to other tissues and organs,2,5,7,12,14,32 and more invasive infection has been seen 7 days after injury.22
Once the bacteria are in the blood stream, they will invade many organs and result in multiple infections. Positive culture can guide us in this unfortunate situation. In our study, more than 39.2% of positive cultures had 3 or more than 3 positive cultures in different organs, and about 36.5% had similar culture results. This was a sign of single bacteria dissemination in different organs. Once this happens, we have to start systemic antibiotics immediately, along with surgical debridement and other modalities (changing the local antibiotic, antibiotic clysis) to treat the situation.
Therefore it is better to diagnose the infection in advance and begin to treat it. For diagnosis, surface swab culture and tissue culture has been recommended.2,14
Tissue culture is more accurate but swab culture is more rapid and is better used in the first few days after injury.14 Concordance of positive swab culture and tissue culture is reported to be about 78% in the first few days after injury.14 Moreover, most cases are from infection with Pseudomonas aeruginosa.
It has been reported from Korea that with time and using antibiotics, the content of infection with Pseudomonas is reduced but content of infection with Klebsiella will increase.7
In our study, 38.5% of the patients had positive burn wound culture and the most frequent bacteria was Staph. Coagulase negative. But the most prevalent bacteria that disseminated through lymph and blood vessels was Pseudomonas aeruginosa, and many similar positive cultures in urine, blood and sputum were detected. In a report from Turkey, Vural et al. mentioned that the most frequently disseminated bacteria was Pseudomonas, and positive culture in urine was 27.9%, blood 7.6% and sputum 1.14%.14 In another report from the UK, the most disseminated bacteria was Staph. (79%).15
In Ethiopia the most disseminated bacteria in burn ICU patients was Staph. (42.8%),2 and in Korea it was Pseudomonas (30.1%).7
The worst thing about infection with these bacteria in burn centres is that they are multiple resistant to several antibiotics and very hard to treat.2,4,5,8,19,33
In our study, the most sensitive antibiotics were Amikacin (91.9%), Ceftazidim (60.5%) and Meropenem (37.7%). There are some reports that resistance to Ciprofluxacin and Amikacin is low and over time will decrease.5
Identifying the most frequent bacteria in burn wound infections and their resistance to antibiotics, and knowing the new modalities for their treatment are the keystones to preventing disseminated infections and mortality in burn patients. Once this happens, aggressive medical and surgical treatment is the keystone for the patients’ survival.
Strict and rigorous application of hygiene rules, early wound dressing, early debridement, together with continuous epidemiological surveillance of burn wound bacteria are important to optimize burn wound infection prevention, and treatment with empiric antibiotic therapy is the next step.
Conclusion
Knowledge of frequent bacterial flora in burn wounds and methods to prevent and treat burn wound infection is one of the first steps in burn wound care.
The most frequent disseminated bacteria in our centre was Pseudomonas aeruginosa and most sensitive antibiotic was Amikacin. More than 39.2% of our positive culture patients had 3 or more than 3 positive cultures, and 36.5% of them had similar culture results, which was a sign of disseminated infection
Acknowledgments
Acknowledgments.the authors wish to thank Mrs. Mitra Ghadarjani, Mrs.Bita Kamranfar, Mrs. Monireh Milani and Mrs. P. Shariatzadeh and Mr. Khaki for their kind and very effective cooperation in conducting the present study. this study was conducted with a grant from Iran University of Medical Sciences, research Vice-President’s Office.
References
- 1.Essayagh M, Essayagh T, Essayagh S, El Hamzaoui S. Epidemiology of burn wound infection in Rabat, Morocco: Three-year review. Med Sante Trop. 2014;24(2):157–164. doi: 10.1684/mst.2014.0315. [DOI] [PubMed] [Google Scholar]
- 2.Sewunet T, Demissie Y, Mihret A, Abebe T. Bacterial profile and antimicrobial susceptibility pattern of isolates among burn patients at Yekatit 12 Hospital Burn Center, Addis Ababa, Ethiopia. Ethiop J Health Sci. 2013;23(3):209–216. doi: 10.4314/ejhs.v23i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 2014;24:10–17. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzopardi EA, Azzopardi E, Camilleri L, Villapalos J. Gram negative wound infection in hospitalised adult burn patients - systematic review and metanalysis. PLoS One. 2014;21: 9(4):e95042. doi: 10.1371/journal.pone.0095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou Y, Zhang Q. Analysis of drug resistance of Pseudomonas aeruginosa and use of antibiotics in burn wards during 6 years. Zhonghua Shao Shang Za Zhi. 2014;30(1):9–14. [PubMed] [Google Scholar]
- 6.Cornelis P, Dingemans J. Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front Cell Infect Microbiol. 2013;14(3):75. doi: 10.3389/fcimb.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HG, Jang J, Choi JE, Chung DC. Blood stream infections in patients in the burn intensive care unit. Infect Chemother. 2013;45(2):134–201. doi: 10.3947/ic.2013.45.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tekin R, Yolbas I, Dal T, Okur MH, Selçuk CT. The evaluation of patients with burns during fifteen years period. Clin Ter. 2013;164(5):385–389. doi: 10.7417/CT.2013.1600. [DOI] [PubMed] [Google Scholar]
- 9.Naqvi SH, Naqvi SH. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg. 2013;51(4):151–152. doi: 10.7196/sajs.1811. [DOI] [PubMed] [Google Scholar]
- 10.Nanvazadeh F, Khosravi AD, Zolfaghari MR, Parhizgari N. Genotyping of Pseudomonas aeruginosa strains isolated from burn patients by RAPD-PCR. Burns. 2013;39(7):1409–1413. doi: 10.1016/j.burns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Faezi S, Safarloo M, Amirmozafari N, Nikokar I. Protective efficacy of Pseudomonas aeruginosa type-A flagellin in the murine burn wound model of infection. APMIS. 2014;122(2):115–127. doi: 10.1111/apm.12101. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Hu X, Liu Y, Wang Y. Systemic inflammatory responses and multiple organ dysfunction syndrome following skin burn wound and Pseudomonas aeruginosa infection in mice. Shock. 2013;40(2):152–159. doi: 10.1097/SHK.0b013e31829aef41. [DOI] [PubMed] [Google Scholar]
- 13.Elmanama AA, Laham NA, Tayh GA. Antimicrobial susceptibility of bacterial isolates from burn units in Gaza. Burns. 2013;39(8):1612–1618. doi: 10.1016/j.burns.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Vural MK, Altoparlak U, Celebi D, Akcay MN. Comparison of surface swab and quantitative biopsy cultures dependent on isolated microorganisms from burn wounds. Eurasian J Med. 2013;45(1):34–38. doi: 10.5152/eajm.2013.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alrawi M, Crowley TP, Pape SA. Bacterial colonisation of the burn wound: a UK experience. J Wound Care. 2014;23(5):274–277. doi: 10.12968/jowc.2014.23.5.274. [DOI] [PubMed] [Google Scholar]
- 16.Fekih Hassen A, Ben Khalifa S, Daiki M. Epidemiological and bacteriological profiles in children with burns. Burns. 2014;40(5):1040–1045. doi: 10.1016/j.burns.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Orban C, Tomescu D. The importance of early diagnosis of sepsis in severe burned patients: outcomes of 100 patients. Chirurgia (Bucur) 2013;108(3):385–388. [PubMed] [Google Scholar]
- 18.Belba MK, Petrela EY, Belba AG. Epidemiology of infections in a burn unit, Albania. Burns. 2013;39(7):1456–1467. doi: 10.1016/j.burns.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Beige F, Baseri Salehi M, Bahador N, Mobasherzadeh S. Plasmid mediated antibiotic resistance in isolated bacteria from burned patients. Jundishapur J Microbiol. 2014;10:e13567. doi: 10.5812/jjm.13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tekin R, Dal T, Bozkurt F, Deveci O. Risk factors for nosocomial burn wound infection caused by multidrug resistant Acinetobacter baumannii. J Burn Care Res. 2014;35(1):e73–e80. doi: 10.1097/BCR.0b013e31828a493f. [DOI] [PubMed] [Google Scholar]
- 21.Coetzee E, Rode H, Kahn D. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg. 2013;51(2):50–53. doi: 10.7196/sajs.1134. [DOI] [PubMed] [Google Scholar]
- 22.Taneja N, Chari P, Singh M, Singh G. Evolution of bacterial flora in burn wounds: key role of environmental disinfection in control of infection. Int J Burns Trauma. 2013;3(2):102–107. [PMC free article] [PubMed] [Google Scholar]
- 23.Bache SE, Maclean M, Gettinby G, Anderson JG. Airborne bacterial dispersal during and after dressing and bed changes on burns patients. Burns. 2015;41(1):39–48. doi: 10.1016/j.burns.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Brisbois EJ, Bayliss J, Wu J, Major TC. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomater. 2014;10(10):4136–4142. doi: 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burlando B, Cornara L. Honey in dermatology and skin care: a review. J Cosmet Dermatol. 2013;12(4):306–313. doi: 10.1111/jocd.12058. [DOI] [PubMed] [Google Scholar]
- 26.Sienkiewicz M, Poznańska-Kurowska K, Kaszuba A, Kowalczyk E. The antibacterial activity of geranium oil against Gram-negative bacteria isolated from difficult-to-heal wounds. Burns. 2014;40(5):1046–1051. doi: 10.1016/j.burns.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Coetzee E, Rode H, Kahn D. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg. 2013;51(2):50–53. doi: 10.7196/sajs.1134. [DOI] [PubMed] [Google Scholar]
- 28.Liao AY, Andresen D, Martin HC, Harvey JG, Holland AJ. The infection risk of plastic wrap as an acute burns dressing. Burns. 2014;40(3):443–445. doi: 10.1016/j.burns.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 29.nagoba BS, Selkar SP, Wadher BJ, Gandhi RC. Acetic acid treatment of pseudomonal wound infections - a review. J Infect Public Health. 2013;6(6):410–415. doi: 10.1016/j.jiph.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Soltan Dallal MM, Safdari R, emadi Koochak H, Sharifi-Yazdi S. A comparison between occlusive and exposure dressing in the management of burn wound. Burns. 2016;42(3):578–582. doi: 10.1016/j.burns.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Chahed J, Ksia A, Selmi W, Hidouri S. Burn injury in children: is antibiotic prophylaxis recommended? Afr J Paediatr Surg. 2014;11(4):323–325. doi: 10.4103/0189-6725.143141. [DOI] [PubMed] [Google Scholar]
- 32.Malone JR, Durica SR, thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132(3):454–459. doi: 10.1542/peds.2013-1384. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Liu P, Xue X, Chen Z, Pei D. Analysis of drug resistance and drug resistance genes of imipenem-resistant Pseudomonas aeruginosa strains isolated from burn wards. Zhonghua Shao Shang Za Zhi. 2014;30(1):25–29. [PubMed] [Google Scholar]


