Abstract
Background
We tested the association of biologic age (BA) measures constructed from different types of biomarkers with mortality and disease in a community-based sample.
Methods
In Framingham Offspring participants at Exams 7 (1998–2001, mean age 62 ± 10) and 8 (2005–2008, mean age 67 ± 9), we used the Klemera–Doubal method to estimate clinical BA and inflammatory BA and computed the difference (∆age) between BA and CA. Clinical ∆age was computed at Exam 2 (1979–1983, mean age 45 ± 10). At Exam 8, we computed measures of intrinsic and extrinsic epigenetic age. Participants were followed through 2014 for outcomes. Cox proportional hazards models tested the association of each BA estimate with each outcome adjusting for covariates.
Results
Sample sizes ranged from 2532 to 3417 participants. In multivariable models, each 1-year increase in clinical ∆age at Exam 2 (hazard ratio [HR] = 1.04–1.06, p < 2 × 10–16) and clinical ∆age and inflammatory ∆age at Exam 7 significantly increased the hazards of mortality and incident cardiovascular disease (HR = 1.01–1.05, p < 2 × 10−7), whereas inflammatory ∆age increased the hazards of cancer (HR = 1.01, p < .05). At Exam 8, increased clinical ∆age, inflammatory ∆age, and extrinsic epigenetic age all significantly increased the hazard of mortality (HR = 1.03–1.05, all p < .05); clinical ∆age and inflammatory ∆age increased cardiovascular disease risk (HR = 1.04–1.05, all p < .01); and clinical ∆age increased cancer risk (HR = 1.03, p < .01) when all three BA measures were included in the model. Intrinsic epigenetic age was not significantly associated with any outcome.
Conclusions
Our findings suggest BA measures may be complementary in predicting risk for mortality and age-related disease.
Keywords: Inflammation, Epigenetics, Aging, Epidemiology
The population is aging worldwide not only due to gains in early-life survival but also due to progress with declining late-life mortality (1). Aging in humans is highly variable with wide differences in health at a given chronologic age. Some adults become frail in early old age, whereas others remain fit in their 90s and beyond (2). Understanding the biologic processes of aging and how these processes confer susceptibility to chronic disease may lead to successful interventions that delay aging and improve health span (3).
Given the complexity of the aging process, different measures of biologic aging (BA) and of successful aging have been constructed to better reflect an individual’s rate of aging by combining clinical biomarkers representative of different physiologic systems (4–7). Several of the measures have been related to risk for mortality (4,6,7) and physical and cognitive decline (5–7). Despite this body of work, there has not been a consensus on a clinical measure of BA. More recently, genomic data have been used to develop age predictors including transcriptomic and DNA methylation molecular signatures (8–10). DNA methylation age predicts mortality independent of chronologic age and other risk factors (11,12) and is associated with some age-related conditions such as brain aging (13), but not with coronary heart disease (14). It is not clear if each of these different measures of BA captures unique information or adds complementary information over and above chronological age (CA) to predict disease risk and life span.
We had the opportunity to examine a measure of clinical BA over several time points in the adult life course in addition to inflammatory and DNA methylation age constructed in later adulthood in a large community-based sample under continuous surveillance. We hypothesized that different types of BA measures (clinical, inflammatory, and genomic) make unique contributions to age-related disease risk and all-cause mortality. Furthermore, we were able to test the association of the different BA measures in the same cohort accounting for important confounders.
Methods
Study Sample
The Framingham Heart Study (FHS) is a community-based longitudinal cohort study initiated in 1948 to study determinants of cardiovascular disease (CVD) and its risk factors. In 1971, 5,124 children of the original participants and spouses of the children were enrolled into the FHS Offspring cohort (15). Offspring participants have been examined every 4–8 years, have completed nine research examinations, and remain under active surveillance for cardiovascular events, cancer, and death. The Boston University Medical Campus Institution Review Board reviews and approves the protocol for each research examination, and informed consent is obtained at every attended examination. Research examinations consist of a physician administered medical history and resting blood pressure, laboratory assessment, and various noninvasive measures of cardiovascular and lung function.
Clinical Biologic Age
Prior reports demonstrated that a combination of clinical biomarkers used to define biologic age (BA) predicted mortality better than CA (4). By definition, BA varies even in a sample of individuals all of the same CA (5). We used six clinical biomarkers representing diverse physiologic systems that were consistently available over three examinations and were used in prior reports: systolic blood pressure, forced expiratory volume at 1 s (FEV1), total cholesterol, fasting glucose, C-reactive protein, and serum creatinine. We chose examinations to evaluate the change in clinical BA from midlife (Examination 2: 1979–1983, mean age 44 years) to later adulthood (Examination 7: 1998–2001, mean age 62 years) and to examine the relationship of clinical BA to other measures of BA available at later examinations (inflammatory BA available at Exams 7 and 8; DNA methylation BA available at Exam 8: 2005–2008, mean age 67 years). Participants were excluded from the clinical BA sample at a given examination if any of the six biomarkers were missing (16%–28% of attendees). FEV1 was the biomarker resulting in missing values 95%–98% of the time. In additional, at Exam 8, we excluded 13 participants due to missing covariates.
Inflammatory Biologic Age
We chose to create an inflammatory BA phenotype because inflammation plays a central role in aging and development of age-related disease (16). We selected inflammatory biomarkers measured at Examinations 7 and 8. The markers function across the inflammation process including acute phase reactants, chemokines, cytokines, selectins, and cell adhesion molecules (Table 1) (17). The samples were obtained fasting, and the details of the assays and measurements have been previously reported with intra- and inter-assay coefficients of variation less than 10% (18). Participants were excluded from the inflammatory BA sample at a given examination if they did not provide a fasting morning sample or if any of the biomarkers were missing (9%–11% of exam attendees). At Exam 8, a further 16 participants were excluded do to missing covariates.
Table 1.
Characteristics of Clinical and Inflammatory Biologic Age Study Samples at Exam 7
| Clinical Biologic Age Sample | N = 2,532 | Inflammatory Biologic Age Sample | N = 3,134 |
|---|---|---|---|
| Chronological age (y) | 61 (9.3) | Chronological age (y) | 62 (9.5) |
| Sex, female | 55% | Sex, female | 53% |
| Clinical BA | 61.0 (11.7) | Inflammatory BA | 61.5 (12.9) |
| ΔAge | 0.0 (7.0) | ΔAge | −0.1 (8.8) |
| Clinical Variablesa | Inflammatory Marker Variablesb | ||
| Systolic blood pressure (mm Hg) | 129 (20) | C-reactive protein (mg/L) | 2.2 (1.0, 5.1) |
| Forced expiratory volume at 1 s (L) | 2.7 (0.8) | Monocyte chemoattractant protein-1 (pg/mL) | 313 (254, 382) |
| Total cholesterol (mg/dL) | 200 (37) | Osteoprotegerin (pmol/L) | 5.4 (4.4, 6.5) |
| Glucose (mg/dL) | 104 (27) | P-selectin (ng/mL) | 36 (29, 45) |
| C-reactive protein (mg/L) | 4.2 (7.4) | Intercellular adhesion molecule 1 (ng/mL) | 242 (211, 283) |
| Creatinine (mg/100 mL) | 1.1 (0.2) | Interleukin-6 (pg/mL) | 2.7 (1.8, 4.3) |
| LP-PLA2 mass (ng/mL) | 288 (230, 361) | ||
| LP-PLA2 activity (nmol/mL/min) | 141 (119, 165) | ||
| Tumor necrosis factor receptor II (pg/mL) | 1,977 (1,666, 2,418) | ||
| Covariatesa | Covariatesa | ||
| Current smoking | 12 | Current smoking | 13 |
| Diabetes | 12 | Diabetes | 13 |
| Hypertension treatment | 32 | Hypertension treatment | 34 |
| Lipid treatment | 20 | Lipid treatment | 21 |
| Prevalent CVD | 5 | Prevalent CVD | 6 |
| Prevalent cancer | 8 | Prevalent cancer | 9 |
Note: BA = biologic age; CVD = cardiovascular disease; LP-PLA2 = lipoprotein-associated phospholipase A2.
aVariables are percentage or mean (SD). bVariables are median, Q1, Q3; C-reactive protein included in both study samples; intersection of clinical biological age and inflammatory biological age samples: N = 2,408.
Methylation Age
Epigenetic changes are a key hallmark of aging (19), and DNA methylation-based biomarkers often referred to as the “epigenetic clock” have been shown to be robust measures of biologic age (9,10). Recent work incorporating blood cell metrics into the epigenetic measures demonstrate significant associations with mortality (12). Therefore, we examined two epigenetic measures we reported previously (12): (a) intrinsic epigenetic age (IEAA) is the residual obtained from a multivariate regression of the Horvath epigenetic age estimate (353 CpGs) on CA and measures of blood cell counts (9) and is constructed to be independent of blood cell count changes that occur with age and (b) extrinsic epigenetic age (EEAA) defined using the Hannum epigenetic age estimate (71 CpGs) (10) and creating a weighted average of the estimate taking into account imputed blood cell types using the Klemera–Doubal approach (20). EEAA is strongly correlated with blood cell counts (12).
Blood samples collected at Examination 8 were used to extract genomic DNA and the Illumina Infinium Human Methylation 450K BeadChip (Illumina, San Diego, CA, USA) was used for DNA methylation measurement as previously reported (11,12).
Incident Events
Participants are under continuous surveillance for cardiovascular events and death. An end point committee of three senior investigators reviews all available information including hospital records, death certificates, and next-of-kin interviews to determine the date and cause of death. Cardiovascular events (coronary heart disease: including coronary insufficiency, myocardial infarction, coronary heart disease death; stroke, heart failure, and coronary or CVD death) are adjudicated by the committee using standardized criteria previously reported (21). A study neurologist adjudicates cerebrovascular outcomes (atherothrombotic infarction, cerebral embolism, intracerebral hemorrhage, subarachnoid hemorrhage, death due to stroke). Cancer cases are identified at routine research examinations and by medical history updates. The vast majority of cancers were validated with pathology reports with less than 5% of cases based on clinical diagnosis or death certificate. Non-melanoma skin cancers were excluded.
Cognitive and Physical Function at Examination 8
At Exam 8, trained technicians administered the mini-mental state examination (MMSE), a 30-point questionnaire used to measure cognitive function including the domains of orientation, attention, recall, and ability to follow simple commands. Hand grip strength was measured in kilograms using a Jamar dynamometer (Sammons Preston, Bolingbrook, IL, USA) obtaining three trials in each hand. Gait speed was measured over a 4-m course to the nearest 0.01 second, and the faster of two normal paced walks was used for analyses.
Covariates
At each research exam, participants were asked about smoking habits. Current smoking was defined as smoking one or more cigarettes per day in the year preceding the exam. Participants were asked about medication use, and lipid-lowering medications were recorded. Diabetes was defined as fasting glucose of 126 mg/dL or higher or use of oral hypoglycemic agents or insulin. Hypertension was defined as blood pressure ≥ 140/90 or use of anti-hypertensive medications.
Statistical Methods
Biologic age estimates
We used the Klemera and Doubal method (20) to compute clinical BA estimate and the inflammatory BA estimate. Compared with other methods for computing BA, the Klemera–Doubal algorithm with CA as one of the biomarkers, showed the best performances in precision of estimation (20) and predictive ability (4). The key idea of the Klemera–Doubal method is to minimize distance between BA and biomarkers in an m-dimensional space (m is number of biomarkers) and also to minimize the variability of BA estimates. This is achieved by running simple linear regressions on CA using biomarkers as outcomes. The BA variable is constructed based on parameter estimates and residuals from these simple linear regressions (20). We defined Δage for each BA measure as the BA minus CA. Thus, individuals with Δage > 0 have greater BA than their CA, whereas individuals with Δage < 0 have younger BA than their CA. Clinical and inflammatory BA were significantly correlated with CA at all exams (all p < 2.2 × 10−16); however, clinical and inflammatory ∆age measures as well as IEAA and EEAA were not correlated with CA (all p > .12).
We examined the distribution of clinical ∆age at Exam 2 by attendance at Exam 7 to determine whether greater clinical ∆age at Exam 2 was associated with attendance at the later exam and with ability to construct clinical and inflammatory BA phenotypes at Exams 7 and 8. Next, we evaluated the correlation between clinical ∆age at Exam 2 and clinical ∆age at Exam 7, nearly 20 years later. Finally, we examined the correlation between pairs of ∆age phenotypes based on the clinical and inflammatory biomarkers both across exams and at the same exam.
We constructed separate Cox proportional hazards models to examine the association of each ∆age estimate at each time point (clinical BA ∆age at Exams 2, 7, 8; inflammatory BA ∆age at Exams 7 and 8; IEAA and EEAA at Exam 8) with mortality, CVD events, and cancer. Participants were followed through 2014. Multivariable-adjusted models included CA, sex, current smoking, diabetes, hypertension treatment, lipid treatment, and for models investigating mortality, prevalent CVD and prevalent cancer. In the models examining CVD or cancer, participants with prevalent disease at the exam at which the BA was measured were excluded. Finally, to investigate whether each measure of BA contributed to event risk independently of the others, we included all BA measures in the same model.
We examined the cross-sectional association of clinical ∆age, inflammatory ∆age, IEAA, and EEAA at Exam 8 with gait speed and grip strength after adjusting for CA, sex, height, and body mass index and with MMSE score after adjusting for CA, sex, and education.
All analyses were performed in R version 3.2.3 using R packages Himsc, psych, pROC.
Results
Characteristics of the individuals included for each BA measure are shown in Table 1 for Exam 7. Sample sizes for each BA measure differ due to availability of the biomarkers used to compute the BA. Sample characteristics for Exams 2 and 8 are presented in Supplementary Tables 1 and 2. Between 51% and 55% of the individuals included were female across all exams. The mean CA of the clinical BA and inflammatory BA subsamples were similar: 61 (9.3) and 62 (9.5) years, respectively. The clinical BA sample was smaller than the inflammatory BA sample mainly due to missing data for the FEV1 component of clinical BA; the two samples were largely overlapping (n = 2,408 in both samples). ΔAge was centered at zero with a wide distribution −20 to 30 (Supplementary Figure 1). CA of the clinical BA sample was 45.0 (10.1) years at Exams 2 and 67 (8.9) years at Exam 8 for all BA samples. The distribution of the individual components of clinical BA differ over the exams in accordance with the increasing CA (eg, mean FEV1 declines and mean systolic blood pressure increases).
Clinical ∆age at Exam 2 was more favorable in participants who returned to Exams 7 and 8, whereas the distribution was shifted to larger ∆ages indicative of advanced aging for participants missing at the later exams (Supplementary Figures 1 and 2). Clinical ∆age at Exam 2 was correlated with clinical ∆age (r = .5) and inflammatory ∆age at Exam 7 (r = .2); similar correlations were observed for ∆age measures at Exam 8 (Supplementary Figures 1 and 2). The correlation between clinical ∆age at Exams 7 and 8 (r = .7) and inflammatory ∆age at Exam 7 and 8 (r = .6) is higher (Supplementary Figure 3). The correlation between clinical and inflammatory ∆age within exam was lower (r = .37 and r = .35 at Exams 7 and 8, respectively) than the correlation of clinical or inflammatory ∆age with its corresponding measure across the two exams. IEAA and EEAA at Exam 8 were not correlated with clinical ∆age or inflammatory ∆age at Exam 8.
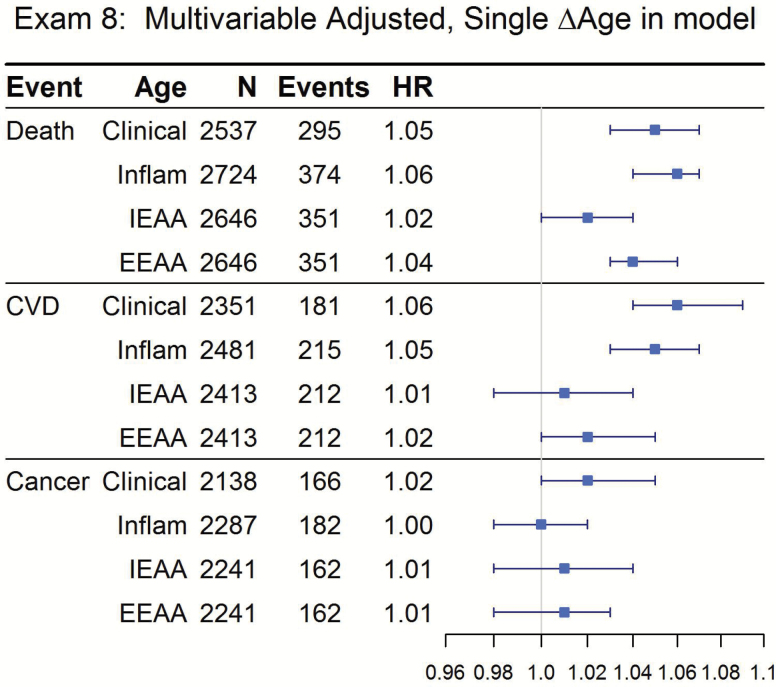
There were large numbers of outcomes for all BA measures at all exams (Figure 1, Table 2, and Supplementary Table 3). In multivariable-adjusted models, each 1-year increase in clinical ∆age and inflammatory ∆age at Exam 7 (older adulthood), significantly increased the hazards of all-cause mortality and incident CVD, whereas inflammatory ∆age also increased the hazard of cancer (Table 2; hazard ratios [HR] = 1.01–1.05, p < 2 × 10−7 except cancer p < .05). Similar increased hazards for increased clinical ∆age in models constructed in midlife (Exam 2, mean age 45 years, median follow-up >32 years) were also observed (Supplementary Table 3; HR = 1.04–1.06, p < 2 × 10−16).
Figure 1.
Incident events according to measures of Δage and epigenetic age: Framingham Offspring Study, Exam 8. Each outcome and each Δage measure is a separate model. Median follow-up time for all outcomes was ≈8 years. Models are multivariable adjusted with covariates: age, sex, current smoking, diabetes, hypertension treatment, lipid treatment, mortality only additionally adjusted for prevalent CVD and prevalent cancer.
Table 2.
Incident Events According to ∆Age: Framingham Offspring Study, Exam 7a
| Clinical ∆Age | Inflammatory ∆Age | |||||
|---|---|---|---|---|---|---|
| Events/N | HR (95% CI) | p Value | Events/N | HR (95% CI) | p Value | |
| MV Adjusted | MV Adjusted | |||||
| Mortality | 490/2,532 | 1.04 (1.03, 1.05) | 1.0 × 10−9 | 718/3,140 | 1.05 (1.04, 1.06) | <2 × 10−16 |
| CVD | 310/2,400 | 1.05 (1.03, 1.06) | 4.7 × 10−7 | 413/2,944 | 1.04 (1.03, 1.05) | 1.8 × 10−13 |
| Cancer | 411/2,329 | 1.01 (0.998, 1.03) | .08 | 510/2,859 | 1.01 (1.00, 1.02) | .014 |
Note: CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio; MV = multivariable. Median follow-up time 14.4–14.7 years across all outcomes. Covariates: age, sex, current smoking, diabetes, hypertension treatment, lipid treatment, mortality only additionally adjusted for prevalent CVD and prevalent cancer.
aEach outcome is a separate model.
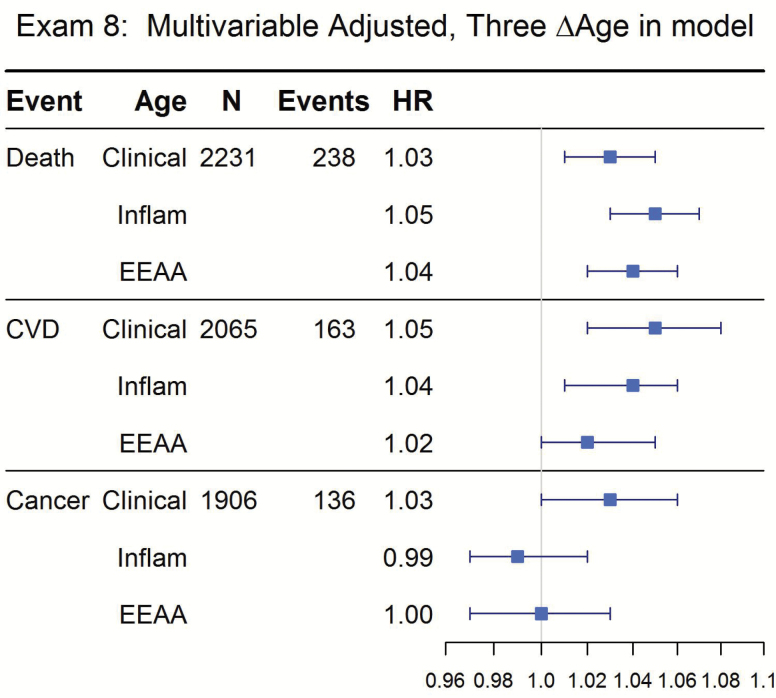
In fully adjusted models, IEAA constructed at Exam 8 was not significantly associated with any outcome (Figure 1, all p > .1). EEAA was significantly associated with mortality (p = 6.1 × 10−8) and incident CVD (p = .04) but not cancer (Figure 1). To determine whether each ∆age measure contributed to the hazard of the outcomes under investigation, all three measures were included in a single model. Increased clinical ∆age, inflammatory ∆age, and EEAA all significantly increased the hazard of mortality (HR = 1.03–1.05, all p < .05); clinical ∆age and inflammatory ∆age increased risk for CVD (HR = 1.04–1.05, all p < .01) and clinical ∆age alone increased risk for cancer (HR = 1.03, p < .01) in the multivariable model with all three measures in the model (Figure 2).
Figure 2.
Incident events according to measures of Δage and epigenetic age: Framingham Offspring Study, Exam 8. Three Δage measures in the model. Median follow-up time for all outcomes was ≈8 years. Models are multivariable adjusted with covariates: age, sex, current smoking, diabetes, hypertension treatment, lipid treatment, mortality only additionally adjusted for prevalent CVD and prevalent cancer.
We examined the cross-sectional associations of BA measures and functions defined with gait speed, hand grip strength, and mini-mental state examination score at Exam 8. Older clinical and inflammatory ∆age and EEAA was associated with slower gait speed and weaker hand grip strength (all p < .05, Supplementary Table 4). Inflammatory ∆age was also associated with lower MMSE score (p < .001). IEAA was not associated with the functional measures.
Discussion
In this large well-characterized community-based cohort followed for over 30 years, our findings of several estimates of BA in the same individuals constructed from clinical, inflammatory and methylation data are threefold. First, midlife clinical BA is correlated with measures of BA in older adult life with the exception of DNA methylation age. Accelerated aging in midlife is associated with lower attendance at later exams, a metric often associated with poorer health. Second, increased clinical and inflammatory aging, corresponding to older BA than CA, results in greater hazard of death and incident CVD across exams even after accounting for CA and important potential confounders. Third, in models that included all three BA measures, increased aging from all three remained significantly predictive of increased mortality, whereas clinical and inflammatory ∆age estimates increased risk for disease. Therefore, our findings suggest the three BA measures may be complementary in predicting risk for mortality and age-related disease. Finally, in cross-sectional analyses, all three BA measures were associated with functional measures of gait speed and grip strength.
Other clinical biomarker measures used to reflect the heterogeneity in human health span including frailty indices with large numbers of clinical and laboratory items have been developed in older adults that relate to mortality. We chose a set of clinical biomarkers reflective of diverse systems including the cardiovascular, pulmonary, metabolic, renal, and inflammatory systems that are easily measured in a clinical setting. The biomarkers represent a subset of biomarkers used in previous reports with demonstrated ability to quantify biologic age even in young adults (4,5) and are among the items in the frailty indices (22,23). Accelerated aging measured using clinical biomarkers in a birth cohort of healthy young adults before the onset of disease distinguished those with evidence of physical and cognitive decline and early signs of vascular aging (5). A similar set of clinical biomarkers measured in a nationally representative sample across midlife (ages 30–59), demonstrated greater risk of death in those with older BA (4). We confirm and extend this work by examining clinical BA at different points across the life span and testing both mortality and incident age-related diseases. Clinical BA using readily available clinical biomarkers may capture underlying physiologic reserve and represent a potentially useful approach to define physical resilience at the whole person level (24). This may be one tool to test interventions (exercise, drug therapies) to determine whether accelerated aging or low physical resilience can be ameliorated which would have important clinical implications.
Chronic low-grade levels of inflammation occur with age, a process defined as “inflamm-aging,” that is believed to accelerate biological aging (16). Multiple underlying mechanisms contribute to chronic inflammation with aging, including dysregulation of the immune system, oxidative stress, chronic infection, and so on (16). Therefore, the inflammatory markers we included in the inflammatory BA measure reflect many of these mechanisms. Older inflammatory BA is associated with risk for mortality as well as incident CVD and measures of physical function. Our results warrant replication in other independent samples. Further study of how inflammatory and other mechanisms such as epigenetics and the environment act together to accelerate or slow biologic aging are needed.
Several estimates of DNA methylation age in blood have been strongly associated with CA and shown to predict mortality (9–12). We had the opportunity to examine epigenetic measures of aging and inflammatory and clinical measures of BA in the same individuals at the same point in the adult life span. We found that EEAA was associated with all-cause mortality but not CVD and cancer. EEAA reflects both epigenetic changes and is correlated with blood cell composition (12) and may be strongly related to mortality because this measure also includes information on changes in blood cell counts with age. Although IEAA was not associated with outcomes in our study, this epigenetic measure has been associated with mortality in a larger meta-analysis that included Framingham participants (12) and with lung cancer susceptibility in women (25). IEAA is based on the Horvath epigenetic age estimate that generalizes to a broad range of tissues and cell types and is not correlated with blood cell counts. More research is needed to understand how the aging epigenome confers risk for age-related disease.
Our study has several limitations. The FHS is predominantly white; therefore, our findings may not be generalizable to other race/ethnic groups. There may be other important biomarkers of inflammation, including the senescence-associated secretory phenotype, and other biologic mechanisms (mitochondrial dysfunction, telomere length, alteration in proteostasis) not represented in this study (26). A previously reported transcriptomic BA signature was associated with indices such as grip strength but was limited in ability to examine mortality (8) and could be considered in future studies. Nonetheless, the study has several strengths including the large sample, community-based setting, ability to examine several biologic mechanisms in the same participants, and the longitudinal follow-up with careful ascertainment of events.
In summary, in our community-based sample, estimates of BA constructed from clinical, inflammatory, and DNA methylation biomarkers provide complementary information in predicting mortality and risk for age-related disease suggesting multiple aging metrics may be needed to capture the multiple dimensions of biological aging. Further study is needed to determine how the mechanisms interact to promote or delay aging.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by National Institute on Aging, R56AG029451 (Principle Investigator [PI], J.M.M.) and 2U01AG023755. The Framingham Heart Study is funded by National Institutes of Health contract N01-HC-25195 and HHSN268201500001I. The inflammatory biomarkers were funded through grants R01-AG028321, RO1-HL64753, R01-HL076784 (PI, E.J.B.). The DNA methylation was funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (PI, D.L.).
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
The authors thank Brian Chen, PhD, special volunteer with National Institute on Aging, for his assistance with the extrinsic and intrinsic epigenetic age measures using the Horvath lab software https://labs.genetics.ucla.edu/horvath/dnamage/. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1. Dong X, Milholland B, Vijg J. Evidence for a limit to human lifespan. Nature. 2016;538:257–259. doi:10.1038/nature19793 [DOI] [PubMed] [Google Scholar]
- 2. Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi:10.1093/gerona/58.3.M232 [DOI] [PubMed] [Google Scholar]
- 3. Burch JB, Augustine AD, Frieden LA et al. . Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi:10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age?J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi:10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belsky DW, Caspi A, Houts R et al. . Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112:E4104–E4110. doi:10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swindell WR, Ensrud KE, Cawthon PM, Cauley JA, Cummings SR, Miller RA; Study of Osteoporotic Fractures Research Group Indicators of “healthy aging” in older women (65–69 years of age): a data-mining approach based on prediction of long-term survival. BMC Geriatr. 2010;10:55. doi:10.1186/1471-2318-10-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters MJ, Joehanes R, Pilling LC et al. ; NABEC/UKBEC Consortium The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. doi:10.1038/ncomms9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi:10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hannum G, Guinney J, Zhao L et al. . Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi:10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marioni RE, Shah S, McRae AF et al. . DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi:10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen BH, Marioni RE, Colicino E et al. . DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8:1844–1865. doi:10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine ME, Lu AT, Bennett DA, Horvath S. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging (Albany NY). 2015;7:1198–1211. doi:10.18632/aging.100864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horvath S, Gurven M, Levine ME et al. . An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17:171. doi:10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med. 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 16. Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2017;73:1218–1225. doi:10.1093/gerona/glw240 [DOI] [PubMed] [Google Scholar]
- 17. Schnabel RB, Lunetta KL, Larson MG et al. . The relation of genetic and environmental factors to systemic inflammatory biomarker concentrations. Circ Cardiovasc Genet. 2009;2:229–237. doi:10.1161/Circgenetics.108.804245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fontes JD, Yamamoto JF, Larson MG et al. . Clinical correlates of change in inflammatory biomarkers: the Framingham Heart Study. Atherosclerosis. 2013;228:217–223. doi:10.1016/j.atherosclerosis.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi:10.1016/j.mad.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 21. D’Agostino RB Sr, Vasan RS, Pencina MJ et al. . General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi:10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 22. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi:10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi:10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71:489–495. doi:10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levine ME, Hosgood HD, Chen B, Absher D, Assimes T, Horvath S. DNA methylation age of blood predicts future onset of lung cancer in the women’s health initiative. Aging (Albany NY). 2015;7:690–700. doi:10.18632/aging.100809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niedernhofer LJ, Kirkland JL, Ladiges W. Molecular pathology endpoints useful for aging studies. Ageing Res Rev. 2017;35:241–249. doi:10.1016/j.arr.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.