Summary
Inhibitory interneurons govern virtually all computations in neocortical circuits and are in turn controlled by neuromodulation. While a detailed understanding of the distinct marker expression, physiology, and neuromodulator responses of different interneuron types exists for rodents and recent studies have highlighted the role of specific interneurons in converting rapid neuromodulatory signals into altered sensory processing during locomotion, attention, and associative learning, it remains little understood whether similar mechanisms exist in human neocortex. Here, we use whole-cell recordings combined with agonist application, transgenic mouse lines, in situ hybridization, and unbiased clustering to directly determine these features in human layer 1 interneurons (L1-INs). Our results indicate pronounced nicotinic recruitment of all L1-INs, whereas only a small subset co-expresses the ionotropic HTR3 receptor. In addition to human specializations, we observe two comparable physiologically and genetically distinct L1-IN types in both species, together indicating conserved rapid neuromodulation of human neocortical circuits through layer 1.
Keywords: neocortical circuits, interneuron types, layer 1 interneurons, neuromodulation, human neocortex, mouse neocortex, whole-cell recordings, genetic markers, cell types, translation, evolution
Graphical Abstract
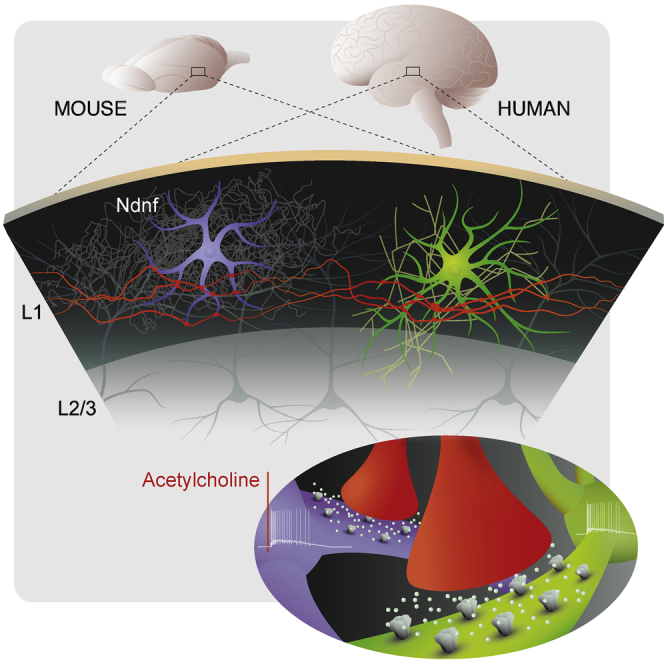
Highlights
-
•
Layer 1 interneurons in human and mouse neocortex respond strongly to acetylcholine
-
•
These rapid responses are mediated by α7 and β2-containing nicotinic receptors
-
•
Human layer 1 comprises neurogliaform cells expressing the conserved marker Ndnf
-
•
Apart from conserved features, human L1 interneurons show a number of specializations
Inhibitory interneurons govern the function of neural circuits and are in turn controlled by neuromodulation. Here, Poorthuis et al. demonstrate that these mechanisms are conserved in layer 1 of human neocortex, where interneurons express nicotinic acetylcholine receptors that mediate fast responses and thereby enable reconfiguration of circuit function at rapid timescales.
Introduction
An ultimate goal of much of modern neuroscience is to understand the function of the human brain, and in particular the human neocortex, which expanded and differentiated substantially during mammalian evolution, mediates many of the capacities that distinguish us from our closest relatives and also plays a central role in psychiatric disorders of human patients (DeFelipe et al., 2002, Marín, 2012, Nelson and Valakh, 2015). Virtually all computations in neocortical circuits are controlled and shaped by the complement of inhibitory interneurons. Different interneuron types show distinct marker expression, physiology, and connectivity with postsynaptic targets and thereby control distinct aspects of circuit function (Kepecs and Fishell, 2014, Wester and McBain, 2014, Poorthuis et al., 2014, Silberberg, 2008). Comparative studies with rodents have revealed that the primate neocortex comprises interneuron types with unique morphologies, along with a greater proportion of interneurons in general and differences in the developmental origin of these cells (DeFelipe et al., 2002, Rakic, 2009). However, despite a few notable exceptions (Jiang et al., 2012, Szegedi et al., 2016, Oláh et al., 2007), we know very little about the physiology and circuit function of distinct human interneuron types.
Here, we take advantage of a well-established source of live slices from human temporal neocortex (Eyal et al., 2016, Verhoog et al., 2013, Testa-Silva et al., 2014) to determine the functional features of human layer 1 interneurons (L1-INs). In rodents, a hallmark of these cells is their strong expression of nicotinic acetylcholine receptors (Christophe et al., 2002, Bennett et al., 2012, Letzkus et al., 2011, Alitto and Dan, 2013, Arroyo et al., 2012), and recent work has shown that these currents are a necessary prerequisite for rapid recruitment of L1-INs, as well as vasoactive intestinal polypeptide (Vip)-expressing cells, by endogenous acetylcholine released during learning, locomotion, and attention (Letzkus et al., 2011, Alitto and Dan, 2013, Pi et al., 2013, Fu et al., 2014, Poorthuis et al., 2014, Letzkus et al., 2015, Kuchibhotla et al., 2017). A second candidate neuromodulator for similar rapid responses is serotonin released from raphe nucleus afferents, which are enriched in layer 1 (Trottier et al., 1996) and thought to predominantly contact interneurons (Chameau and van Hooft, 2006). The HTR3 receptor is the only known fast, ionotropic serotonin receptor, which in rodents is expressed in approximately 10% of L1-INs in frontal cortex (Zhou and Hablitz, 1999), and the majority in somatosensory cortex (Lee et al., 2010). How recruitment by these neuromodulators affects the circuit depends on L1-IN connectivity. Two physiologically and molecularly distinct types of L1-INs have been identified in rodents (Wozny and Williams, 2011, Chu et al., 2003, Tasic et al., 2016, Cruikshank et al., 2012, Zhu and Zhu, 2004), which display differential connectivity in the local circuit (Jiang et al., 2013, Jiang et al., 2015) and potentially different functions in vivo (Palmer et al., 2012, Letzkus et al., 2015). Here, we determine these attributes in human neocortex and present analogous, age- and area-matched data from wild-type and transgenic mice (see Supplemental Experimental Procedures) to directly link our findings to the large and dynamic rodent literature.
Results
Nicotinic Responses in Human Temporal Neocortex Layer 1 Interneurons
Exploiting the fact that L1-INs can be robustly targeted without genetic markers, we obtained whole-cell recordings in acute slices of healthy human temporal neocortex that needed to be removed to gain access to the underlying pathology and was donated by adult patients (10 male, 3 female, age range 19–52 years, average 36.6 ± 2.9). Layer 1 was identified as the area of low cell density immediately below the pia mater (Figure 1A), and post hoc analyses of neuron density ensured that only neurons located more than 20 μm from the L1/L2 border were included in the dataset.
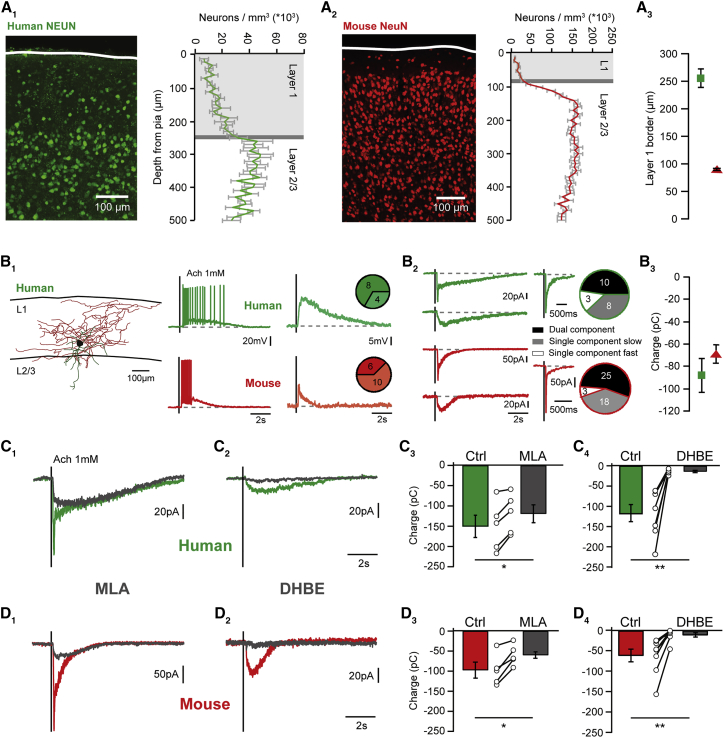
Figure 1.
Nicotinic Responses in Human Layer 1 Interneurons
(A) Neuron density relative to the pia was used to define layer 1 in human (green, 7 slices, 3 patients) and mouse neocortex (red, 29 slices, 3 mice) (A1, A2). Layer 1 thickness was 254.2 ± 16.3 μm for human and 90.7 ± 2.0 μm for mouse (A3).
(B) All human and mouse L1-INs showed rapid voltage responses to local pressure application of acetylcholine, and displayed supra- or subthreshold responses in similar proportions (p = 0.25, Fisher’s exact test) (B1). Nicotinic currents showed biphasic time courses in approximately half of the human and mouse cells (top left), with a rapid, large amplitude initial current and a slower second component (B2). A second major L1-IN fraction displayed exclusively slow currents (bottom left), while the remaining minority showed only the rapid component (center). The proportion of these response types was similar in human and mouse (right, p = 0.58, Fisher exact test). (B3) The charge of nicotinic responses was similar in the two species (p = 0.39, Kruskal-Wallis test, n = 21 versus n = 46, −88.2 ± 15.5 versus −69.3 ± 8.2 pC).
(C and D) Bath application of the selective α7 receptor antagonist MLA (10 nM) abolished the fast current in human (C1) and mouse (D1) L1-INs with biphasic currents. C3, D3 MLA reduced the response charge in humans (p < 0.05, n = 5, Wilcoxon signed-rank test, −150.4 ± 27.3 versus −119.2 ± 22.0 pC) and mice (p < 0.05, n = 5, Wilcoxon signed-rank test, −97.6 ± 20.0 versus −60.0 ± 7.9 pC), although its effect on peak amplitude was more pronounced (Figure S1). The β2-containing receptor antagonist DHBE (1 μM) selectively blocked the slow current (C2, D2), which carried most of the charge in humans (C4, p < 0.01, n = 7, Wilcoxon signed-rank test, −118.2 ± 22.7 versus −12.1 ± 2.4 pC, tested on 3 biphasic and 4 monophasic slow currents, see Figure S1) and mice (D4, p < 0.01, n = 8, Wilcoxon signed-rank test, −66.5 ± 12.0 versus −8.25 ± 4.1 pC, tested on 1 biphasic and 7 monophasic slow currents). Error bars indicate SEM. See also Figure S1.
To address whether human L1-INs express nicotinic receptors, we pressure applied acetylcholine (1 mM) in the presence of the muscarinic antagonist atropine (400 nM). All L1-INs tested in current clamp (n = 12) were depolarized by acetylcholine, with the majority showing suprathreshold responses (8 out of 12 neurons, Figures 1B1 and S1). Voltage-clamp recordings revealed that many L1-INs displayed clearly bi-phasic nicotinic currents (10 out of 21), with a rapid component and a much slower second component (Figure 1B2). The remaining L1-INs had either exclusively slow (n = 8) or fast nicotinic currents (n = 3). Consistent with observations from other areas of rodent cortex (Christophe et al., 2002, Bennett et al., 2012), comparison recordings from temporal neocortex of adult mice produced very similar results (Figures 1 and S1).
To define the nicotinic acetylcholine receptor subtypes mediating these responses, we used pharmacology. In both species, the rapid component of biphasic cholinergic currents was blocked by methyllycaconitine (MLA; 10 nM, Figures 1C, 1D, and S1), identifying α7 receptors as the underlying conductance (Ward et al., 1990). In contrast, the slow component of biphasic currents, and slow monophasic currents were completely abolished by dihydro-beta-erythroidine (DHBE; 1 μM, Figures 1C, 1D, and S1), indicating β2-containing heteromeric receptors (Cordero-Erausquin et al., 2000). The rapid component had a much greater amplitude in general, and in particular in mouse L1-INs (Figure S1), whereas the slow current carried by far most charge in both species (Figures 1C and 1D, cf. Bennett et al., 2012). While the rise time of α7 currents was indistinguishable between human and mouse L1-INs, the slow current displayed both longer rise and decay times in human L1-INs (Figure S1), which may suggest differential modulation or subcellular localization of the receptors or could alternatively be due to morphological differences. Importantly, we could not detect an impact of the patients’ smoking history on these currents (p > 0.05 for Kruskal-Wallis tests on response amplitude and charge when comparing current or former smokers with non-smokers), in contrast to larger and more prevalent nicotinic currents in layer 6 pyramidal neurons of smokers (Verhoog et al., 2016). Taken together, these results indicate that human L1-INs display strong expression of α7 and β2-containing nicotinic acetylcholine receptors, consistent with similar observations in unidentified human interneurons (Alkondon et al., 2000), and with the high density of nicotine binding sites in human layer 1 (Sihver et al., 1998). Interestingly, fast α7 receptors have been suggested to mediate rapid cholinergic synaptic transmission (Bennett et al., 2012, Smiley et al., 1997), while the slower currents through β2-containing receptors are likely recruited by a form of volume transmission, indicating that a large fraction of L1-INs may thus be recruited by both modes of cholinergic signaling. Together with their pronounced excitability (Figure 3, see below), and the enrichment of cholinergic synapses in human layer 1 (Smiley et al., 1997), our results thus indicate that L1-INs can be rapidly recruited by endogenous acetylcholine release during arousal, attention, and learning in the intact human brain in a fashion similar to that found in behaving mice (Arroyo et al., 2014, Poorthuis et al., 2014, Letzkus et al., 2015).
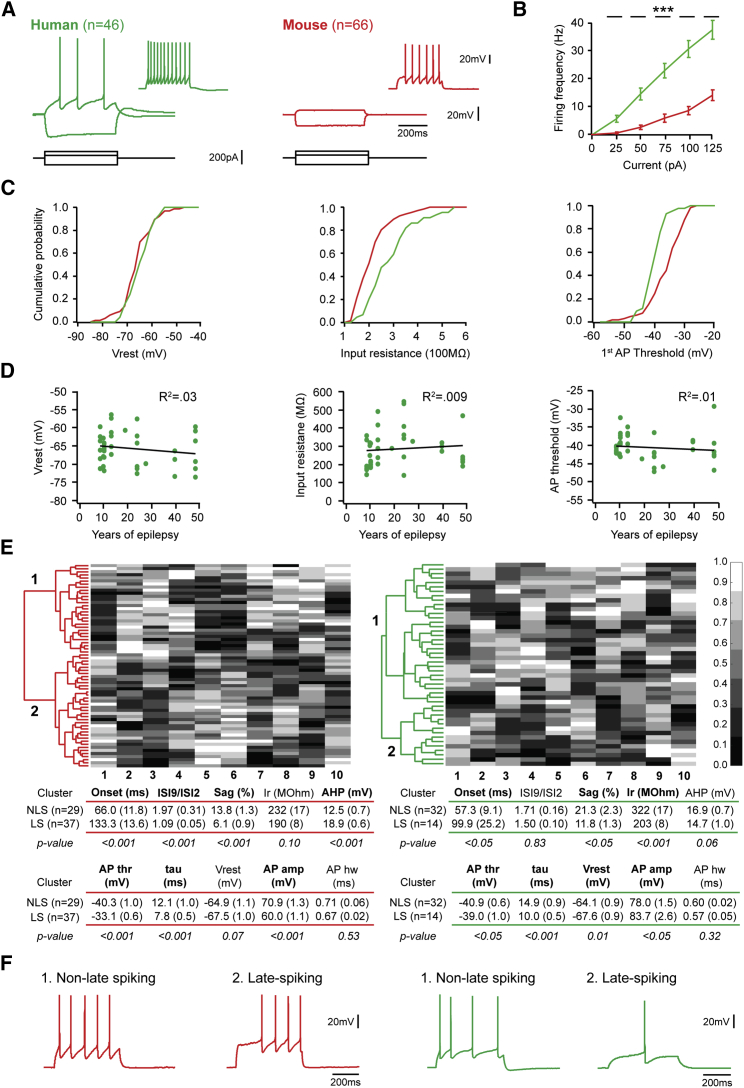
Figure 3.
Intrinsic Properties and Subtypes of Human Layer 1 Interneurons
(A) Responses of example human (green) and mouse (red) L1-IN to current injections of −100, +25, and +75 pA (inset).
(B) Human neurons fired at higher frequencies across the entire range of injected currents (p < 0.001 for all current amplitudes, Kruskal-Wallis test, n = 46 human neurons and n = 66 mouse neurons).
(C) While the resting membrane potential of human and mouse L1-INs was similar (Vrest, −65.2 ± 0.7 versus −66.4 ± 0.8 mV, Kruskal-Wallis test, p = 0.28), human neurons displayed higher input resistance (286.0 ± 14.9 versus 208.4 ± 8.9 MΩ, Kruskal-Wallis test, p < 0.001) and lower action potential threshold compared to mice (−40.4 ± 0.5 versus −36.4 ± 0.7 mV, Kruskal-Wallis test, p < 0.001), with both factors likely contributing to the greater excitability of human L1-INs.
(D) These parameters showed no correlation with the disease history of the patients (p > 0.2, see also Figure S4).
(E) (left) Unsupervised hierarchical clustering on a set of active and passive physiological properties (Ward’s method on normalized datasets without prior assumption on cluster number) yielded a non-late-spiking and a late-spiking cluster in the mouse, which also differ in most other parameters (table presents mean, SEM and results of Kruskal-Wallis test). In addition to differences in spiking accommodation, voltage sag, and action potential afterhyperpolarization (Chu et al., 2003, Tasic et al., 2016, Jiang et al., 2013, Jiang et al., 2015), the late-spiking L1-INs display more depolarized action potential threshold, faster membrane time constant and lower action potential amplitude. (Right) The same unbiased analysis on human L1-INs yielded similar clusters, with a population of late-spiking cells that display smaller voltage sag, more depolarized action potential threshold, and faster membrane time constant. In addition, input resistance and resting membrane potential displayed similar trends in both species, whereas spiking accommodation, action potential afterhyperpolarization, and amplitude varied in a distinct manner between the two clusters in mouse and human. Calibration bar indicates value rank between 0 and 1.
(F) Example firing patterns. Error bars indicate SEM.
See also Figures S4 and S5.
Conserved Absence of HTR3 Responses in Human Layer 1 Interneurons
A second candidate ionotropic receptor for fast neuromodulation is the serotonin HTR3 receptor, which in rodents is expressed in approximately 10% of L1-INs in frontal cortex (Zhou and Hablitz, 1999), and the majority in somatosensory cortex (Lee et al., 2010). In human L1-INs, pressure application of the selective HTR3 receptor agonist mCPBG (100 μM) yielded no detectable current (Figure 2A, n = 10), and similar results were observed when using serotonin (Figure S2, n = 6). As a control for recording conditions, we verified that acetylcholine elicited robust responses in a subset of these experiments (Figure 2A). Similar to the human data, L1-INs in adult mouse temporal neocortex displayed no response to mCPBG (Figure 2A, n = 10). These data suggest that in both species, the large majority of L1-INs is not under rapid control by serotonin.
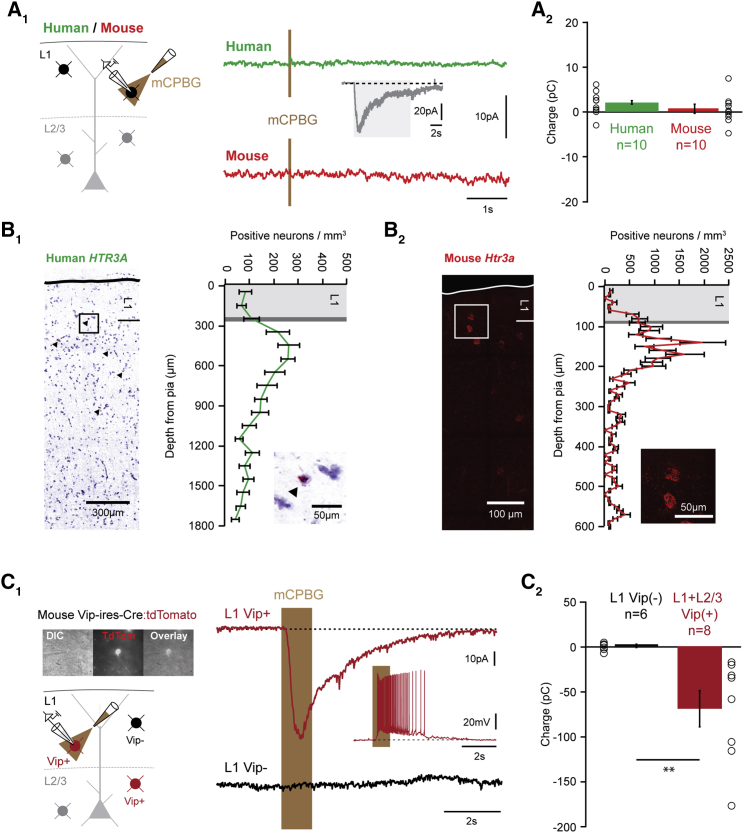
Figure 2.
Conserved Absence of HTR3 Responses in Human Layer 1 Interneurons
(A) Pressure application of the selective Htr3 receptor agonist mCPBG (100-ms application displayed, 1 s and 10 s also tested) caused no response in L1-INs of either species, in contrast to robust responses when acetylcholine was applied to the same cells (inset, 10-s application) (A1). On average, the Htr3 receptor mediated charge was 1.81 ± 0.82 pC in human (n = 10), and 0.53 ± 1.01 pC in mouse (n = 10), and similar data were obtained with application of serotonin (A2) (Figure S2).
(B) In situ hybridization for HTR3A in human (B1) and mouse neocortex (B2). Note that while the density peaks in layer 2/3, a small population of positive neurons is also present in layer 1. The image in B1 is a montage of images assembled using Pannoramic Scan software.
(C) Vip positive interneurons in layer 1 and layer 2/3 were targeted using a cross of transgenic mouse lines. Example responses of a Vip positive and negative L1-IN to mCPBG application. While Vip negative L1-INs showed no response, Vip positive L1-INs and L2-3-INs displayed large inward currents that led to robust firing (inset). Similar data were obtained with application of serotonin (Figure S2) (C1). The average charge of mCPBG responses in Vip negative L1-INs was 0.13 ± 1.86 pC (n = 6), whereas Vip positive interneurons in layer 1 and layer 2/3 displayed responses of −69.88 ± 0.30 pC (n = 8, p < 0.01, Kruskal-Wallis test). Error bars indicate SEM (C2 ).
See also Figures S2 and S3.
However, the number of recordings presented here (n = 26 in total) does not rule out the presence of a small population of responsive L1-INs as reported for rat frontal cortex (Zhou and Hablitz, 1999). Consistent with this, in situ hybridization in human and mouse temporal neocortex indicated that, while most Htr3a receptor positive neurons are located in layer 2/3, there is also expression in a small set of L1-INs (Figures 2B and S3). We hypothesized that these could be Vip-positive interneurons, which express Htr3a receptors (Férézou et al., 2002, Lee et al., 2010) and represent a small population of L1-INs in mouse neocortex (Prönneke et al., 2015, Xu et al., 2010). Recordings from Vip interneurons in a cross of transgenic mouse lines revealed robust inward currents in response to mCPBG and serotonin both in layer 1 and layer 2/3 (Figures 2C and S2). In contrast, unlabeled, Vip negative L1-INs displayed no response. In line with this result, Vip expression in human and mouse neocortex recapitulates the Htr3a profile and is confined to a small population of L1-INs (Figure S3). In conclusion, these data suggest that, while all L1-INs respond to acetylcholine, only the small subset that expresses VIP is under dual control by the serotonergic and cholinergic systems in both species.
Intrinsic Properties of Human Layer 1 Interneurons
The intrinsic physiological properties of human L1-INs determine how neuromodulatory input is converted to circuit modulation by these cells. Our data indicate that human L1-INs (n = 46) display a markedly steeper input-output function compared to the mouse (n = 66), with more than twice the action potential frequency across the whole range of injected current and significantly lower rheobase (Figures 3A–3C and S4). Both higher input resistance and lower action potential threshold of human L1-INs contribute to this effect, while we find no difference in resting membrane potential. In addition, we observe differences in several other intrinsic parameters, including voltage sag and spike frequency accommodation that are of interest for mechanistic models of human neocortex (Figure S4). Importantly, we found no indication that the disease history of human patients had an impact on these results (Figure S4). Consistent with this, greater excitability, lower action potential threshold, and higher input resistance and voltage sag have been reported in a study comparing layer 2/3 neurogliaform cells from healthy macaque and rat (Povysheva et al., 2007). Together, this suggests that these properties represent specializations of certain types of primate interneurons that need to be taken into account when modeling the human neocortex.
Conserved Interneuron Subtypes and Marker Expression in Human Layer 1
Rodent data have shown that L1-INs can be further divided into physiological subtypes (Wozny and Williams, 2011, Chu et al., 2003, Tasic et al., 2016), which display differential connectivity in the local circuit (Jiang et al., 2013, Jiang et al., 2015) and potentially different functions in vivo (Letzkus et al., 2015). To address whether human L1 comprises similar interneuron subtypes in an unbiased way, we employed unsupervised hierarchical clustering on a comprehensive set of active and passive physiological properties (Figure 3E). For the mouse, this analysis defined two clusters that differ in 7 parameters and correspond to the well-described rodent late-spiking and non-late-spiking L1-INs characterized by differences in latency of the first action potential at rheobase, as well as in spike frequency accommodation, voltage sag, and action potential afterhyperpolarization (Chu et al., 2003, Tasic et al., 2016, Jiang et al., 2013, Jiang et al., 2015). The same analysis on human data yielded two clusters that differ in action potential latency, indicating that late-spiking and non-late-spiking interneurons are also present in layer 1 of human neocortex (Figures 3E and 3F). Out of the remaining 9 parameters, 3 showed a corresponding cluster distribution in both species (voltage sag, action potential threshold, membrane time constant), and 2 showed a trend for similarity (input resistance, resting membrane potential), whereas spike frequency accommodation, action potential afterhyperpolarization, and amplitude were clearly distinct between the clusters across species, as well as between human and mouse L1-INs in general (Figure S4). There was no difference in action potential latency between human and mouse late-spiking or non-late-spiking cells (p > 0.05, Kruskal-Wallis test). No detectable difference was in addition observed in nicotinic responses between late-spiking and non-late-spiking L1-INs of either species (Figure S5), indicating that both types are under similar cholinergic control. Interestingly, most rodent analyses of late-spiking interneurons have reported little firing accommodation (Chu et al., 2003, Jiang et al., 2013, Jiang et al., 2015), potentially suggesting that late-spiking, accommodating L1-INs are a specialization of human neocortex that may be mechanistically related to the larger voltage sag of these cells (Figure S4).
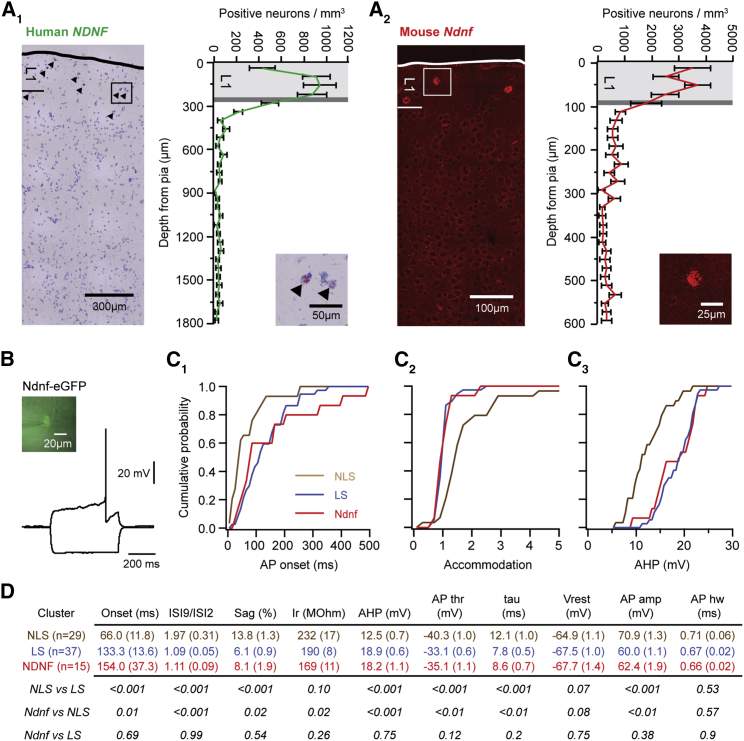
These results indicate that human layer 1 comprises two distinct types of interneurons, which share a range of properties with the respective populations in mice. To address whether this conservation extends to the molecular identity of L1-INs, we utilized neuron-derived neurotrophic factor (Ndnf), a recently described, selective genetic marker for mouse L1 neurogliaform interneurons in visual cortex (Tasic et al., 2016). Intriguingly, in situ hybridization revealed that NDNF-expressing cells are highly enriched in human layer 1, with a steep drop in density at the border to layer 2 (Figures 4A and S3). Mouse neocortex showed a very similar expression profile (Figures 4A and S3), and we next used a transgenic mouse line expressing eGFP under the Ndnf promoter (Gong et al., 2003) to determine how Ndnf-positive L1-INs relate to the two interneuron subtypes identified in unbiased recordings (Figure 3). Among other properties, Ndnf-positive L1-INs showed long action potential onset latencies, little spiking accommodation, and large action potential afterhyperpolarization (Figures 4B–4D), very similar to the late-spiking cluster. Moreover, a quantitative comparison revealed that Ndnf-positive L1-INs differ from the non-late-spiking cluster in the same properties as the late-spiking cluster, whereas no differences between Ndnf-positive L1-INs and the late-spiking cluster were found (Figures 4B–4D). The observed convergence between marker expression and clustering as two independent approaches provides strong evidence that late-spiking L1-INs are Ndnf-positive neurogliaform cells and highlights the power and sensitivity of a clustering approach based on a comprehensive set of properties. Taken together, these data indicate that human neocortex expresses a conserved marker for L1 neurogliaform cells and comprises a population of late-spiking interneurons, suggesting that human layer 1 contains a set of neurogliaform cells or a physiologically and genetically related interneuron type. In turn, identification of NDNF will facilitate future research on these interneurons, for instance, by enabling postmortem analyses in different brain disorders and more precise translation of mouse data to the human brain.
Figure 4.
Neuron-Derived Neurotrophic Factor Is a Conserved Marker for Human Layer 1 Neurogliaform Cells
(A) In situ hybridization for NDNF in human neocortex (left) and quantification of the distribution of positive neurons along cortical depth (12 slices from 2 patients). The density of NDNF-positive neurons is high in layer 1 (light gray) and drops off steeply at the border to layer 2 (dark gray) (A1). In situ hybridization for Ndnf in mouse temporal neocortex (16 slices from 3 animals) shows a similar expression profile, indicating that Ndnf is a conserved marker for layer 1 interneurons (A2). The image in A1 is a montage of images assembled using Pannoramic Scan software.
(B) Whole-cell current-clamp slice recording using an Ndnf-eGFP mouse line.
(C) To determine whether Ndnf positive layer 1 interneurons correspond to one of the populations identified by unbiased clustering of the entire layer 1 interneuron population (Figure 3), we compared the distribution of action potential onset (C1), spiking accommodation (C2), and action potential afterhyperpolarization (C3) in the three populations. Ndnf interneurons display robust similarity with the late-spiking cluster in all three parameters.
(D) Statistical comparison of the 10 attributes used for clustering between the three populations indicates that Ndnf interneurons and cells in the late-spiking cluster differ from the non-late-spiking cluster in the same properties, whereas no difference could be detected between the late-spiking and Ndnf populations. Error bars indicate SEM.
Discussion
Layer 1 is the unique site in neocortex where a variety of long-range projections from cortical, higher-order thalamic and neuromodulatory sources impinges onto the apical dendrites of local pyramidal neurons and the sparse set of L1-INs (Cauller, 1995, Douglas and Martin, 2004). In line with this organization, recent data from rodents indicate that L1-INs in sensory cortex are specialized for encoding contextual information such as primary reinforcers (Letzkus et al., 2011), interhemispheric interactions (Palmer et al., 2012), and multimodal processing (Ibrahim et al., 2016). While a mechanistic understanding of such processes in the human brain is still elusive, our aim here was first to determine the functional properties of human L1-INs and second to test to what extent data from the mouse—where further rapid progress is expected due to a range of high-resolution in vivo approaches—is relevant to the function of human neocortex. In contrast to recent studies reporting differences between human and rodent pyramidal neurons in terms of membrane properties (Eyal et al., 2016), synaptic plasticity (Verhoog et al., 2013), and communication (Testa-Silva et al., 2014), our results indicate that key properties of L1-INs at the level of physiology, marker expression, and neuromodulation are conserved in human neocortex. This suggests that, despite the striking evolution of neocortical structure, and particularly of the inhibitory system (DeFelipe et al., 2002), L1-INs carry out comparable functions in the two species. However, future work is required to elucidate whether human non-late-spiking L1-INs show similarities to the single-bouquet cells found in the rodent (Jiang et al., 2013, Jiang et al., 2015). In addition, it will be important to determine whether the two human L1-IN types can be further subdivided based on additional markers, morphological properties, and connectivity (Tasic et al., 2016, Jiang et al., 2015). Moreover, our data also reveal differences between human and mouse L1-INs, which together with a previous study comparing layer 2/3 neurogliaform interneurons between macaque and rat (Povysheva et al., 2007) identify specializations of these interneuron types in the primate brain such as the greater voltage sag that can profoundly affect their physiological properties.
In addition to the evolutionary relevance, a more precise understanding of the physiology of different identified human interneurons types is also a key prerequisite for biomedical research. Maladaptive changes in both inhibitory interneurons (Marín, 2012, Nelson and Valakh, 2015) and cholinergic neuromodulation (Levin, 2013) are thought to be core features of a range of human brain disorders and the present results together with the available data on human fast-spiking interneurons and neurogliaform cells in deeper layers (Jiang et al., 2012, Szegedi et al., 2016, Oláh et al., 2007) also provide a first framework for a cell-type-specific, mechanistic understanding of the underlying pathophysiology that can be used to build quantitative disease models and to evaluate the relevance of data obtained in other model systems such as tissue derived from induced pluripotent stem cells.
Experimental Procedures
Further details and an outline of resources used in this work can be found in Supplemental Experimental Procedures. All procedures on mice were performed in accordance with institutional guidelines and were approved by the Regierungspräsidium Darmstadt. All procedures on human tissue were performed with the approval of the Medical Ethical Committee of the VU University Medical Centre, and in accordance with Dutch license procedures and the declaration of Helsinki.
Brain Slice Preparation and Patch-Clamp Recordings
Healthy human brain tissue was obtained from anterior medial temporal cortex with written informed consent of patients undergoing surgical treatment (10 male, 3 female, age range 19–52 years, age 36.6 ± 2.9 years). Experimental mice (85 ± 5 days, age range 40–162 days) of both sexes were sacrificed by decapitation under anesthesia. Brain slices were prepared using standard procedures. Whole-cell recordings were performed at 31°C–34°C, and agonists were pressure-applied to the soma of the recorded neuron.
Analysis of Intrinsic Properties and Interneuron Subtypes
Neurons were subjected to current steps (500 ms, −100 to 250 pA), and passive and active properties were determined from the resulting voltage traces. Unsupervised clustering was performed on ranked data using Ward’s method.
In Situ Hybridization and Immunohistochemistry
In situ hybridization and immunohistochemistry was performed using fluorescent labeling in mouse tissue and chromogenic staining in human cortex according to standard procedures. Cells were detected and their location relative to the pia mater was determined.
Statistics
Datasets were tested for normality (Kolmogorov-Smirnov test) and subjected to the tests indicated in the text. A result was considered significant when the p value was lower than 0.05. Since effect size was unknown, sample size could not be pre-specified. No randomization procedure or blinding of experimenter was used in the experimental design.
Acknowledgments
We thank all members of the Letzkus lab, Erin Schuman, Maria Sol Fustinana Gueler, Julijana Gjorgjieva, and members of the FENS Kavli Network of Excellence for comments and discussions, Or Shahar for help with in situ hybridization, Ioannis Kramvis for help with human slicing, Brigitte Sinke and Hans Lodder for outstanding technical assistance, Florian Vollrath for expert help with image processing, Friedrich Kretschmer and Georgi Tushev for assistance with programming and data analysis, and Julia Kuhl for artwork. This work was supported by the Max Planck Society, the European Research Council (StG 335587 to J.J.L.), the German Research Foundation (CRC 1193 – B02 to J.J.L.), the Minna James Heineman Foundation (to J.J.L.), and the Netherlands Organization for Scientific Research (NWO Rubicon, 825.13.015 to R.B.P.).
Author Contributions
R.B.P. and J.J.L. initiated the project, R.B.P. performed most experiments, K.M., M.W., A.W., and M.B.V. performed experiments, R.B.P., S.J., and J.J.L. performed data analyses. R.B.P. and J.J.L. conceived the project and wrote the manuscript with input from H.D.M. All authors contributed to the experimental design and interpretation and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.03.111.
Supplemental Information
References
- Alitto H.J., Dan Y. Cell-type-specific modulation of neocortical activity by basal forebrain input. Front. Syst. Neurosci. 2013;6:79. doi: 10.3389/fnsys.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M., Pereira E.F., Eisenberg H.M., Albuquerque E.X. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. J. Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo S., Bennett C., Aziz D., Brown S.P., Hestrin S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons. J. Neurosci. 2012;32:3859–3864. doi: 10.1523/JNEUROSCI.0115-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo S., Bennett C., Hestrin S. Nicotinic modulation of cortical circuits. Front. Neural Circuits. 2014;8:30. doi: 10.3389/fncir.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C., Arroyo S., Berns D., Hestrin S. Mechanisms generating dual-component nicotinic EPSCs in cortical interneurons. J. Neurosci. 2012;32:17287–17296. doi: 10.1523/JNEUROSCI.3565-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauller L. Layer I of primary sensory neocortex: Where top-down converges upon bottom-up. Behav. Brain Res. 1995;71:163–170. doi: 10.1016/0166-4328(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Chameau P., van Hooft J.A. Serotonin 5-HT(3) receptors in the central nervous system. Cell Tissue Res. 2006;326:573–581. doi: 10.1007/s00441-006-0255-8. [DOI] [PubMed] [Google Scholar]
- Christophe E., Roebuck A., Staiger J.F., Lavery D.J., Charpak S., Audinat E. Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J. Neurophysiol. 2002;88:1318–1327. doi: 10.1152/jn.2002.88.3.1318. [DOI] [PubMed] [Google Scholar]
- Chu Z., Galarreta M., Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J. Neurosci. 2003;23:96–102. doi: 10.1523/JNEUROSCI.23-01-00096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M., Marubio L.M., Klink R., Changeux J.P. Nicotinic receptor function: New perspectives from knockout mice. Trends Pharmacol. Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- Cruikshank S.J., Ahmed O.J., Stevens T.R., Patrick S.L., Gonzalez A.N., Elmaleh M., Connors B.W. Thalamic control of layer 1 circuits in prefrontal cortex. J. Neurosci. 2012;32:17813–17823. doi: 10.1523/JNEUROSCI.3231-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J., Alonso-Nanclares L., Arellano J.I. Microstructure of the neocortex: Comparative aspects. J. Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- Douglas R.J., Martin K.A. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Eyal G., Verhoog M.B., Testa-Silva G., Deitcher Y., Lodder J.C., Benavides-Piccione R., Morales J., DeFelipe J., de Kock C.P., Mansvelder H.D., Segev I. Unique membrane properties and enhanced signal processing in human neocortical neurons. eLife. 2016;5:1–18. doi: 10.7554/eLife.16553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Férézou I., Cauli B., Hill E.L., Rossier J., Hamel E., Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J. Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Tucciarone J.M., Espinosa J.S., Sheng N., Darcy D.P., Nicoll R.A., Huang Z.J., Stryker M.P. A cortical circuit for gain control by behavioral state. Cell. 2014;156:1139–1152. doi: 10.1016/j.cell.2014.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Zheng C., Doughty M.L., Losos K., Didkovsky N., Schambra U.B., Nowak N.J., Joyner A., Leblanc G., Hatten M.E., Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Ibrahim L.A., Mesik L., Ji X.Y., Fang Q., Li H.F., Li Y.T., Zingg B., Zhang L.I., Tao H.W. Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron. 2016;89:1031–1045. doi: 10.1016/j.neuron.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Zhu J., Liu Y., Yang M., Tian C., Jiang S., Wang Y., Guo H., Wang K., Shu Y. Enhancement of asynchronous release from fast-spiking interneuron in human and rat epileptic neocortex. PLoS Biol. 2012;10:e1001324. doi: 10.1371/journal.pbio.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang G., Lee A.J., Stornetta R.L., Zhu J.J. The organization of two new cortical interneuronal circuits. Nat. Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Shen S., Cadwell C.R., Berens P., Sinz F., Ecker A.S., Patel S., Tolias A.S. Principles of connectivity among morphologically defined cell types in adult neocortex. Science. 2015;350:aac9462. doi: 10.1126/science.aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A., Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla K.V., Gill J.V., Lindsay G.W., Papadoyannis E.S., Field R.E., Sten T.A., Miller K.D., Froemke R.C. Parallel processing by cortical inhibition enables context-dependent behavior. Nat. Neurosci. 2017;20:62–71. doi: 10.1038/nn.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Hjerling-Leffler J., Zagha E., Fishell G., Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus J.J., Wolff S.B., Meyer E.M., Tovote P., Courtin J., Herry C., Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. [DOI] [PubMed] [Google Scholar]
- Letzkus J.J., Wolff S.B., Lüthi A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron. 2015;88:264–276. doi: 10.1016/j.neuron.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Levin E.D. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem. Pharmacol. 2013;86:1145–1152. doi: 10.1016/j.bcp.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Nelson S.B., Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oláh S., Komlósi G., Szabadics J., Varga C., Tóth E., Barzó P., Tamás G. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex. Front. Neural Circuits. 2007;1:4. doi: 10.3389/neuro.04.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L.M., Schulz J.M., Murphy S.C., Ledergerber D., Murayama M., Larkum M.E. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 2012;335:989–993. doi: 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- Pi H.J., Hangya B., Kvitsiani D., Sanders J.I., Huang Z.J., Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis R.B., Enke L., Letzkus J.J. Cholinergic circuit modulation through differential recruitment of neocortical interneuron types during behaviour. J. Physiol. 2014;592:4155–4164. doi: 10.1113/jphysiol.2014.273862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva N.V., Zaitsev A.V., Kröner S., Krimer O.A., Rotaru D.C., Gonzalez-Burgos G., Lewis D.A., Krimer L.S. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J. Neurophysiol. 2007;97:1030–1039. doi: 10.1152/jn.00794.2006. [DOI] [PubMed] [Google Scholar]
- Prönneke A., Scheuer B., Wagener R.J., Möck M., Witte M., Staiger J.F. Characterizing VIP neurons in the barrel cortex of VIPcre/tdTomato mice reveals layer-specific differences. Cereb. Cortex. 2015;25:4854–4868. doi: 10.1093/cercor/bhv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihver W., Gillberg P.G., Nordberg A. Laminar distribution of nicotinic receptor subtypes in human cerebral cortex as determined by [3H](-)nicotine, [3H]cytisine and [3H]epibatidine in vitro autoradiography. Neuroscience. 1998;85:1121–1133. doi: 10.1016/s0306-4522(97)00652-0. [DOI] [PubMed] [Google Scholar]
- Silberberg G. Polysynaptic subcircuits in the neocortex: Spatial and temporal diversity. Curr. Opin. Neurobiol. 2008;18:332–337. doi: 10.1016/j.conb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Smiley J.F., Morrell F., Mesulam M.M. Cholinergic synapses in human cerebral cortex: An ultrastructural study in serial sections. Exp. Neurol. 1997;144:361–368. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Szegedi V., Paizs M., Csakvari E., Molnar G., Barzo P., Tamas G., Lamsa K. Plasticity in single axon glutamatergic connection to GABAergic interneurons regulates complex events in the human neocortex. PLoS Biol. 2016 doi: 10.1371/journal.pbio.2000237. Published online November 9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B., Menon V., Nguyen T.N., Kim T.K., Jarsky T., Yao Z., Levi B., Gray L.T., Sorensen S.A., Dolbeare T. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat. Neurosci. 2016;19:335–346. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa-Silva G., Verhoog M.B., Linaro D., de Kock C.P., Baayen J.C., Meredith R.M., De Zeeuw C.I., Giugliano M., Mansvelder H.D. High bandwidth synaptic communication and frequency tracking in human neocortex. PLoS Biol. 2014 doi: 10.1371/journal.pbio.1002007. Published online November 25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier S., Evrard B., Vignal J.P., Scarabin J.M., Chauvel P. The serotonergic innervation of the cerebral cortex in man and its changes in focal cortical dysplasia. Epilepsy Res. 1996;25:79–106. doi: 10.1016/0920-1211(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Verhoog M.B., Goriounova N.A., Obermayer J., Stroeder J., Hjorth J.J., Testa-Silva G., Baayen J.C., de Kock C.P., Meredith R.M., Mansvelder H.D. Mechanisms underlying the rules for associative plasticity at adult human neocortical synapses. J. Neurosci. 2013;33:17197–17208. doi: 10.1523/JNEUROSCI.3158-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoog M.B., Obermayer J., Kortleven C.A., Wilbers R., Wester J., Baayen J.C., De Kock C.P., Meredith R.M., Mansvelder H.D. Layer-specific cholinergic control of human and mouse cortical synaptic plasticity. Nat. Commun. 2016;7:12826. doi: 10.1038/ncomms12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J.M., Cockcroft V.B., Lunt G.G., Smillie F.S., Wonnacott S. Methyllycaconitine: A selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- Wester J.C., McBain C.J. Behavioral state-dependent modulation of distinct interneuron subtypes and consequences for circuit function. Curr. Opin. Neurobiol. 2014;29:118–125. doi: 10.1016/j.conb.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozny C., Williams S.R. Specificity of synaptic connectivity between layer 1 inhibitory interneurons and layer 2/3 pyramidal neurons in the rat neocortex. Cereb. Cortex. 2011;21:1818–1826. doi: 10.1093/cercor/bhq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Roby K.D., Callaway E.M. Immunochemical characterization of inhibitory mouse cortical neurons: Three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.M., Hablitz J.J. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J. Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Zhu J.J. Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J. Neurosci. 2004;24:1272–1279. doi: 10.1523/JNEUROSCI.4805-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.