Summary
The objective was to critically review the data and assess the implications of NexoBrid [NexoBrid-NXB formerly Debrase Gel Dressing-DGD]a in the special field of deep hand burns. Detailed analysis of endpoints in the treatment of hand burn patients was conducted as part of a multi-center, open label, randomized, controlled two-arm study to evaluate the safety and efficacy of NXB enzymatic debridement, comparing it to the current standard of care (SOC). These results were compared to a large cohort of patients treated with NXB in a previous, single arm study. Thirty-one burned hands were treated with NXB and 41 hand burns were in the SOC group. In the NXB group, 4 out of 31 hand burns (12.9%) required some excisional debridement compared to 29 out of the 41 (70.7%) in the SOC group (p<0.0001). Mean percentage of burn wound area excised in the NXB group was 4.4 ± 13.1% compared to 52.0 ± 41.4% in the SOC group (p<0.0001). None of the NXB-treated hands required escharotomy compared to 4 out of the 41 (9.7%) in the SOC group. NXB enzymatic debridement demonstrated a statistically significant reduction in burn wound excision and auto-grafting compared to SOC, and seems to prevent the need for emergency escharotomy.
a DGD is produced by MediWound and distributed under the name NexoBrid®
Keywords: hands; burns; eschar removal; enzymatic debridement; enzymatic escharotomy; enzymatic surgery; MIM,; minimally invasive modality of burn care
Abstract
Le but était de réaliser une révision attentive des données et d’évaluer la place de Nexobrid (Nexobrid-NXB, précédemment Debrase Gel Dressing-DGD) dans l’indication particulière des brûlures profondes de la main. Une analyse détaillée des objectifs dans le traitement des brûlures de la main a été conduite en partie par une étude multicentrique, ouverte, randomisée, contrôlée avec 2 groupes pour évaluer la sécurité et l’efficacité de ce débridement enzymatique par rapport aux soins habituels (Standard of Care ou SOC). Ces résultats ont été comparés à une vaste cohorte de patients traités par NXB dans une étude précédente sur un seul groupe. 31 mains brûlées furent traitées par NXB et 41 dans le groupe SOC. Dans le groupe NXB, 4 sur 31 mains brûlées soit 12,9 % nécessitèrent une excision partielle, alors que 29 sur 41 dans le groupe SOC (70,7 %) (p < 0,0001). La moyenne des zones brûlées excisées dans le groupe NXB était de 4,4 (+ ou - 13,1 %) comparée aux 52,0 (+ ou - 41,4 % du groupe SOC) (p <0,0001). Aucune des mains traitées par NXB ont nécessité une excision totale, comparée à 4 sur 41 du groupe SOC (9,7 % du groupe). Le débridement enzymatique NXB montre une réduction statistiquement significative de l’indication d’excision avec autogreffe par rapport au groupe traité classiquement et semble prévenir la nécessité d’une escarrotomie en urgence.
Introduction
Burns represent one of the most severe cutaneous traumas, where the magnitude of burned tissue (burn eschar), its depth, surface area and anatomical area such as hands, joints and face are prognostic factors for final functional and aesthetic outcomes. In order to minimize burn eschar-related local and systemic complications such as inflammation, infection and scarring, its removal (debridement) as early as possible is the first crucial step of burn care, as it is for any wound care.
The most direct, effective and prevalent method for eschar removal is excisional surgery, layer by layer tangential excision.1-5 Burn injuries of the hands have a special place in the field of burn care, being very common, involving 30% to 60% of all burn patients and requiring special attention and treatment.1-8
Due to the special anatomy of the hand in which numerous delicate and important structures are crowded into a small limited space and covered by scarce subdermal soft tissue and thin skin, tissue swelling may lead to increased interstitial pressure, causing burn induced compartment syndrome (BICS) with additional collateral damage to the vascular bed, skin, nerves and muscles. Early and accurate diagnosis of burn depth, and adequate surgical debridement with or without escharotomy of the burned tissue in hands is challenging, technically demanding and may cause considerable morbidity and additional tissue loss, especially in light of previous studies which demonstrated that often viable tissue is unnecessarily excised.9
These problems have driven motivated clinicians and researchers since the end of World War II to look for the holy grail of an effective, non-surgical or enzymatic debriding means, especially for hand burns.10-16
Traditionally, ‘conservative’ non-surgical debridement (NSD) is a lengthy process, based on inflammatory and autolytic processes where maceration slowly liquefies and destroys the eschar. This process is noxious per se, and combined with the delay in releasing mounting interstitial/compartment pressure, it is prone to lead to other systemic and local complications, especially escalating local tissue death, infection, inflammation and scarring.17-22
Selective and rapid enzymatic debridement, before the development of the inflammatory/infectious processes, preserves the non-injured tissue, in particular the dermis with its collagen matrix and epithelial cellular elements. When provided with proper conditions for epithelial proliferation and propagation, the newly debrided collagen dermal bed allows spontaneous and complete re-epithelialization, generally in two to three weeks, with good functional and aesthetic results, in the same way as in the healing of a skin-graft donor site. In full thickness burns, the debrided wound bed that cannot epithelialize spontaneously may serve as a recipient surface for grafting.23-24
NexoBrid (NXB), formerly known as Debriding Gel Dressing (DGD, Debridase or Debrase), is an enzymatic debriding orphan drug derived from the Pineapple Bromelain group of enzymes. The lyophilized dry powder enzyme is mixed with a vehicle gel to be applied onto the burn wound and covered with an occlusive dressing for 4 hours. The active enzymes cannot penetrate intact keratin, its activity is limited to a few hours, and together with its eschar specificity, it has been proven to be a safe and effective debriding agent that usually completes the debridement phase in a single 4-hour application. NXB specificity and selectivity to burn eschar offers the physician the option to apply it on burned surfaces and to completely debride the eschar without the need for an initial diagnosis of burn depth and without committing to a diagnosis-based surgical treatment plan involving excisional debridement and skin grafting.
Such selective debridement allows maximal exploitation of the dermal and epidermal salvaged component’s regenerative potential and subsequent wound healing by dermal epithelialization, offering an option for a minimally invasive modality (MIM) for burn care. Autogeneous grafting can be reserved only for full thickness defects devoid of spontaneous epithelialization potential and for non-healing wounds.23-27
A previous retrospective analytical study demonstrated that enzymatic debridement decreased both the number of deep hand burn injuries that eventuated in an operative procedure, and the wound area grafted in those who did undergo skin grafting.28
Due to the unique importance and high incidence of hand burn trauma, and in order to corroborate past data on the possible contribution of effective and selective enzymatic debridement in the treatment of hand burns, a further detailed analysis of treated deep hand burns in the population of a multi-center, open label, randomized, two-arm controlled prospective clinical study was conducted.29 The study was designed to evaluate the safety and enzymatic debriding efficacy of NXB, and to compare NXB-treated hands to those treated by standard of care (SOC) within this study, in view of the retrospective study results.
Materials and methods
Randomized Control Trial (RCT)
The recent study addressed patients suffering from deep burns of the hand, a sub-group of patients in a phase III, multicenter, open label, randomized, two-arm study that was conducted in accordance with Good Clinical Practice.
One hundred and eighty-two patients, 4 to 55 years of age, hospitalized in burn units, with deep partial thickness (DPT) and/or full thickness (FT) burns ranging from 5%-30% total body surface area (TBSA), met the inclusion criteria and were enrolled.
Following signing of an informed consent form (ICF), burn etiology, treatments prior to admission, and general description of the burn wound (locations, %TBSA, burn depth, description of the eschar) were recorded. Photographs of the burn wounds were taken prior to and after cleansing/blister removal and wound characterization (Fig. 1), and before and after debridement. Eligible patients were randomized in a 1:1 ratio to receive NXB or SOC treatment. Compartment pressure was measured in severalb circumferential extremity wounds prior to NXB or SOC treatments.
Fig. 1.
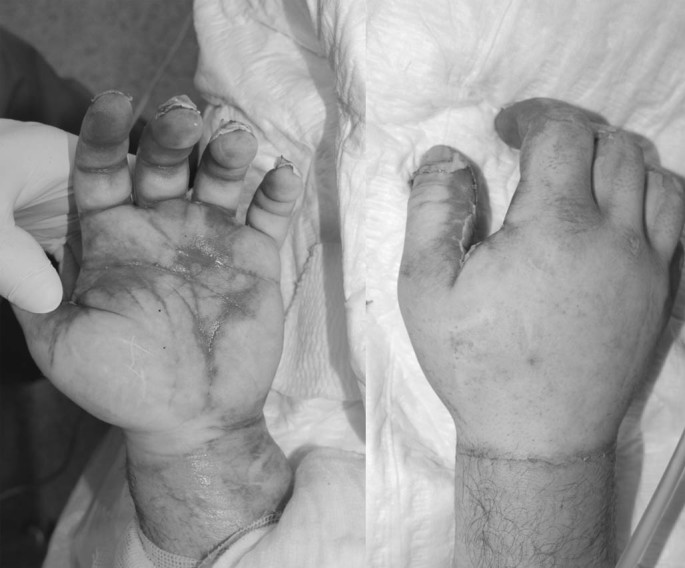
Treatment procedures: all DPT and FT burns of each patient were treated. Wounds were cleansed, blisters removed and all wounds were dressed with moisturizing, antibacterial solutions. Prior to debridement with NXB, the patients received analgesic medication, as commonly practiced during an extensive dressing change, to ensure a pain-free procedure (as also mandated by the protocol).
NXB treatment: NXB lyophilized enzyme powder and the vehicle gel were mixed at 1:10 ratio at the bedside ≤15 min prior to use and applied evenly onto the eschar at a thickness of ~1.5-3 mm (circa 2gr enzyme powder mixed in 20gr of gel vehicle per 1% adult TBSA). Following application of NXB (Fig. 2), the wounds were covered with an occlusive film dressing for 4 hours, which was then removed using aseptic techniques. The treated area was scraped with a sterile tongue depressor in order to remove dissolved eschar and NXB remnants. This was followed by a short wet to dry soaking to remove all remaining remnants (Fig. 3).
Fig. 2.
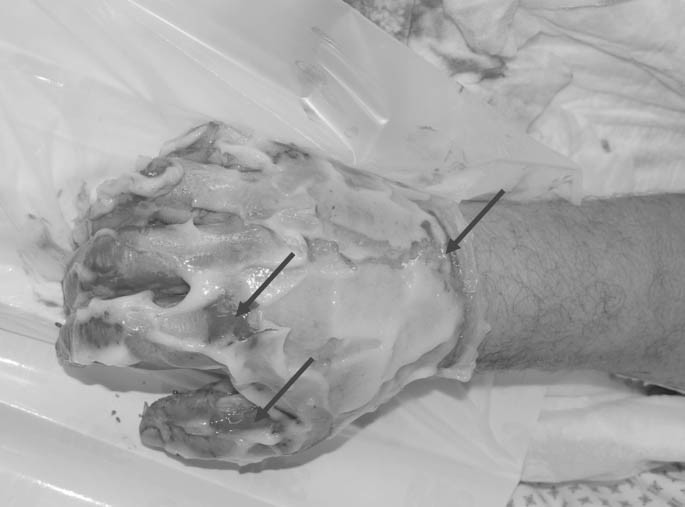
Fig. 3.
SOC treatment: patients in the SOC group were treated with surgical or non-surgical debridement and wound care strategies according to burn depth, wound characterization, each center’s clinical practice and the investigator’s judgment. Surgical procedures included the following: tangential excision, minor excision, fascial avulsion, dermabrasion, brushing or Versajet™. Non-surgical procedures included covers with topical medication, wet-to dry dressings or bathing to induce maceration and autolysis of eschar.
Subsequent to debridement (NXB or SOC), all wounds were assessed for debridement efficacy. One repeat NXB treatment was allowed per wound if the investigator judged it to be beneficial.
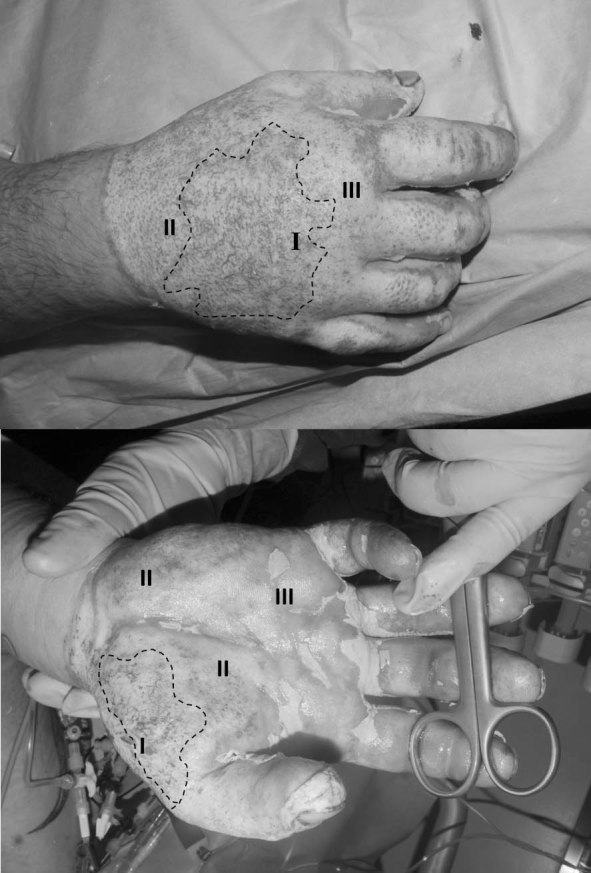
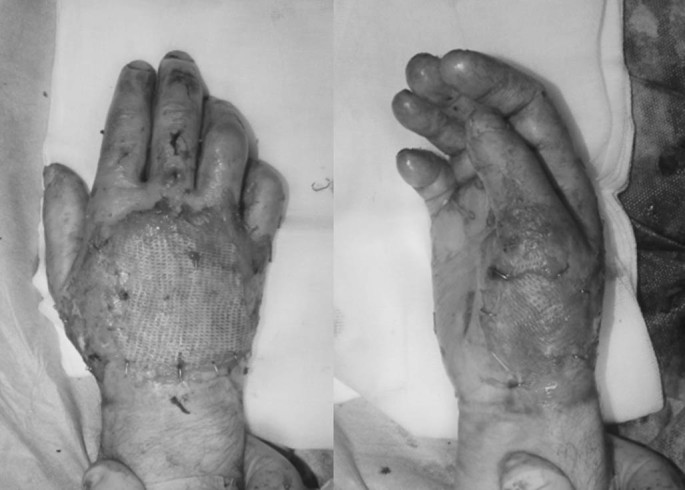
Full thickness skin defects requiring permanent coverage were covered by split thickness autologous skin grafts as soon as the patient’s condition and hospital resources allowed (Figs. 4 and 5). Mixed dermal wounds were treated with protective biological covers, temporary skin substitutes or topical medications, all aiming to protect and moisturize the wound, providing good conditions for epithelialization over dermis. Mixed depth (full and deep partial thickness) burns were often allowed to maximize their spontaneous epithelialization potential and areas that were not closed in ~3 weeks were then grafted. All hands were treated from admission by occupational therapists with early mobilization and splinting as needed.
Fig. 4.
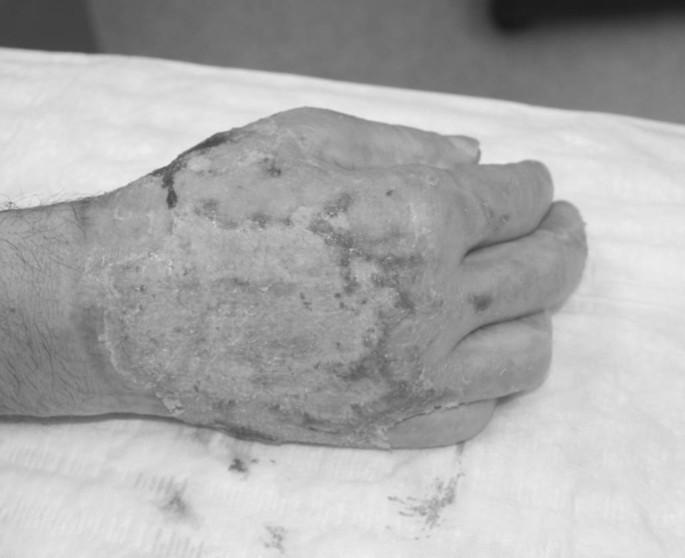
Fig. 5.
Following discharge, treated wounds were assessed on a weekly basis for wound closure (percent of wound surface epithelialized) until complete wound closure (above 95% of the wound surface) was reached. Post wound closure, patients were followed up for three months to assess maintenance of wound closure. Photographs of the treated wounds were taken post debridement, after auto-grafting if performed, at hospital discharge, and at weekly and monthly visits.
Several endpoints were selected as measures of NXB efficacy:
The percentage of wounds that necessitated surgical excision in each group in the first surgery, and the area excised (as a % of wound TBSA).
The percentage of DPT wounds that were closed by autologous skin grafting (as a measure for NXB selectivity, assuming selective debridement would spare the viable dermis which would then have the potential for spontaneous epithelialization with less need for autologous skin grafting. FT wounds on the other hand always need to be autografted regardless of the debridement technique) and the area grafted (as a % of the wound TBSA).
Time to complete eschar removal (debridement).
Time to complete wound closure.
Blood loss.
Time to hospital discharge.
Escharotomy and compartment interstitial pressure in circumferential extremity wounds.
All data were transferred to eCRFs and cross-checked against the patients’ medical files.
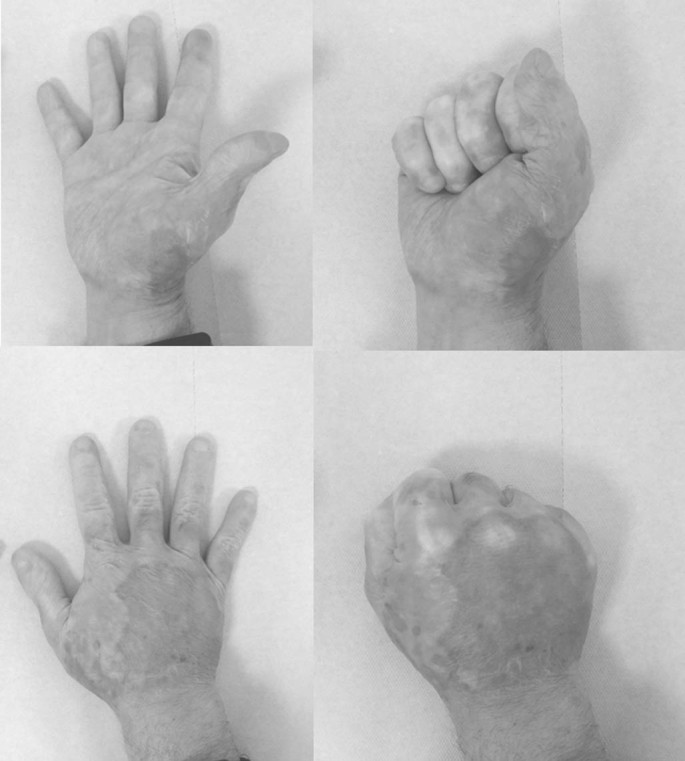
Long-term assessment: long-term assessment of scar quality and patients’ quality of life was carried out 2-4 years after injury by assessors masked to the original treatment assignment (Fig. 6). Scar quality was assessed using the Modified Vancouver Scar Scale (MVSS) that includes scar color, height, pliability and pigmentation as well as pain and pruritus.30,31 The patients’ quality of life was evaluated using the Short Form (SF)-36 questionnaire for adults,32,33 and the Burns Outcome Questionnaire (BOQ) for children.34,35
Fig. 6.
Statistical methods: statistical analysis for efficacy was based on data summaries and inferential statistics. Data was summarized with respect to demographic and baseline characteristics, efficacy observations and measurements. A two-way analysis of variance (ANOVA) model was used to assess the treatment effect for continuous variables except for time to event. For wound-based variables, a repeated measurement ANOVA model was used. For time to event variables, the logrank test was used for treatment comparison. In addition, median and distribution were estimated via the Kaplan-Meier method. Descriptive statistics were provided as well. The hazards ratio was estimated via the Cox regression model. For all time to event variables, two start times were taken: injury date and Informed Consent Form sign date (ICF).
For timely eschar removal, a subject who did not reach 90% eschar removal was considered a treatment failure. The failure rate in both groups was estimated. The difference in failure rate and its 90% confidence interval (CI) was also estimated. Categorical efficacy variables were analyzed using Fisher’s exact test.
The assessment of safety was based mainly on the frequency of adverse events and on the observation of clinically significant abnormal laboratory values. Other safety data (e.g. vital signs and special tests) were summarized as appropriate. Additional post-hoc wound closure analyses were performed, applying Spearman correlation for % wound autografted and time to complete wound closure, and Cox regression model (hazard ratio) for time to complete wound closure adjusted to % of wound autografted.
All statistical analyses were performed using SAS® Version 8 or higher. All data and analyses were reviewed by the European and American regulatory authorities, EMA and FDA respectively.
‘Retrospective’ study: hands
This was the first clinical NXB study, a prospective, single arm with retrospective data collection as previously described. 27,2827,28 The patient cohort of >300 was heterogeneous, containing children and adults, burns of all etiologies (flame, scald, contact, electrical, chemical) and with different post injury, pre admission periods of up to one week. Patients with non-thermal burns, incomplete files, missing informed consent, photographs or data, or lost to follow up were omitted from this study.
One hundred and fifty-four patients whose files were complete, containing the entire treatment course from admission to complete wound closure (on hospital discharge or follow up visits), including pre debridement and post debridement photographs, made up the final study group.
The study series, patients (n=154) aged six months to 82 years old, suffered burn wounds in 639 locations. NXB treatment was applied to the 401 deep burns (DPT and FT). This series had a similar number of patients under the age of sixteen (n=75) and over sixteen (n=79). In the entire series, 69% were males (65.2% of the children and 72.2% of adults) and 31% females (34.8% of the children and 27.8% of adults). 57 patients (23 under sixteen years old, and 34 over the age of sixteen), with 69 hand wounds, were analyzed as a separate subset.
Results
Randomized control trial
Thirty-two centers from Europe, Israel, Brazil, Australia and India underwent study initiation procedures, and 26 centers enrolled patients. Of the 182 enrolled patients, 52 suffered from 72 deep hand burns: 25 of these patients suffering from 31 hand burns were treated with NXB and 27 suffering from 41 hand burns were treated by SOC.
In eight circumferential wounds, clinically suspected as BICS, interstitial/compartment pressure measurements were taken and were found to be elevated prior to treatment. Six were treated with NXB and 2 were treated by SOC.
NXB application reduced elevated interstitial pressure below the threshold of 25 mmHg without the need for surgical intervention.
As for the SOC-treated wounds, in the 2 hands with elevated interstitial pressure and 2 additional hands where BICS was diagnosed clinically (swelling, pain, reduced pulse, reduced pO2 etc.) normal pressure was restored by surgical escharotomy or immediate excision.
A cohort of patients with DPT wounds only was additionally analyzed in order to investigate the dermal tissue sparing effect of NXB (as FT wounds and mixed wounds containing FT and DPT components should always be grafted regardless of the eschar removal technique).
The DPT subpopulation included 24 of the 31 burned hands in the NXB group and 21 of the 41 burned hands in the SOC group.
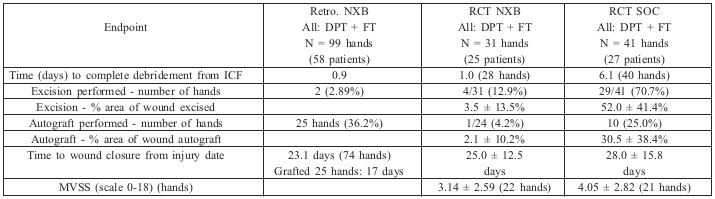
A post-hoc analysis of hand wounds treated with NXB and SOC demonstrated the following results (Table I):
A similar number of wounds had successful eschar removal (defined as ≥90% eschar removal per subject) in both groups: 90.3% (28/31) in the NXB group and 97.6% (40/41 wounds) in the SOC group (p = 0.1843).
Time to achieve successful eschar removal was significantly shorter in the NXB group: 1.04 ± 1.40 days from ICF signing (2.57 ± 1.95 days from injury) compared to the SOC group with 6.08 ± 5.17 days from ICF signing (7.75 ± 5.41 days from injury) (p<0.0001).
Four of the 31 hand wounds in the NXB group (12.9%) required a later surgical excision before autografting compared to 29 out of 41 hand burns that necessitated surgical excision (70.7%) in the SOC group (p<0.0001).
The percentage of burn wound area necessitating surgical excision in the NXB group was 4.4 ± 13.1% compared to 52.0 ± 41.4% in the SOC group (p<0.0001).
None of the NXB-treated hands required escharotomy compared to 4 out of 41 (9.7%) in the SOC group.
Time to wound closure from injury date was similar and slightly shorter in the NXB group: 25.0 ± 12.5 days compared to 28.0 ± 15.8 days in the SOC group.
In the DPT subpopulation:
One of the 24 (4.2%) hand wounds in the NXB group was autografted compared to 10 out of 20 (50%) in the SOC group (p=0.0005).
The percentage of burn wound area autografted in the NXB group was 2.1 ± 10.2% compared to 30.5 ± 38.4% in the SOC group (p=0.0017).
Long-term follow up: mean MVSS score was slightly lower (better results) in the NXB group compared to SOC: 3.14 ± 2.59 vs. 4.05 ± 2.82, respectively, without a statistically significant difference (p=0.27). In QoL assessments, the overall results in adults (SF-36 questionnaire) were comparable between the NXB and SOC groups as indicated by the physical component score (51.1 and 51.3, respectively) and the mental component score (52.3 vs. 49.1, respectively). In pediatric patients, BOQ scores were similar, 118.7 in the NXB arm and 121.6 in the SOC arm.
Table I.
Retrospective study
The data collected in the RCT was in line with the data previously generated in the retrospective study regarding debridement efficacy, surgical interventions and healing time (Table 1). Complete debridement in a single 4-hour application was achieved in 89.2% of the area treated with NXB.
None of the patients suffered any NXB- or debridementrelated adverse events of their deep hand burns other than local pain in the treated area upon NXB application when not relieved properly by analgesia.
After debridement, 25 hands (36.2%) showed full thickness defects that required permanent coverage (autografting). Only 2 hands (2.89% of the entire cohort of deeply burned hands) necessitated further debridement prior to grafting.
Clean residual viable dermis was found to comprise the debrided wound bed in the other 44 burn wounds, which were treated with topical medication and healed by spontaneous epithelialization. The mean area of hand burn wounds that were initially estimated as deep and in need of excision and grafting was 1.4 + 0.8 %TBSA. The overall calculated mean wound area that required skin grafting was 0.4 + 0.6 %TBSA: only 28.6% of the original diagnoses of deep wound area in need of grafting, a statistically significant difference (p<0.001).
Graft take was more than 90% in all grafted hand wounds and did not require additional grafting procedures. Debridement was initiated at an average 0.9 days post admission, completed in 4 hours and was followed by topical wound care aimed at early healing.
Mean time to wound closure of all hands was 23 days. Hand wounds that underwent surgical grafting had a mean time to wound closure of 17 days.
Of the 69 hands treated with NXB, many had circumferential burns. In the majority of cases, compartment pressure was not measured and the clinical picture post-NXB treatment was such that high interstitial/compartment pressure was not suspected and measurements were not required (Fig. 1). In 4 patients (7 hands), pressure was measured, with pre-debridement values ranging from 35-75 mmHg, and all below 25 mmHg post NXB treatment. All hands were treated from admission by occupational and physical therapists with early mobilization and splinting as needed.
Discussion
Deep burns of the hands are very common, challenging and cause major early and late functional and aesthetic morbidities and sequelae.
Surgical treatment (excisional debridement-escharectomy, escharotomy and autografting) of the burned hand is especially challenging due to the important and delicate crowded anatomy, thin skin, minimal sub-dermal tissue and its functional and cosmetic importance. Non-surgical debridement means (autolysis with or without slow acting chemicals/enzymes) are not fast and effective enough to replace or minimize surgical intervention. In these two studies (and others published), NXB enzymatic debridement was shown to be effective and selective, combining the advantage of timely, very early effective eschar removal with the benefit of sparing dermal viable tissue, significantly reducing surgery. NXB demonstrated a statistically significant reduction in the need for burn wound excision and auto-grafting compared to the current SOC, significantly decreasing both the incidence and area of burn wounds that had to be surgically excised, as well as the area that had to be grafted in those who did undergo skin grafting. In the entire NXBtreated hand burn population reported here, no surgical escharotomy was needed, compared to ~10% in the SOC-treated hands. These results are in accordance with the results of a controlled animal study where the ability of NXB to rapidly release BICS was demonstrated.36 It is methodically impossible to pool these two studies, but it is interesting to compare the results of the phase III, multi-center, RCT to the data from the first, previous uncontrolled single center study:
Debridement efficacy: the seemingly reduced efficacy in the retrospective study vs. the more recent RCT may be due to the learning curve in diagnosing the enzymatically debrided bed that differs from the surgically excised tissues. Despite this, only 2 hands needed additional excision in the retrospective study.
Reduced autografting: the higher incidence of autografting in the retrospective study compared to the RCT may also be due to the learning curve of attempting to treat the exposed bed towards epithelialization instead of the wellpracticed excision and autografting. The skills of correct assessment of the enzymatically debrided bed and taking care of the epithelializing dermal bed were honed and implemented in future studies, with a significant reduction in excision and autografting. Not having an SOC control arm in the first study, one can only extrapolate and speculate the reduction in surgery (excisional debridement and autografting) as could be expected in deeply burned hands.28
Time to wound closure: the shorter time seen in the retrospective study (23 days) was probably due to increased and earlier autografting vs. the RCT (NXB 25.0 ± 12.5 days vs. 28.0 ± 15.8 days in the SOC) where the investigators were more skilled in epithelializing techniques and more confident in later grafting.
Long term, final outcome: unfortunately, a comparison between the two studies cannot be performed as these data are missing in the retrospective study. It is interesting to note that in the RCT study, despite the reduced surgery and longer time to wound closure, scar final outcome (MVSS) in the NXB group was at least as good (better, but not statistically significant) as in the SOC group. This may be due to the increased salvage of dermal component in the NXB arm and earlier mobilization (as less grafting was performed, there was less need to immobilize hands in order to protect grafts from shearing forces in the first days).
Enzymatic escharotomy: the effective BICS relief achieved with NXB is consistent in both studies (0 surgical escharotomy in a total of 100 hands vs. 9.7% in the RCT SOC arm) and seen also in reduced post NXB pressure when measured. Similar results were observed in a controlled enzymatic escharotomy study in a burn pig model, in which NXB resolved burn induced compartment syndrome within 30-45 minutes after application.35
Strengths of this study
These two studies include a large number of patients, burn centers and physicians across the globe, thus increasing its generalization and the external validity of the data. The RCT was designed and regulated with the assistance of regulatory agencies such as the European EMA and American FDA, thus enhancing its methodology and quality. The results of both studies were reviewed by the EMA and FDA as part of the product development process.
This review summarizes the results of two different studies, providing an insight into the development process of a new treatment strategy that is based on data and experience gained during the development of a mere debriding tool.
Weaknesses of this study
This manuscript reviews two different studies in two different stages of the development process, not only of the debriding tool but also of different treatment modalities that differ from the currently accepted deep hand burn SOC. Only the RCT was comparative and randomized, introducing heterogeneity between studies. Due to the obvious differences between enzymatic, surgical and non-surgical debridement, it was impossible to mask the patients and investigators to the treatment arm in the RCT, thus introducing the potential for observer bias. However, when long-term scar quality was assessed, the observers were masked to the original study treatment. The lack of a large cohort of long-term results does not allow a definitive answer regarding the superiority of the minimally invasive modality that is based on the healing of salvaged native dermis.
Summary and conclusion
NXB seems to provide an additional alternative to the demanding treatment of the burned hand. A safe, non-surgical debriding tool that offers early burn eschar removal on admission, releases (or prevents) BICS with increased preservation of native dermis and/or subcutaneous tissue over the intricate tendinous and vascular structures may provide a minimally invasive modality for the care of deeply burned hands.
Additionally, the use of a fast, effective and selective nonsurgical debriding means as a first line therapy may allow for an increase in surge capacities in a burn mass casualty scenario where skilled surgeons and surgical facilities are the main established bottlenecks, while still achieving safe and early eschar removal.
Acknowledgments
Competing interests.The RCT study was supported by Medi-Wound LTD.
Ethics.The study was conducted in accordance with Good Clinical Practice, with the approval of the local Helsinki committee.
References
- 1.Salisbury RE. Thermal burns. McCarthy JG (ed): Plastic Surgery. 1990:787–830. [Google Scholar]
- 2.Still JMJ, Law EJ. Primary excision of the burn wound. Clin Plast Surg. 2000;27(1):23–47. [PubMed] [Google Scholar]
- 3.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10(12):1103–1108. [PubMed] [Google Scholar]
- 4.Monafo WW, Aulenbacher CE, Pappalardo C. Early tangential excision of the eschars of major burns. Arch Surg. 1972;104:503–508. doi: 10.1001/archsurg.1972.04180040117020. [DOI] [PubMed] [Google Scholar]
- 5.Sheridan RL, Tompkins RG, Burke JF. Management of burn wounds with prompt excision and immediate closure. J Intensive Care Med. 1994;9(1):6–17. doi: 10.1177/088506669400900103. [DOI] [PubMed] [Google Scholar]
- 6.Rowland SA. Fasciotomy: The treatment of compartment syndrome. Green DP, Hotchkiss RN. 1993:661–694. [Google Scholar]
- 7.Silverstein P, Maxwell P, Duckett L. Enzymatic debridement. The art and science of burn care. 1987:75–81. [Google Scholar]
- 8.Van Zuijlen PP, Kreis RW, Vloemans AF, Groenevelt F, Mackie DP. The prognostic factors regarding long-term functional outcome of fullthickness hand burns. Burns. 1999;25(8):709–714. doi: 10.1016/s0305-4179(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Gurfinkel R, Rosenberg L, Cohen S, Cohen A. Histological assessment of tangentially excised burn eschars. Can J Plast Surg. 2010;18(3):e33–e36. [PMC free article] [PubMed] [Google Scholar]
- 10.Wachtel TL, Parks SN, Dimick AR. Early enzymatic debridement and grafting of burned hands: evaluating the travase fast-graft method in deep dermal burns. JBCR. 1983;4(5):325–330. [Google Scholar]
- 11.Klasen HJ. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns. 2000;26(3):207–222. doi: 10.1016/s0305-4179(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 12.Dimick AR. Experience with the use of proteolytic enzyme (Travase) in burn patients. J Trauma. 1977;17(12):948–955. doi: 10.1097/00005373-197712000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gant TD. The early enzymatic debridement and grafting of deep dermal burns to the hand. Plast Reconstr Surg. 1980;66(2):185–190. doi: 10.1097/00006534-198008000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Krizek T, Robson M, Koss N. Emergency non-surgical escharotomy in the burned extremity. Orthop Rev. 1975;4:53–55. [Google Scholar]
- 15.Levick P, Brough M, Vasilescu C, Laing J. Treatment of full-thickness burns with travase: results of a clinical trial. Burns. 1978;4(4):281–284. [Google Scholar]
- 16.Pennisi VR, Capozzi A. Travase: observations and controlled study of the effectiveness in burn debridement. Burns. 1975;1(3):274–278. [Google Scholar]
- 17.Soroff HS, Sasvary DH. Collagenase ointment and polymyxin B sulfate/ bacitracin spray versus silver sulfadiazine cream in partial-thickness burns: a pilot study. J Burn Care Rehabil. 1994;15(1):13–17. doi: 10.1097/00004630-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Starley IF. The treatment of paediatric burns using topical papaya. Burns. 1999;25(7):636–639. doi: 10.1016/s0305-4179(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 19.Vincent J. [Traitement Fibrinolytique actuel par des Enzymes pour les soin des Plaies] Enzymatic debridement in modern wound care. Journal des Plaies et Cicatrisations. 1999;17(17):14–17. [Google Scholar]
- 20.Singh G. Debridement of the burn wound with sutilains ointment. Burns, 1980;7(1):41–48. [Google Scholar]
- 21.Pennisi VR, Abril F, Capozzi A. The combined efficacy of travase and silver sulphadiazine in the acute burn. Burns. 1976;2(3):169–172. [Google Scholar]
- 22.Ozcan C. Enzymatic debridement of burn wound with collagenase in children with partial-thickness burns. Burns, 2002;28(8):791–794. doi: 10.1016/s0305-4179(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 23.Levenson S. Supportive therapy in burn care. Debriding agents. J Trauma. 1979;19(11 Suppl):928–930. [PubMed] [Google Scholar]
- 24.Hansbrough JF. Wound healing in partial-thickness burn wounds treated with collagenase ointment versus silver sulfadiazine cream. J Burn Care Rehab. 1995;16(3 Pt 1):241–247. doi: 10.1097/00004630-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg L. The Minimal Invasive Modality (MIM or the PNN-Primum Non Nocere Protocol) for burn treatment: A preliminary report. The ISBI Meeting, Jerusalem, Israel. 1998 [Google Scholar]
- 26.Rosenberg L. A Debrase based minimal invasive modality (MIM or the PNN-Primum Non Nocere Protocol) for burn treatment: A preliminary report. The John A. Boswick, M.D. Burn and Wound Care Symposium, Maui, Hawaii. 2002 [Google Scholar]
- 27.Rosenberg L. Safety and efficacy of a proteolytic enzyme for enzymatic burn debridement: a preliminary report. Burns. 2004;30:843–850. doi: 10.1016/j.burns.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Krieger Y, Bogdanov-Berezovsky A, Gurfinkel R, Silberstein E. Efficacy of enzymatic debridement of deeply burned hands. Burns. 2012;38:108–111. doi: 10.1016/j.burns.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E. novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. . Burns. 2014;40(3):466–474. doi: 10.1016/j.burns.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Brusselaers N. Burn scar assessment: a systematic review of different scar scales. J Surg Res. 2010;164(1):115–123. doi: 10.1016/j.jss.2010.05.056. [DOI] [PubMed] [Google Scholar]
- 31.Forbes-Duchart L. Determination of inter-rater reliability in pediatric burn scar assessment using a modified version of the Vancouver Scar Scale. J Burn Care Res. 2007;288(3):460–467. doi: 10.1097/BCR.0b013E318053D3BB. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10(5):405–413. doi: 10.1023/a:1012588218728. [DOI] [PubMed] [Google Scholar]
- 33.Moi AL. Impaired generic health status but perception of good quality of life in survivors of burn injury. J Trauma. 2006;61(4):961–968. doi: 10.1097/01.ta.0000195988.57939.9a. [DOI] [PubMed] [Google Scholar]
- 34.Stubbs TK. Psychosocial impact of childhood face burns: a multicenter, prospective, longitudinal study of 390 children and adolescents. Burns. 2011;37(3):387–394. doi: 10.1016/j.burns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Van Baar ME. Quality of life after burns in childhood (5-15 years): Children experience substantial problems. Burns. 2011;37(6):930–938. doi: 10.1016/j.burns.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Krieger Y. Escharotomy using an enzymatic debridement agent for treating experimental burn-induced compartment syndrome in an animal model. J Trauma. 2005;58(6):1259–1264. doi: 10.1097/01.ta.0000169867.08607.f1. [DOI] [PubMed] [Google Scholar]


