TO THE EDITOR:
Polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome is a rare plasma cell disorder additionally characterized by extravascular volume overload, sclerotic bone lesions, and plasmacytomas. Although high-dose melphalan followed by peripheral blood autologous stem cell transplantation (ASCT) has been suggested as a first-line treatment,1 its long-term outcomes are limited. Here, we report long-term outcomes for 36 patients with POEMS syndrome who underwent first ASCT from January 2004 to September 2016 with periodic neurologic and clinical assessment. The study was approved by the Human Investigation Review Committee of the Chiba University Graduate School of Medicine.
Patients younger than age 65 years with rapidly progressing disease were scheduled to receive ASCT. The median age was 49 years (range, 16-65 years). Thirteen patients (36%) demonstrated a poor performance score (PS) of 3 to 4. Thirty-four patients (95%) received more than 1 prior induction therapy that included thalidomide in 25 (70%), lenalidomide in 5 (14%), and bortezomib in 5 (14%). Median time from diagnosis to ASCT was 8 months (range, 3-106 months). The drug used for conditioning was high-dose melphalan 200 mg/m2, but 3 patients received reduced doses of 140 mg/m2 because of poor PS. The median infused dose of CD34+ cells was 2.30 × 106 cells per kg. Patients’ characteristics are provided in Table 1.
Table 1.
Patients’ characteristics
| Characteristic | No. | % |
|---|---|---|
| Total No. of patients | 36 | |
| Median age at ASCT, y (range) | 49 (16-65) | |
| Sex | ||
| Female | 14 | 39 |
| Male | 22 | 61 |
| Monoclonal protein | ||
| IgA-L | 19 | 52.8 |
| IgG-L | 12 | 33.3 |
| IgG-L + IgG-K | 1 | 2.8 |
| BJP-L | 1 | 2.8 |
| Unknown | 3 | 8.3 |
| Polyneuropathy | 36 | 100 |
| Organomegaly | 32 | 88.9 |
| Endocrinopathy | 20 | 55.6 |
| Plasmacytoma | 2 | 5.6 |
| Osteosclerotic lesion | 25 | 69.4 |
| Skin lesions | 34 | 94.4 |
| Median percent of bone marrow plasma cells at diagnosis (range) | 2.5 (0.3-10.0) | |
| Median time from diagnosis to ASCT, months (range) | 7.6 (3-105) | |
| ECOG PS at ASCT | ||
| 1-2 | 23 | 63.9 |
| 3-4 | 13 | 36.1 |
| No. of pre-ASCT induction regimens | ||
| None | 2 | 5.6 |
| 1 | 13 | 36.1 |
| 2 | 8 | 22.2 |
| ≥3 | 13 | 36.1 |
| Pretransplant regimens | ||
| Thalidomide-based | 25 | 69.4 |
| Lenalidomide-based | 5 | 13.9 |
| Botezomib-based | 5 | 13.9 |
| Melphalan-based | 7 | 19.4 |
| Steroids only | 14 | 38.9 |
| Other | 13 | 36.1 |
| Hematologic response at ASCT | ||
| CR | 6 | 16.7 |
| Non-CR | 27 | 75 |
| Not evaluable | 3 | 8.3 |
| Ascites or pleural effusion at ASCT | 21 | 58.3 |
| Median serum albumin at ASCT, mg/dL (range) | 3.85 (2.9-4.9) | |
| Median serum VEGF at diagnosis, pg/mL (range) | 4425 (848-31 700) | |
| Median serum VEGF at ASCT, pg/mL (range) | 1410 (26-7870) | |
| Conditioning regimen | ||
| Melphalan 200 mg/m2 | 33 | 91.7 |
| Melphalan 140 mg/m2 | 3 | 8.3 |
| Median dose of CD34+ × 106 cells per kg (range) | 2.30 (1.44-4.6) | |
BJP-L, Bence-Jones protein-λ; CR, complete response; ECOG, Eastern Cooperative Oncology Group; IgA-L, immunogloblin A–λ; IgG-K, immunogloblin G–κ.
Median time to engraftment was 13 days (range, 11-21 days). Grade 3 to 4 nonhematologic toxicities included engraftment syndrome (ES; n = 7 [19.4%]) and infection (n = 4 [11.1%]). ES was observed in older patients (median age for ES vs no ES, 57 vs 48 years; P = .03) and patients with pre-ASCT extravascular volume overload (ES vs no ES: 33% vs 0%; P = .02). No significant difference was observed in serum vascular endothelial growth factor (VEGF) levels or hematologic response rate before ASCT among patients with or without ES. All patients responded well to corticosteroids. With 1 early death as a result of severe sepsis, the 1-year nonrelapse mortality rate was 2.8% (95% confidence interval [CI], 0.2%-12.6%).
Response was assessable in 33 patients. The hematologic response rate, defined as negative M components by immunofixation, was 12% (n = 4) before ASCT, which increased to 64% (n = 21) after ASCT. Median serum VEGF level before ASCT in 33 patients with assessable response was 1490 pg/mL (range, 26-7870 pg/mL), which significantly decreased to 395 pg/mL (range, 56-3290 pg/mL; P < .001) at 6 months after ASCT and to 385 pg/mL (range, 60-3310 pg/mL; P < .001) at 12 months after ASCT. VEGF response rate, defined as a reduction in serum VEGF levels to <1000 pg/mL,2 was 36% before ASCT, which increased to 85% at 6 months after ASCT and to 90% at 12 months after ASCT.
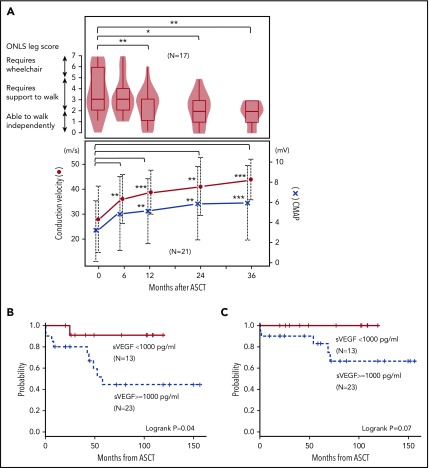
Furthermore, neurologic evaluations were performed in 21 patients. The compound motor action potential amplitude and motor conduction velocity of the median nerve started improving at 6 months and increased continuously to 36 months after ASCT (Figure 1A). The overall neuropathy limitation score3 leg scale in 17 patients demonstrated gradual improvement and reached statistical significance at 12 months after ASCT (Figure 1A). The proportion of patients who could walk independently (score <3) was 25% before ASCT, which significantly increased to 50% by 24 months after ASCT.
Figure 1.
Neurological improvement and survival after ASCT. (A) Upper panel shows overall neuropathy limitations score (ONLS) leg score, and lower panel shows conduction velocity and compound muscle action potential (CMAP) of the median motor nerve. (B) Clinical PFS and (C) overall survival categorized by serum VEGF level at ASCT.
At the last follow-up, 31 patients were alive, with a median follow-up of 72 months. The 5-year overall survival rate was 90.1% (95% CI, 71.6%-96.8%). Nine patients experienced clinical relapse/progression, defined as new symptoms attributable to POEMS syndrome, with a 5-year clinical progression-free survival (PFS) of 63.2% (95% CI, 41.4%-78.8%), and 5-year clinical relapse rate was 34.0% (95% CI, 16.1%-52.9%), with a median time from ASCT of 42.9 months (range, 0.3-58.6 months). The most frequent clinical manifestation was extravascular volume overload (n = 7) followed by peripheral neuropathy (n = 3) and pulmonary hypertension (n = 1). Notably, 5 patients were unable to achieve hematologic response, and all patients subsequently developed an increase in VEGF before or at clinical relapse/progression. A median time interval from ASCT to VEGF increase and from VEGF increase to clinical relapse/progression was 17.9 months (range, 2.9-39.2 months) and 13.5 months (range, 0-55.7 months), respectively. Salvage therapies were thalidomide-based in 6 patients, thalidomide plus radiation therapy in 1, lenalidomide followed by bortezomib-based therapy in 1, and oral medication for pulmonary hypertension in 1. Second ASCT was performed in 2 patients for whom salvage therapy was successful. With a median follow-up period of 70 months after ASCT, 4 patients died (cerebral infarction, n = 2; disease progression, n = 2; supplemental Table 1, available on the Blood Web site).
Age, PS, monoclonal heavy-chain subtypes, serum VEGF levels at diagnosis, hematologic response before ASCT, and serum albumin levels were not significantly different among patients with relapse/progression compared with those without relapse/progression except for high serum VEGF level before ASCT (median, 4280 vs 941 pg/mL; P = .005). Patients who achieved VEGF response before ASCT demonstrated a significantly better 5-year clinical PFS (90.9% vs 47.4%; P = .04; Figure 1B) and had a trend toward better 5-year overall survival (100% vs 84.8%; P = .07; Figure 1C).
This study demonstrated toxicity and survival equivalent to those in 3 previous cohort studies.4-6 We updated our previous data, which demonstrated that neuropathy continued to improve with time after ASCT.7 This was consistent with previous reports published by Karam et al8 from the Mayo Clinic; however, our periodic assessment further confirmed that neurologic improvement started as early as 6 months after ASCT and continued to improve for 2 years. In addition, the overall neuropathy limitation score demonstrated gradual improvement for the leg scale, which reached a statistically significant level at 12 months after ASCT. These observations highlight the advantage of ASCT, which keeps patients free from neurotoxic medications by substantial remission. Although the result is encouraging, it also suggests that even though neurologic improvement starts early, an objective physical improvement requires more than a year after ASCT.
Because of the limited number of patients from our single-center experience, we were unable to determine adverse factors for survival as shown in previous reports,9 except for high serum VEGF levels before ASCT. Our current results supported our previous data by demonstrating that suppressing serum VEGF levels to within the normal range (≤1040 pg/mL) may prolong PFS.2 The clinical impact of pre-ASCT VEGF levels was also suggested by Li et al.6 Our results confirmed the benefit of pre-ASCT induction therapy followed by high-dose melphalan for better PFS. Although the statistical power was insufficient to determine a survival benefit, we believe that VEGF response is a reliable surrogate marker for a pre-ASCT treatment goal. Primary refractory patients with a suboptimal VEGF response may be good candidates for post-ASCT maintenance therapy; however, benefits must be weighed against neurotoxicity.
More than half the patients with clinical relapse/progression were unable to achieve hematologic response, which was compatible with previous reports.9 Detailed profiling further demonstrated that all of the patients developed VEGF elevation before or at clinical relapse/progression, supporting the predictive efficacy of VEGF in our previous results.2 Furthermore, the prognosis for those refractory to salvage therapies was poor and included death as a result of cerebral infarction, which is also a symptom associated with the disease.10-12 This was different from the Mayo Clinic data, which showed a fairly good prognosis.13 This discrepancy may be the result of a different treatment strategy used for patients who had rapidly progressing disease and a longer observation period in our cohort.
Patients with emerging VEGF elevation were at high risk of clinical relapse/progression and should be considered for appropriate salvage therapy. However, optimal treatment strategy for salvage regimens, including novel myeloma agents, is still under investigation.14,15 We also performed a second transplantation in 2 patients for whom salvage therapy was successful, suggesting that a second ASCT might be a promising option.
In conclusion, induction chemotherapy followed by high-dose melphalan and ASCT is a promising treatment that demonstrates prompt neurologic improvement, which subsequently translates into prolonged PFS in patients with POEMS syndrome.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank patients and their families who shared their experiences with us and also our colleagues in the Department of Neurology and Hematology for providing excellent care for the patients.
Authorship
Contribution: C.N. and S.K. designed the research; C.O. wrote the manuscript; C.O., C.K.-M., S. Misawa, and E.S. collected and analyzed patient data; Y.N., N.O.-H., E.T., T.M., S.T., S. Mitsukawa, Y.T., N.M., M.T., N.S., S. Misawa, and T.I. helped collect data and prepare the manuscript; and all authors approved the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chikako Ohwada, Chiba University Hospital, 1-8-1, Inohana, Chuo-ku, Chiba 260-8670, Japan; e-mail: chikako_ohwada@faculty.chiba-u.jp.
References
- 1.Dispenzieri A. POEMS syndrome: 2017 Update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92(8):814-829. [DOI] [PubMed] [Google Scholar]
- 2.Misawa S, Sato Y, Katayama K, et al. Vascular endothelial growth factor as a predictive marker for POEMS syndrome treatment response: retrospective cohort study. BMJ Open. 2015;5(11):e009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham RC, Hughes RA. A modified peripheral neuropathy scale: the Overall Neuropathy Limitations Scale. J Neurol Neurosurg Psychiatry. 2006;77(8):973-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Lacy MQ, Hayman SR, et al. Peripheral blood stem cell transplant for POEMS syndrome is associated with high rates of engraftment syndrome. Eur J Haematol. 2008;80(5):397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook G, Iacobelli S, van Biezen A, et al. High-dose therapy and autologous stem cell transplantation in patients with POEMS syndrome: a retrospective study of the Plasma Cell Disorder sub-committee of the Chronic Malignancy Working Party of the European Society for Blood & Marrow Transplantation. Haematologica. 2017;102(1):160-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Duan MH, Wang C, et al. Impact of pretransplant induction therapy on autologous stem cell transplantation for patients with newly diagnosed POEMS syndrome. Leukemia. 2017;31(6):1375-1381. [DOI] [PubMed] [Google Scholar]
- 7.Kuwabara S, Misawa S, Kanai K, et al. Neurologic improvement after peripheral blood stem cell transplantation in POEMS syndrome. Neurology. 2008;71(21):1691-1695. [DOI] [PubMed] [Google Scholar]
- 8.Karam C, Klein CJ, Dispenzieri A, et al. Polyneuropathy improvement following autologous stem cell transplantation for POEMS syndrome. Neurology. 2015;84(19):1981-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kourelis TV, Buadi FK, Gertz MA, et al. Risk factors for and outcomes of patients with POEMS syndrome who experience progression after first-line treatment. Leukemia. 2016;30(5):1079-1085. [DOI] [PubMed] [Google Scholar]
- 10.Dacci P, Lessi F, Dalla Bella E, Morbin M, Briani C, Lauria G. Ischemic stroke as clinical onset of POEMS syndrome. J Neurol. 2013;260(12):3178-3181. [DOI] [PubMed] [Google Scholar]
- 11.Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD Jr. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Xiong L, Zu H. Recurrent stroke as the clinical onset of POEMS syndrome. J Clin Neurol. 2017;13(2):199-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120(1):56-62. [DOI] [PubMed] [Google Scholar]
- 14.Cai QQ, Wang C, Cao XX, Cai H, Zhou DB, Li J. Efficacy and safety of low-dose lenalidomide plus dexamethasone in patients with relapsed or refractory POEMS syndrome. Eur J Haematol. 2015;95(4):325-330. [DOI] [PubMed] [Google Scholar]
- 15.Vannata B, Laurenti L, Chiusolo P, et al. Efficacy of lenalidomide plus dexamethasone for POEMS syndrome relapsed after autologous peripheral stem-cell transplantation. Am J Hematol. 2012;87(6):641-642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.