Abstract
The absorption characteristics of the dermis were reviewed in the terahertz range from 0.2 to 2 THz. The absorption magnitude of the dermis was higher than that of the epidermis model owing to the inclusion of collagen fibers. The heat denaturation of the collagen and the decrease of water content in the dermis caused a decrease in the absorption magnitude of the dermis. We verified that the absorption magnitude of collagen sheets at 1 THz similarly decreased by nearly 43% upon the heat treatment at approximately 70° C. When the heat-treated sheet was used as a scaffold for cell culture, the average growth rate of the fibroblast increased. These findings suggest that variation in the ability of cell growth in the dermis can be predicted using the absorption of the collagen or the difference between the absorptions of dermis and water.
OCIS codes: (300.0300) Spectroscopy; (300.6495) Spectroscopy, terahertz
1. Introduction
Nondestructive testing using terahertz waves with frequencies from 0.1 to 10 THz is an emerging technology in science and industry for evaluating the properties of a material or component [1, 2]. Differences in the properties such as crystallinity and the interaction between large molecules can be analyzed from a fingerprint spectrum, which is a specific characteristic of each substance in the terahertz band. The fingerprint spectra of various substances have therefore been investigated and collected to establish the spectral database.
In the biological field, the technologies have been used for distinguishing between normal and cancer [3] or burned tissues [4], and for the evaluation of biological molecules [5]. Recently, in vivo studies on the nondestructive testing of surface tissues such as skin and eyes have been conducted with the development of terahertz time-domain spectrometers [6, 7]. The terahertz time-domain spectrometers have also been compared [8] and their testing accuracy has been improved. Furthermore, the investigation of the dominant factors affecting the spectra in biological samples [9] and the collection of the spectral data are in progress [10].
Dermis is a representative tissue where environmental exposure, for example, chemical and electromagnetic fields, occurs. Therefore, the state of the dermis, i.e., normal and abnormal conditions, is important measure of the effects of environmental exposure. When we employ the dermis models or the cultured dermal-cell tissues used for the evaluation of environmental exposure, we must evaluate the sample using various parameters such as water content, collagen state, and cell growth rate to verify the variation in the biological state of the samples used. Although there are some evaluation methods for each condition [11–14], there are few studies to investigate the complex relationship among the conditions, or the separation of the plural conditions from the composite data as described below, to the best of the authors’ knowledge. As we mentioned above, terahertz spectroscopy allows us to observe the higher-order structure of biological tissues or complex interactions between large molecules, which may be a measure of the composite conditions in dermal samples [15]. Furthermore, water content can be analyzed from absorption attributed to the dielectric relaxation of water at low frequencies. If terahertz spectroscopy can analyze water content and collagen conditions separated from dermal composite condition, and can predict a biological activity, such as the ability of cell growth, the spectroscopic method would be more useful for evaluating the samples used in environmental exposure studies.
Therefore, in this study, we systematically characterize the absorption properties in the THz range under normal and abnormal (here, heated) dermis conditions while considering absorption factors such as water and collagen. Furthermore, we characterize the absorption properties in THz range of the collagen sheets that were used as the scaffold of the dermis cells. Then we evaluate the relationship between the collagen and the growth rate of the cells. The results suggest that we can distinguish variations in the cell growth ability from the terahertz absorption of collagen before cell growth.
2. Measurement samples and systems
Dermis samples were cut from the lower reticular layer of a frozen porcine skin block. The thicknesses of the samples were from 200 to 350 μm, and the diameter of the samples was approximately 3 mm. The thickness of each sample was measured with a micrometer. The undried samples were used for measuring absorption spectra at room temperature (approximately 25°C). When analyzing the heat damage, some dermis samples were heated to 50–70°C for approximately 1 to 2 min inside two quartz glasses with a silicone spacer before the absorption measurement. The measurement data were acquired at a relative humidity of approximately 30–40%, and included the effect of water evaporation changed by the humidity.
We also prepared an epidermis model, i.e., epidermis cells incubated for 13 days with multiple layers (Epi-model, LabCyte) and collagen sheets with thickness of approximately 5 μm (Collagen Vitrigel VIT-C001, Asahi Glass Co., Ltd.). To consider the absorption properties of the collagen included in the dermis, the collagen sheets were soaked in 100 μl of phosphate-buffered saline (PBS) in a dish and heated to 37, 50, 65, or 70°C in a water bath for 7 (or 2) min. The heated collagen sheets were then naturally dried. The absorption spectra of the prepared samples were measured in the band from 0.2 to 2 THz using a transmission terahertz time-domain spectrometer (Rayfact SpecTera RS-01020, Tochigi Nikon).
In addition, we investigated the relationship between the heat denaturation of the collagen and the cell growth of fibroblasts (NB1RGB, RIKEN BRC). The collagen sheets were set into wells of a 96-well microplate. The cells were seeded at 4,000 cells per well on the 96-well microplates and incubated in minimum essential medium (MEM) α with GlutaMAX (Thermo Fisher Scientific Inc.) and 10% fetal bovine serum (FBS, Thermo Fisher Scientific Inc.) at 37°C in a 100% humidified 5% CO2 atmosphere. After incubation for 3 days, an MTT viability assay [13, 14] was performed. 20 μl of assay reagent (CellTiter 96, Promega Co.) was added to the wells of the 96-well microplate with cells, followed by incubation for 2 h to record the absorbance at 490 nm using a microplate reader (iMark, Bio-Rad Laboratories). The magnitude of the absorbance corresponds to cell viability.
3. Measurement results
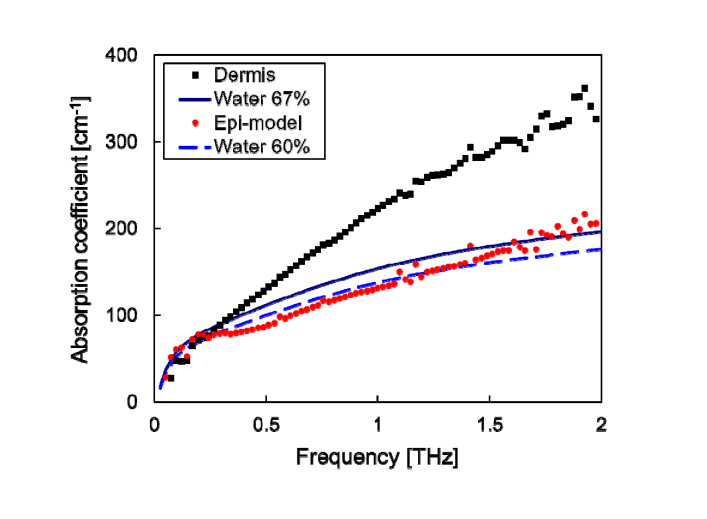
We firstly measured the absorption coefficients of the epidermis model and the dermis. As shown in Fig. 1, the absorption of the dermis was higher than that of the epidermis model, especially at frequencies higher than 0.3 THz. We fitted a curve of liquid water spectrum [16] multiplied by fitting coefficient (variable) to the data at frequencies from 0.2 to 0.3 THz. The fitting coefficients were determined by a least squares method. The fitting coefficients of the epidermis model and dermis were 0.6 and 0.67, respectively. Those coefficients were close to the weight ratios of water to the tissues (approximately 60–75%), which were measured using samples cultured on the same plate or cut from the same block. Because the spectral shape of the sample including water is affected by the fast relaxation of water molecules [17], the fitting coefficients were used as indices indicating the water content of the dermis samples in this study. The absorption magnitude of the epidermis model agreed with that of 60% liquid water, whereas the absorption magnitude of the dermis disagreed with that of 67% water at higher frequencies. The result of the epidermis model agreed with that of the porcine epidermis [18] regardless of the tissue level. At higher frequencies, the absorption of proteins such as collagen can be observed [19]. The epidermis and dermis mainly include keratin and collagen, respectively. The absorption magnitudes of the protein depend on the kind, content, and hydration state of the proteins. The absorption spectrum of a cell is similar to that of water, even though there is a difference owing to hydration [20]. We considered that the collagen caused the large absorption in the dermis, because more than 70% of the dry weight of a normal dermis consists of collagen, and collagen has hydrophobic bonding; there is little suppression of molecular vibration by hydration.
Fig. 1.
Absorption coefficient spectra of the epidermis model and dermis. The absorption coefficients of 67% and 60% water were calculated from that of 100% bulk water.
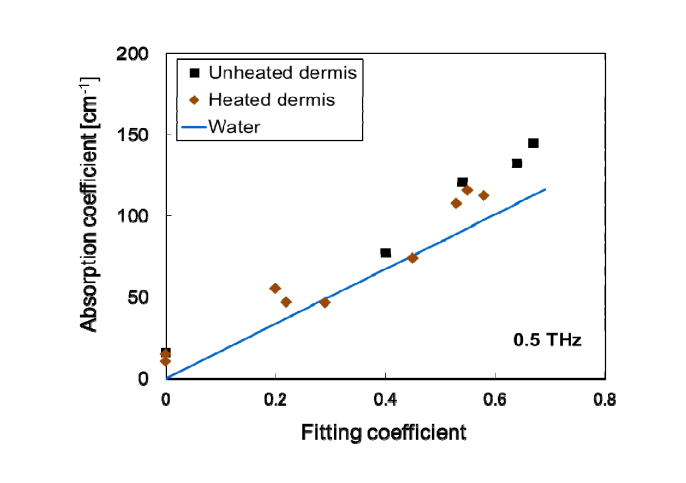
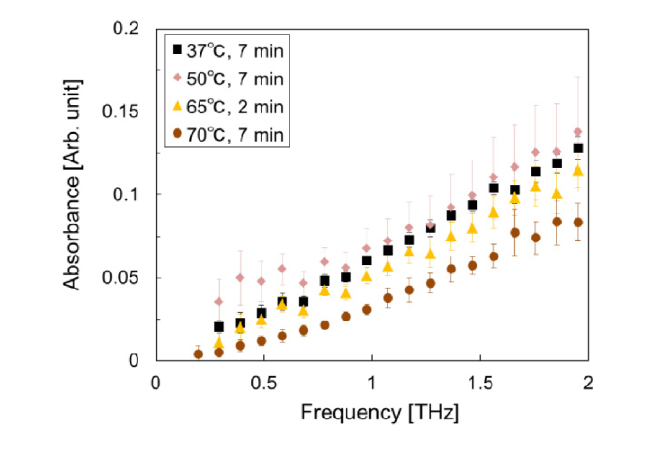
To verify the dependence of the spectral variation on the change in the collagen structure (from collagen to gelatin) in the dermis, we examined the absorption of dermis samples unheated and temporarily heated to 50–70°C. The denaturation temperature of porcine skin is approximately 66°C. The temperature and heating time were changed in order to investigate the dependence on the conditions. Figure 2 shows the relationship between the absorption coefficient of unheated or heated dermis at 0.5 THz and the fitting coefficient mentioned above. The absorption of the dermis decreased with the heat treatment including water evaporation. Furthermore, the difference in absorption values between the dermis and waterwas reduced by approximately 68% by the heating and drying. Similarly, we examined the absorption of collagen sheets temporarily heated to 37, 50, 65, or 70°C in a water bath for 7 (or 2) min. The time of the 65°C heating was 2 min to obtain intermediate data between those of 50 and 70°C. The absorbance of collagen without water in the whole frequency range decreased at heat treatment temperatures above 65°C, as shown in Fig. 3. We measured absorbance here because it is difficult to precisely measure thin sheet thickness d to calculate absorption coefficient α. The absorbance is nearly equal to αdlog10e. The collagen fiber has a broad absorption with a peak at around 3 THz [19]. We therefore considered that the absorption magnitude decreased owing to the denaturation of collagen (disorder of the triple helical structure with a larger dipole moment). It is important to note that the absorption change of the heated and dried dermis includes the effect of the hydration in the denaturated parts owing to the breaking of hydrophobic interactions between collagen molecules.
Fig. 2.
Relationship between the water content and the absorption coefficient of the unheated or heated dermis at 0.5 THz. Absorption coefficients of water were calculated by multiplying the absorption coefficients of bulk water by fitting coefficients (water content rate).
Fig. 3.
Absorbance spectra of the collagen sheets temporarily heated to 37, 50, 65, or 70°C. The error bars show the standard deviation of four measurements.
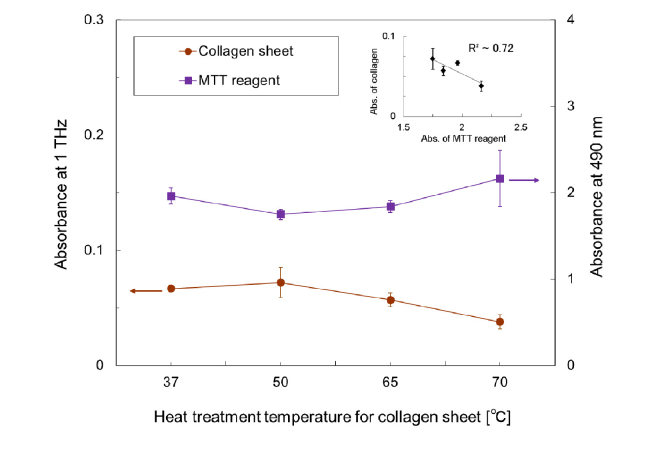
On the basis of the above-mentioned results, we investigated the relationship between the collagen absorption and the growth ability of fibroblast cells on the collagen by MTT viability assay. A fibroblast is a type of cell that synthesizes an extracellular matrix, such as that of collagen, depending on the situation in the dermis. The cellular growth rate, therefore, strongly depends on the collagen state in the dermis. For the cell culture, we used the collagen sheets heat-treated before cell seeding as a scaffold, whose absorption properties were checked as described above. In the MTT viability assay, the absorbance data were recorded after incubating the cells with the assay reagent for 2 h. Figure 4 shows the absorbance of the assay reagent at 490 nm, which indicate cell viability, and the absorbance of the heat-treated collagen sheets at 1 THz. The absorbance data were acquired four times for each measurement. The inset shows the correlation between cell viability and THz absorption of the collagen sheet with the square of the correlation coefficient R2. We found that the average absorbance of the collagen sheets was slightly larger at treatment temperature of 50°C with a large standard deviation. Low-temperature heat treatment had the possibility of changing the collagen orientation. The 65°C and 70°C heating caused reductions of approximately 15% and 43% in the absorbance of the collagen sheet, respectively. In comparison with the average growth rate of fibroblasts (the absorbance at 490 nm in the MTT assay), the temperature dependence seemed to be opposite. R2 was approximately 0.72. This suggests that the terahertz absorption of collagen is related to the growth of fibroblast cells, which generally exist in dermis. Therefore, we considered that we can predict future fibroblast growth rate variation (or the regeneration conditions) in cultured dermal-cell tissue and the dermis model using the absorption of collagen and the difference between the absorptions of the dermis sample and water, respectively, as shown in Fig. 2.
Fig. 4.
Relationship between the absorbance values of the collagen sheets temporarily heated to 37, 50, 65, or 70°C before cell seeding at 1 THz and the absorbance of MTT reagent at 490 nm, which indicate the viability of cells grown on each heat-treated sheet. The error bars show the standard deviation of four measurements. Inset shows the correlation between the absorptions of collagen sheet and MTT reagent with the square of the correlation coefficient R2.
4. Summary
The absorption spectra of the epidermis model and dermis were reviewed in the terahertz band. The absorption of the dermis was determined mainly from those of water and collagen. We quantitatively verified that the heat denaturation of the collagen was related to the decrease in absorption of the dermis and also the growth rate of the fibroblasts grown on the collagen. The possibility of clearly distinguishing the present and future conditions of the dermis on the basis of the collagen absorption property in the tissue by terahertz spectroscopy was shown. The analysis method is also useful for evaluating the effects of environmental exposure.
Acknowledgments
The authors express their thanks to K. Sasaki of NICT, S. Otsuki, and K. Kawase of RIKEN for their support.
Funding
The Ministry of Internal Affairs and Communications, Japan.
Disclosures
The authors declare that there are no conflicts of interest related to this article.
References and links
- 1.Wallace V. P., Taday P. F., Fitzgerald A. J., Woodward R. M., Cluff J., Pye R. J., Arnone D. D., “Terahertz pulsed imaging and spectroscopy for biomedical and pharmaceutical applications,” Faraday Discuss. 126, 255–263, discussion 303–311 (2004). 10.1039/b309357n [DOI] [PubMed] [Google Scholar]
- 2.Amenabar I., Lopez F., Mendikute A., “In introductory review to THz non-destructive testing of composite mater,” J. Infrared Millim. Te. 34(2), 152–169 (2013). 10.1007/s10762-012-9949-z [DOI] [Google Scholar]
- 3.Nakajima S., Hoshina H., Yamashita M., Otani C., Miyoshi N., “Terahertz imaging diagnostics of cancer tissues with a chemometrics technique,” Appl. Phys. Lett. 90(4), 041102 (2007). 10.1063/1.2433035 [DOI] [Google Scholar]
- 4.Arbab M. H., Dickey T. C., Winebrenner D. P., Chen A., Klein M. B., Mourad P. D., “Terahertz reflectometry of burn wounds in a rat model,” Biomed. Opt. Express 2(8), 2339–2347 (2011). 10.1364/BOE.2.002339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laman N., Harsha S. S., Grischkowsky D., Melinger J. S., “High-resolution waveguide THz spectroscopy of biological molecules,” Biophys. J. 94(3), 1010–1020 (2008). 10.1529/biophysj.107.113647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilmink G. J., Ibey B. L., Tongue T., Schulkin B., Laman N., Peralta X. G., Roth C. C., Cerna C. Z., Rivest B. D., Grundt J. E., Roach W. P., “Development of a compact terahertz time-domain spectrometer for the measurement of the optical properties of biological tissues,” J. Biomed. Opt. 16(4), 047006 (2011). 10.1117/1.3570648 [DOI] [PubMed] [Google Scholar]
- 7.Ouchi T., Kajiki K., Koizumi T., Itsuji T., Koyama Y., Sekiguchi R., Kubota O., Kawase K., “Terahertz imaging system for medical applications and related high efficiency terahertz devices,” J. Infrared Milli. Terahz. Waves 35(1), 118–130 (2014). 10.1007/s10762-013-0004-5 [DOI] [Google Scholar]
- 8.Mizuno M., Iida H., Kinoshita M., Fukunaga K., Shimada Y., Otani C., “Classification of terahertz spectrometer for transmittance measurements of refractive materials,” IEICE ELEX 13(18), 20160532 (2016). 10.1587/elex.13.20160532 [DOI] [Google Scholar]
- 9.Wallace V. P., Fitzgerald A. J., Pickwell E., Pye R. J., Taday P. F., Flanagan N., Ha T., “Terahertz pulsed spectroscopy of human Basal cell carcinoma,” Appl. Spectrosc. 60(10), 1127–1133 (2006). 10.1366/000370206778664635 [DOI] [PubMed] [Google Scholar]
- 10.Pickwell E., Cole B. E., Fitzgerald A. J., Pepper M., Wallace V. P., “In vivo study of human skin using pulsed terahertz radiation,” Phys. Med. Biol. 49(9), 1595–1607 (2004). 10.1088/0031-9155/49/9/001 [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa N., Matsumoto M., Sakai S., “In vivo measurement of the water content in the dermis by confocal Raman spectroscopy,” Skin Res. Technol. 16(2), 137–141 (2010). 10.1111/j.1600-0846.2009.00410.x [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Nadiarynkh O., Plotnikov S., Campagnola P. J., “Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure,” Nat. Protoc. 7(4), 654–669 (2012). 10.1038/nprot.2012.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann T., “Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays,” J. Immunol. Methods 65(1-2), 55–63 (1983). 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- 14.Barltrop J. A., Owen T. C., Cory A. H., Cory J. G., “5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators,” Bioorg. Med. Chem. Lett. 1(11), 611–614 (1991). 10.1016/S0960-894X(01)81162-8 [DOI] [Google Scholar]
- 15.Chopra N., Yang K., Upton J., Abbasi Q. H., Qaraqe K., Philpott M., Alomainy A., “Fibroblasts cell number density based human skin characterization at THz for in-body nanonetworks,” Nano Commun. Netw. 10, 60–67 (2016). 10.1016/j.nancom.2016.07.009 [DOI] [Google Scholar]
- 16.Rønne C., Thrane L., Åstrand P.-O., Wallqvist A., Mikkelsen K. V., Keiding S. R., “Investigation of the temperature dependence of dielectric relaxation in liquid water by THz reflection spectroscopy and molecular dynamics simulation,” J. Chem. Phys. 107(14), 5319–5331 (1997). 10.1063/1.474242 [DOI] [Google Scholar]
- 17.Yada H., Nagai M., Tanaka K., “Origin of the fast relaxation component of water and heavy water revealed by terahertz time-domain spectroscopy,” Chem. Phys. Lett. 464(4–6), 166–170 (2008). 10.1016/j.cplett.2008.09.015 [DOI] [Google Scholar]
- 18. Mizuno M., Yaekashiwa N., Watanabe S., “Distinction of dermal conditions related to collagen state using THz spectroscopy,” Proc. 42nd Int. Conf. on IRMMW-THz, TD. 6 (2017). 10.1109/IRMMW-THz.2017.8067197 [DOI] [Google Scholar]
- 19.Mizuno M., Yamada A., Fukunaga K., Kojima H., “Far-infrared spectroscopy of salt penetration into a collagen fiber scaffold,” J. Biol. Phys. 41(3), 293–301 (2015). 10.1007/s10867-015-9379-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiraga K., Ogawa Y., Suzuki T., Kondo N., Irisawa A., Imamura M., “Characterization of Dielectric Responses of Human Cancer Cells in the Terahertz Region,” J. Infrared Milli. Terahz. Waves 35(5), 493–502 (2014). 10.1007/s10762-014-0067-y [DOI] [Google Scholar]