Abstract
Chronic Granulomatous Disease is one of the classic primary immunodeficiencies of childhood. While the incidence and severity of bacterial and fungal infections have been greatly reduced in this patient population, much remains to be learned about the pathophysiology of the disease, particularly for autoinflammatory manifestations. In this review, we examine the epidemiology, pathophysiology, and genetic basis for CGD.
Chronic granulomatous disease (CGD) was first identified in the 1950s in a 12-month-old Minnesotan child who presented with a constellation of findings, including chronic suppurative lymphadenitis, hepatosplenomegaly, pulmonary infiltrates, and eczematoid dermatitis. In the classic 1959 description of “fatal granulomatous disease of childhood,” Bridges et al reported that “no satisfactory therapy has been found, however, and the disease has relentlessly progressed through severe debilitation to ultimate death over a period of several years.” [1] Much has changed since 1959; through a combination of antibacterial, antifungal, and immunomodulatory prophylaxis, the outlook for many patients with CGD is much greater, whether through lifelong prophylaxis or bone marrow transplantation.
In this review, we briefly discuss the epidemiology, genetics, and pathophysiology of CGD; we focus first on the genetic mutations responsible for the disease and then on the pathophysiologic ramifications of these mutations. Three specific themes will emerge. First, the type of mutation partially determines the range of symptoms experienced by patients. Second, residual nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity is a strong predictor of subsequent CGD-related complications. Third, although the primary defect in patients with CGD leads to impairment of the cellular respiratory burst, additional downstream physiologic effects seem to have profound influences on morbidity and death.
EPIDEMIOLOGY AND CLINICAL IMPACT
CGD is one of the classic primary immunodeficiencies of childhood; the disease is diagnosed in most children in the first 1 to 3 years of life. Although it varies according to ethnicity, the estimated incidence of CGD is 1 in ~200000 live births [2]. Given the annual US birth cohort of nearly 4 million infants, approximately 20 children each year are born with CGD. By comparison, severe combined immunodeficiency occurs in approximately 40 live births per year in the United States. Estimates of the incidence of CGD in Europe and Asia are similar, although some populations are affected more often, including the Arab population of Israel, in which the incidence is estimated to be 1.5 per 100000 live births [3]. As is discussed later, males are affected more commonly than females (~2:1) because of the predominant mode of genetic transmission.
Children typically present with acute bacterial infection, such as suppurative lymphadenitis (classically caused by Serratia marcescens infection), recurrent staphylococcal infection, failure to thrive, or recurrent lower respiratory tract infection [4]. The disease also is diagnosed in a proportion of children as a result of noninfectious complications, such as very-early-onset inflammatory bowel disease. The incidences of infectious and noninfectious complications in patients with CGD are described elsewhere in this supplement. Survival rates have increased steadily over time. In the 1960s, the mortality rate was >60% by 7 years of age, whereas data published in the last 10 years suggest that at least 50% of these patients remain alive ≥25 years after diagnosis. The diagnostic and prophylactic measures that contribute to this increased survival rate are also described elsewhere in this supplement.
GENETIC FEATURES
Any pathologic mutation within the 5 genes that encode the subunits of the NADPH oxidase system (also known as NOX2) can result in CGD. The most common cause of CGD is a defect in the CYBB gene (gp91phox), located on the short arm of the X chromosome (Xp21.1-p11.4). Multiple types of mutations of the CYBB gene (eg, deletion, frameshift, nonsense, missense, and splice-site mutations) can lead to either absent or reduced production of gp91phox. CYBB-related CGD is inherited in an X-linked recessive manner; however, it is estimated that 10% to 15% of gp91phox mutations are the result of new germline mutations [5].
Although the genetic basis for CGD in nearly two-thirds of patients with the disease lies in the CYBB locus, approximately 25% of patients in Europe and North America carry biallelic mutations (typically a premature stop codon) in neutrophil cytosolic factor 1 (NCF1, 7q11.23), which results in impairment in the p47phox component of the NADPH oxidase system [5]. Genetic variability in NCF1 has been found also in patients with an unrelated autoinflammatory process such as systemic lupus erythematosus or Sjogren syndrome [6]. NCF1-related CGD is inherited in an autosomal recessive fashion; in this scenario, typically both parents are asymptomatic carriers of a single NCF1 mutation [5].
Mutations in CYBB (a membrane-bound component of cytochrome b558) and NCF1 (a cytosolic factor) account for 90% of CGD cases in Europe and North America. The remaining cases, also inherited in an autosomal recessive manner, include genetic defects in any of a second membrane-bound component, p22phox (CYBA, 16q24.3), or in 2 additional cytosolic components, p67phox (NCF2) and p40phox (NCF4), which represent <5% of CGD cases [5].
Males with 1 mutation in CYBB or biallelic mutations in CYBA or NCF1, NCF2, or NCF4 result in clinical manifestations of CGD. In addition, female carriers of a single CYBB mutation have impaired NADPH oxidase activity in a portion of phagocytes, most commonly from 20% to 80%, as a result of variation in inactivation of the X chromosome (lyonization) that possesses the CYBB mutation [7]. This active carrier state can lead to a range of symptoms, including aphthous ulcers, arthralgias, and cutaneous photosensitivity [4]. In a recent study of 162 X-linked CGD carriers, Marciano et al [8] found that infectious complications were highly likely in those with <10% dihydrorhodamine 123 (DHR) oxidase activity. Even patients with 10% to 20% DHR activity remained at a substantially higher risk of infection. For this reason, it might be prudent to manage CGD carriers in much the same way as those with CGD when their DHR activity falls below 20% [8].
Additional complications can arise when the Xp21.1 gene (CYBB) region possesses a more substantial gene deletion. Deletions large enough to span both the CYBB gene and the Kell locus (XK gene) lead to a contiguous gene-deletion syndrome [9]. Mutations in this region are associated with McLeod syndrome, an X-linked recessive disorder characterized by abnormalities in both the neuromuscular and hematopoietic systems, which lead to hemolytic anemia with acanthocytosis and chorea (ie, neuroacanthocytosis). In addition, the gene loci for Duchenne muscular dystrophy and retinitis pigmentosa are near the CYBB locus [9]; therefore, mutations in this region should prompt clinicians to consider CGD, as a result of a contiguous gene defect, particularly if clinical symptoms referable to CGD are present (e.g., colitis or frequent infections).
PATHOPHYSIOLOGY: THE NADPH OXIDASE SYSTEM
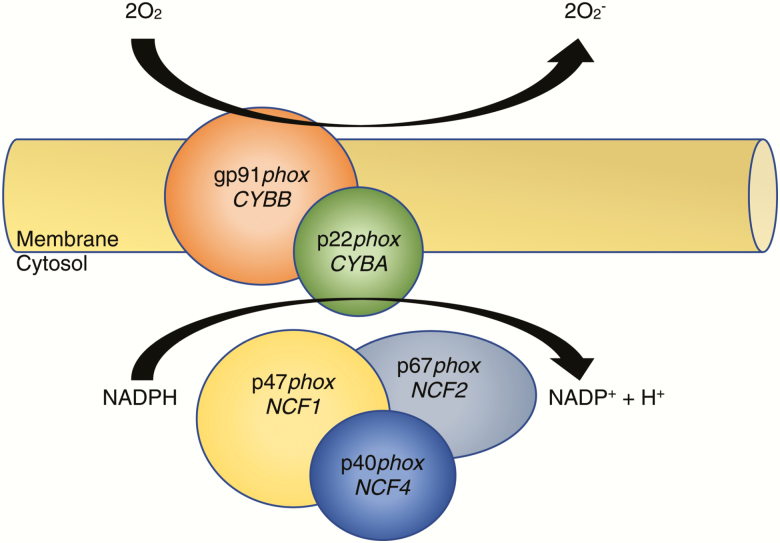
The term “respiratory burst” was coined initially by Baldridge and Gerrard in 1933 [10] after discovering that neutrophils consume large quantities of oxygen during phagocytosis. Initially thought to be related to the mitochondrial oxidative phosphorylation pathway, the mechanism of respiratory burst was characterized distinctly when classic mitochondrial inhibitors (eg, cyanide) left the process unaffected [11, 12]. Additional insights came when neutrophils were found to have the capacity to engulf bacteria but had impaired killing ability in the absence of oxygen [12]. The critical underlying molecular component responsible for respiratory burst was finally discovered to be a membrane-bound flavocytochrome with an absorbance maximum near 558 nm, and thus it was named “flavocytochrome b558” [13, 14]. Flavocytochrome b558 is a heterodimer that stoichiometrically comprises 1 p22phox and 1 gp91phox molecule (Figure 1) [15, 16]. This component is situated primarily within the membranes of specific granules (~85%), and a smaller percentage is localized to the cell’s plasma and secretory granule membranes [17, 18].
Figure 1.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system. Ninety percent of patients with chronic granulomatous disease carry mutations in either CYBB (gp91phox) or NCF1 (p47phox). Mutations in either the membrane or cytosolic domain lead to a disruption of respiratory burst in phagocytes.
The full NADPH oxidase system comprises the membrane-bound heterodimer (p22phox + p91phox) and 3 cytosolic subunits, p47phox (NCF1), p67phox (NCF2), and p40phox (NCF4) (Figure 1). After activation, cytosolic members translocate to the membrane-bound portion and assemble a functional 5-component oxidase complex [19, 20]. Activated NADPH oxidase forms a unique electron-transport chain that can generate microbicidal superoxide anion and other killing agents, such as hypochlorous acid, after electron transfer from NADPH to 2 molecules of O2 [21, 22]. A fully competent NADPH oxidase complex also depends on association of the neutrophil cytosol-derived small G-protein Rac2, which binds guanosine triphosphate and enables oxidase activation and superoxide formation [23].
The deficiency in NADPH oxidase function results in a surprisingly narrow spectrum of microbial susceptibility [24]. This biochemical perturbation relates, in part, to impaired killing of specific catalase-positive organisms with superoxide anion and other reactive oxygen species by neutrophils, monocytes, macrophages, and eosinophils. Catalase-positive organisms (eg, Staphylococcus aureus, Pseudomonas, Aspergillus fumigatus, Candida albicans, and Enterobacteriaceae such as Klebsiella spp and Serratia spp) can degrade host-produced hydrogen peroxide before its ultimate conversion to hypochlorous acid by myeloperoxidase. A striking example of disparate susceptibilities in different types of CGD was demonstrated by Kaplan et al [25]. In their elegant description, primary cells of patients with CGD did not kill S aureus, but killing of Streptococcus strains was preserved. Patients with CGD also experience a wide range of impairment in their ability to generate phagocyte-derived reactive oxygen intermediates (ROIs), typically ranging from undetectable (0%) to <30%. As a result, patients with a less severe mutation in NADPH oxidase–encoded genes have greater residual ROI production and less severe disease [26]. An additional important insight about the pathophysiology of this disease came when Quie et al [27] found that phagocytosis and intracellular killing are uncoupled in patients with CGD; only intracellular killing is affected. Although many aerobic organisms produce catalase, a relatively small group of catalase-positive organisms play a significant role in CGD morbidity and mortality. However, catalase is not the only critical virulence factor for organisms that disproportionately affect patients wtih CGD; the genetic deletion of catalase in either S aureus or Aspergillus nidulans does not appear to alter virulence in vitro or in vivo [28, 29].
In addition to having direct cytotoxic effects, the production of reactive oxygen species seems to have importance for other innate immune functions. For example, patients with CGD display reduced expression and function of Toll-like receptors, complement receptors, and chemokine receptors that correlate with disease severity [30]. In addition, neutrophils also use extracellular means of microbicidal activity through the use of neutrophil extracellular traps [31]. This mechanism depends on normal ROI production by NADPH oxidase and is dysfunctional in patients with CGD [31, 32]. Moreover, recent work by Marzaioli et al [33] found that monocyte–dendritic cell differentiation and maturation are also affected by disturbances in the NADPH oxidase system. NOX5 and p22phox, but not gp91phox/NOX2, seem to regulate the differentiation of monocytes into dendritic cells.
HYPERINFLAMMATION
Inflammatory manifestations are common in patients with CGD and are noted most frequently in the gastrointestinal tract, urogenital tract, lungs, and eyes [34–36]. Because these complications are common and can precede the onset of infectious susceptibilities, it is important to consider the diagnosis of CGD in patients with atypical inflammatory disease or multisystem inflammation (eg, Behçet disease). Important to note is that patients with X-linked CGD are twice as likely to develop inflammatory disease than those with autosomal recessive disease [37].
Granuloma formation is a classic inflammatory finding in patients with CGD. Distinct from those related to other diseases, CGD-related granulomata typically are noncaseating, nestled within fibrotic tissues in the setting of acute or chronic inflammation [38]. In patients with CGD, granulomata have a propensity to affect hollow viscera, most notably the colon, stomach, and bladder. They do not seem to be related to infection, because microorganisms usually are not found in CGD granulomas, and the lesions generally respond to treatment with steroids or other immunomodulators such as cyclosporine [39–41].
Dysfunctional CGD neutrophils also can promote hyperinflammation because of impairment of normal inflammation-resolution mechanisms. In addition to host defense, neutrophils are important for phagocytic clearance of apoptotic cells, which are identified by external presentation of phosphatidylserine residues on their cell surface [42, 43]. This process, termed “efferocytosis,” is critical for quenching ongoing inflammation and tissue necrosis. Efferocytosis (“to take to the grave or bury”) prevents cytotoxic damage from dying cells and results in production of the antiinflammatory cytokine transforming growth factor β by macrophages [44–47]. Evidence for impaired efferocytosis exists in a murine model of CGD [48–50]. It is interesting to note that Fernandez-Byanapalli et al found that interferon γ improved both the host-defense mechanism and clearance of apoptotic cells in a mouse model of CGD [48].
CONCLUSIONS
Although the molecular defects responsible for CGD are quite straightforward, the immunologic and inflammatory consequences of reduced NADPH oxidase activity are profound and can lead to life-threatening infections and life-altering autoinflammatory symptoms, even in some asymptomatic carriers. Future work focused on the pathophysiology of CGD will most certainly benefit from the use of novel tools, such as the CRISPR-Cas9 genome-editing tool [51], to explore additional immunologic manifestations of NADPH oxidase defects and the development of new therapeutic targets.
Notes
Supplement sponsorship. This article appears as part of the supplement “Chronic Granulomatous Disease,” sponsored by Horizon Pharma USA, Inc.
Potential conflicts of interest. N. L. R. has received grant funding from the Jeffrey Modell Foundation and is a contributor with paid royalties to UpToDate, and C. B. C. has received grant funding from Novartis, GSK, Merck, and Horizon, is a consultant for Theravance, GSK, Horizon, and Premier, and has received royalties from UpToDate. M. B. J.: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bridges RA, Berendes H, Good RA. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child 1959; 97:387–408. [PubMed] [Google Scholar]
- 2. Kuhns DB, Alvord WG, Heller T et al. . Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010; 363:2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolach B, Gavrieli R, de Boer M et al. . Chronic granulomatous disease: clinical, functional, molecular, and genetic studies. The Israeli experience with 84 patients. Am J Hematol 2017; 92:28–36. [DOI] [PubMed] [Google Scholar]
- 4. Thomsen IP, Smith MA, Holland SM, Creech CB. A comprehensive approach to the management of children and adults with chronic granulomatous disease. J Allergy Clin Immunol Pract 2016; 4:1082–8. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman R. Hematology: Basic Principles and Practice. 6th ed, Philadelphia: Saunders/Elsevier; 2013: xxxi:2343. [Google Scholar]
- 6. Zhao J, Ma J, Deng Y et al. . A missense variant in NCF1 is associated with susceptibility to multiple autoimmune diseases. Nat Genet 2017; 49:433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battersby AC, Cale AM, Goldblatt D, Gennery AR. Clinical manifestations of disease in X-linked carriers of chronic granulomatous disease. J Clin Immunol 2013; 33:1276–84. [DOI] [PubMed] [Google Scholar]
- 8. Marciano BE, Zerbe CS, Falcone EL et al. . X-linked carriers of chronic granulomatous disease: illness, lyonization, and stability. J Allergy Clin Immunol 2018; 141:365–71. [DOI] [PubMed] [Google Scholar]
- 9. Watkins CE, Litchfield J, Song E et al. . Chronic granulomatous disease, the McLeod phenotype and the contiguous gene deletion syndrome—a review. Clin Mol Allergy 2011; 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldridge CW, Gerard RW. The extra respiration of phagocytosis. Am J Physiol 1933; 103:235–6. [Google Scholar]
- 11. Sbarra AJ, Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem 1959; 234:1355–62. [PubMed] [Google Scholar]
- 12. Selvaraj RJ, Sbarra AJ. The role of the phagocyte in host-parasite interactions. VII. Di- and triphosphopyridine nucleotide kinetics during phagocytosis. Biochim Biophys Acta 1967; 141:243–9. [DOI] [PubMed] [Google Scholar]
- 13. Cross AR, Rae J, Curnutte JT. Cytochrome b-245 of the neutrophil superoxide-generating system contains two nonidentical hemes. Potentiometric studies of a mutant form of gp91phox. J Biol Chem 1995; 270:17075–7. [DOI] [PubMed] [Google Scholar]
- 14. Segal AW, West I, Wientjes F et al. . Cytochrome b-245 is a flavocytochrome containing FAD and the NADPH-binding site of the microbicidal oxidase of phagocytes. Biochem J 1992; 284(pt 3):781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dinauer MC, Orkin SH, Brown R et al. . The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 1987; 327:717–20. [DOI] [PubMed] [Google Scholar]
- 16. Dinauer MC, Pierce EA, Bruns GA et al. . Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest 1990; 86:1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997; 89:3503–21. [PubMed] [Google Scholar]
- 18. Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol 1983; 97:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curnutte JT, Berkow RL, Roberts RL et al. . Chronic granulomatous disease due to a defect in the cytosolic factor required for nicotinamide adenine dinucleotide phosphate oxidase activation. J Clin Invest 1988; 81:606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curnutte JT, Scott PJ, Mayo LA. Cytosolic components of the respiratory burst oxidase: resolution of four components, two of which are missing in complementing types of chronic granulomatous disease. Proc Natl Acad Sci U S A 1989; 86:825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun 1996; 64:3512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosen H, Klebanoff SJ. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med 1979; 149:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abo A, Webb MR, Grogan A, Segal AW. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem J 1994; 298 (pt 3):585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lazarus GM, Neu HC. Agents responsible for infection in chronic granulomatous disease of childhood. J Pediatr 1975; 86:415–7. [DOI] [PubMed] [Google Scholar]
- 25. Kaplan EL, Laxdal T, Quie PG. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics 1968; 41:591–9. [PubMed] [Google Scholar]
- 26. Kuhns DB, Alvord WG, Heller T et al. . Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010; 363:2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quie PG, White JG, Holmes B, Good RA. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest 1967; 46:668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Messina CG, Reeves EP, Roes J, Segal AW. Catalase negative Staphylococcus aureus retain virulence in mouse model of chronic granulomatous disease. FEBS Lett 2002; 518:107–10. [DOI] [PubMed] [Google Scholar]
- 29. Chang YC, Segal BH, Holland SM et al. . Virulence of catalase-deficient Aspergillus nidulans in p47(phox)−/− mice. Implications for fungal pathogenicity and host defense in chronic granulomatous disease. J Clin Invest 1998; 101:1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hartl D, Lehmann N, Hoffmann F et al. . Dysregulation of innate immune receptors on neutrophils in chronic granulomatous disease. J Allergy Clin Immunol 2008; 121:375–382.e9. [DOI] [PubMed] [Google Scholar]
- 31. Brinkmann V, Reichard U, Goosmann C et al. . Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 32. Fuchs TA, Abed U, Goosmann C et al. . Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marzaioli V, Hurtado-Nedelec M, Pintard C et al. . NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood 2017; 130:1734–45. [DOI] [PubMed] [Google Scholar]
- 34. Marciano BE, Rosenzweig SD, Kleiner DE et al. . Gastrointestinal involvement in chronic granulomatous disease. Pediatrics 2004; 114:462–8. [DOI] [PubMed] [Google Scholar]
- 35. Barese CN, Podestá M, Litvak E et al. . Recurrent eosinophilic cystitis in a child with chronic granulomatous disease. J Pediatr Hematol Oncol 2004; 26:209–12. [DOI] [PubMed] [Google Scholar]
- 36. Mahdaviani SA, Mohajerani SA, Rezaei N et al. . Pulmonary manifestations of chronic granulomatous disease. Expert Rev Clin Immunol 2013; 9:153–60. [DOI] [PubMed] [Google Scholar]
- 37. Magnani A, Brosselin P, Beauté J et al. . Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J Allergy Clin Immunol 2014; 134:655–662.e8. [DOI] [PubMed] [Google Scholar]
- 38. Schäppi MG, Jaquet V, Belli DC, Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol 2008; 30: 255–71. [DOI] [PubMed] [Google Scholar]
- 39. Chin TW, Stiehm ER, Falloon J, Gallin JI. Corticosteroids in treatment of obstructive lesions of chronic granulomatous disease. J Pediatr 1987; 111:349–52. [DOI] [PubMed] [Google Scholar]
- 40. Barton LL, Moussa SL, Villar RG, Hulett RL. Gastrointestinal complications of chronic granulomatous disease: case report and literature review. Clin Pediatr (Phila) 1998; 37:231–6. [DOI] [PubMed] [Google Scholar]
- 41. Rosh JR, Tang HB, Mayer L et al. . Treatment of intractable gastrointestinal manifestations of chronic granulomatous disease with cyclosporine. J Pediatr 1995; 126:143–5. [DOI] [PubMed] [Google Scholar]
- 42. Gardai SJ, McPhillips KA, Frasch SC et al. . Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005; 123:321–34. [DOI] [PubMed] [Google Scholar]
- 43. Hoffmann PR, deCathelineau AM, Ogden CA et al. . Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol 2001; 155:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays Biochem 2003; 39:105–17. [DOI] [PubMed] [Google Scholar]
- 45. Henson PM. Dampening inflammation. Nat Immunol 2005; 6:1179–81. [DOI] [PubMed] [Google Scholar]
- 46. Gong D, Shi W, Yi SJ et al. . TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol 2012; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freire-de-Lima CG, Xiao YQ, Gardai SJ et al. . Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 2006; 281:38376–84. [DOI] [PubMed] [Google Scholar]
- 48. Fernandez-Boyanapalli R, McPhillips KA, Frasch SC et al. . Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-γ in a nitric oxide-dependent manner. J Immunol 2010; 185:4030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fernandez-Boyanapalli R, Frasch SC, Riches DW et al. . PPARγ activation normalizes resolution of acute sterile inflammation in murine chronic granulomatous disease. Blood 2010; 116:4512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fernandez-Boyanapalli RF, Frasch SC, McPhillips K et al. . Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 2009; 113:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Ravin SS, Li L, Wu X et al. . CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci Transl Med 2017; 9. [DOI] [PubMed] [Google Scholar]