Abstract
BACKGROUND
Multiple definitions are used to characterize orthostatic hypotension (OH), but the degree to which these definitions correspond with orthostatic symptoms is unknown.
METHODS
We analyzed data from African American Study of Kidney Disease and Hypertension (AASK), a randomized trial of African Americans with hypertension and kidney disease, to characterize the relationship between OH definitions and self-reported syncope, dizziness, or light-headedness. Orthostatic changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), or heart rate (HR) were determined each visit after standing 2:45 minutes. OH was defined using the consensus definition (a drop in SBP ≥20 mm Hg or DBP ≥10 mm Hg) or an often used clinical substitute based on HR (an increase ≥20 bpm).
RESULTS
Among 1,094 participants (mean age 54.5 ± 10.7 years, 38.9% female), there were 52,636 visits (mean 48/person). Mean resting SBP, DBP, and HR at baseline were 147.7 ± 22.3 mm Hg, 92.2 ± 13.4 mm Hg, and 71.1 ± 11.7 bpm, respectively. While the OH consensus definition was associated with syncope (odds ratio 2.49; 95% confidence interval: 1.13, 5.51), dizziness (1.89; 1.53, 2.33), and light-headedness (1.84; 1.52, 2.23), the clinical HR definition was only associated with dizziness (1.28; 1.07, 1.52). None of the OH components (SBP, DBP, or HR) reflected a natural threshold in the prevalence of symptoms; definitions using each of the 3 components were highly specific (≥96%) with low sensitivity (1–5%).
CONCLUSIONS
While the consensus definition was more strongly associated with symptoms, OH definitions did not reflect natural thresholds in symptoms and were insensitive. This implies that the absence of OH using either consensus or clinical definitions does not exclude orthostatic symptoms, which has implications for evaluating clinical events like falls.
CLINICAL TRIALS REGISTRATION
Trial Number: NCT01206062 (clinicaltrials.gov).
Keywords: blood pressure, diastolic blood pressure, dizziness, heart rate, hypertension, light-headedness, orthostatic hypotension, syncope, systolic blood pressure
Orthostatic hypotension (OH) is an important clinical sign associated with a number of adverse outcomes, such as falls, syncope, and mortality.1–4 OH is highly prevalent among older adults2 and is considered a potential adverse consequence of hypertension treatment.5,6 While OH is defined by consensus as a drop in systolic blood pressure (SBP) of ≥20 mm Hg or a drop in diastolic blood pressure (DBP) of ≥10 mm Hg,7,8 an increase in heart rate (HR) of ≥20 beats per minute (bpm) with standing is also often used clinically to represent OH.9,10 According to guidelines, OH should be assessed in adults exhibiting falls or postural dizziness11,12 to help health professionals determine the necessity of interventions such as volume resuscitation, BP augmentation, or medication adjustment.13 However, the extent to which cut points used in consensus definitions or in clinical practice related to symptoms of OH is unknown.
The African American Study of Kidney Disease and Hypertension (AASK) trial examined the effects of 2 mean arterial pressure goals (102 to 107 mm Hg or ≤92 mm Hg) and 3 first-line drug therapies on kidney disease progression in African American adults with hypertension and chronic kidney disease over a 5-year period.14 Throughout the trial, participants were assessed for OH and asked about orthostatic symptoms related to BP treatment, namely, syncope, light-headedness, or dizziness.
We utilized data from the AASK trial to: (i) examine the relationship between components of the consensus definition of OH with self-reported syncope, light-headedness, or dizziness, (ii) characterize the continuous relationship between participant symptoms and components of the consensus definition, and (iii) determine the sensitivity and specificity of components of the consensus definition with regards to orthostatic symptoms. We hypothesized that components of the OH definition would be related to orthostatic symptoms, that the OH definition would demonstrate a progressive, nonlinear relationship with self-reported symptoms, and that the OH definition would be both sensitive and specific for orthostatic symptoms.
METHODS
Study participants
A detailed description of the AASK trial was reported.14 In brief, AASK participants were self-identified African American, age 18–70 years, with chronic kidney disease attributed to hypertension (a DBP of more than 95 mm Hg and a glomerular filtration rate of 20 to 65 ml per minute). Major exclusion criteria were diabetes (i.e., a fasting glucose >140 mg per deciliter, a random glucose >200 mg per deciliter, or diabetes treatment; this definition was consistent with the contemporary diagnosis of diabetes at the time this study was performed), a urinary protein-to-creatinine ratio of >2.5, malignant hypertension in the preceding 6 months, secondary hypertension, or heart failure. In a prior pilot study that used renal biopsies to confirm the diagnosis of kidney disease, the overwhelming majority of participants screened using the criteria above had renal vascular lesions consistent with the clinical diagnosis of hypertensive nephrosclerosis.15
Study design
Between February 1995 and September 1998, 1,094 AASK participants were randomized to 1 of 2 goals, either intensive BP control (mean arterial pressure ≤ 92 mm Hg) or standard control (mean arterial pressure of 102–107 mm Hg), and to 1 of 3 first-line agents: metoprolol (a sustained-release beta blocker), ramipril (an angiotensin-converting enzyme inhibitor), or amlodipine (a dihydropyridine calcium-channel blocker). BP goals were met by first maximizing the dose of each participants’ drug assignments (based on tolerance and safety thresholds) after which other antihypertensive drugs (furosemide, doxazosin, clonidine, and hydralazine or minoxidil) were sequentially added to their regimen. Participants returned for BP measurements monthly for the first 6 months and then every 2 months for the duration of the trial. Trial participants were followed until 30 September 2001.14
Orthostatic hypotension
On the day of the BP measurements, participants were instructed to refrain from caffeine, smoking, and exercise at least 30 minutes prior to and until completion of the BP assessment. Participants were asked to sit quietly for 5 minutes with their feet flat on the floor in an upright, but comfortable posture. With their palm turned upward, the radial pulse was palpated and counted for 30 seconds and multiplied by 2 to determine the HR over a full-minute period. Next, standardized BP assessments were performed by trained, certified observers using a random-zero sphygmomanometer. Three BP measurements were obtained in the seated position after 5 minutes of rest. Seated BP or HR was based on the mean value of the 3 measurements.
Participants were subsequently asked to stand quietly for 2 minutes. After the 2 minutes, the observer raised the participant’s arm for 15 seconds and then placed their arm on an adjacent table. Pulse was taken immediately afterward for 30 seconds and multiplied by 2 to convert units to bpm. At 2 minutes and 45 seconds, a single standing BP assessment was performed. OH was defined using cut points established in the 1996 consensus definition, namely, a drop in SBP ≥ 20 mm Hg or a drop in DBP ≥ 10 mm Hg.7,8 In addition, we evaluated a clinical definition for OH based on a rise in HR ≥20 bpm.9,10 These 3 components, SBP, DBP, or HR, were examined individually and in combination.
Orthostatic symptoms
At each visit, staff were instructed to “ask the participant if he or she has any symptoms,” and to ask explicitly about: “loss of consciousness,” “dizzy or feeling faint,” or “lightheaded on standing.” Staff entered a “yes” or “no” based on each participant’s response. The exact text may be found in Supplementary Material M1.
Other covariates
Age and sex were ascertained via self-report. Body mass index was derived from standardized measurements of height and weight. Creatinine and glucose were measured in serum at baseline using standard assays.14 Baseline SBP or DBP was the first recorded BP for each participant after qualifying for the study.
Medications were recorded at each visit in the following categories: angiotensin-converting enzyme inhibitors or angiotensinogen receptor blockers, beta blockers, calcium-channel blockers, diuretics, centrally acting alpha agonists, peripherally acting alpha antagonists, and vasodilators. In addition, number of antihypertension medication classes was recorded and categorized based on the distribution of values as 0–1, 2, 3, 4, or 5+ classes of medications. Medication data were missing for 6,386 participant-visits.
Statistical analysis
Study population characteristics were described using means (SD) and proportions, overall and by quartile of postural change in SBP, DBP, or HR. Generalized estimating equations (binomial family, logit link, exchangeable correlation structure) with a robust variance estimator were used to determine the association between OH definitions and syncope, dizziness, and/or light-headedness. Models were adjusted for age, sex, BP goal assignment, and BP agent assignment. These analyses were repeated in strata of BP goal in sensitivity analyses. These analyses were also repeated with adjustment for concurrent antihypertensive use (both type and number of classes) in the subgroup that was not missing these data (N = 46,268).
The proportion reporting syncope, light-headedness, and dizziness were plotted according to continuous changes in SBP, DBP, and HR determined after going from sitting to standing positions. These figures were also modeled via generalized estimating equations described above adjusted for age, sex, BP goal assignment, and BP drug assignment.
We determined the changes in SBP, DBP, and HR that reflected the 1st, 2nd, 3rd, 5th, 25th, 50th, 75th, 95th, 97th, 98th, and 99th percentiles. We also determined the sensitivity and specificity of consensus cut points with respect to participant symptoms of syncope, dizziness, and light-headedness. In addition, we evaluated the association (odds ratio [OR]) and 95% confidence interval (CI) of every cut point with any of the 3 symptoms. These associations were performed via the generalized estimating equations described above to account for repeat measurements. We also examined alternative cut points based on maintaining a similar population percentile (1st or 99th), a specificity of 99% for any symptoms, or the highest magnitude of association based on a rolling average derived from 3 consecutive ORs.
All analyses were conducted with STATA version 14.0 (Stata Corporation, College Station, TX).
RESULTS
In this population of 1,094 African Americans with hypertension and chronic kidney disease, the mean age was 54.5 (SD, 10.7) years; 38.9% were female. The mean sitting SBP at baseline was 150.3 (SD, 23.8) mm Hg, the mean sitting DBP was 95.5 (SD, 14.2), and the mean body mass index was 30.6 (SD, 6.6) kg/m2 (Table 1). Participants provided on average 48 (SD, 21.4; range 3 to 153) visits for a total of 52,636 visits with both BP, HR, and symptoms assessments. There were 2,810 visits (i.e., 5.3%) meeting at least 1 of the 3 definitions of OH. Characteristics were evaluated across quartiles of postural change in SBP, DBP, and HR (Supplementary Table S1). The only characteristic with a notable trend across categories was proportion female, lower with higher changes in SBP, DBP, or HR.
Table 1.
Baseline characteristics of the AASK trial, N = 1,094
| Mean (SD) or % | |
|---|---|
| Age, year | 54.5 (10.7) |
| Female, % | 38.9 |
| Mean SBP, mm Hg | 150.3 (23.9) |
| Mean DBP, mm Hg | 95.5 (14.2) |
| Mean heart rate, mm Hg | 72.0 (12.6) |
| Body mass index, kg/m2 | 30.6 (6.6) |
| Serum creatinine, mg/dl | 2.0 (0.7) |
| Serum glucose, mg/dl | 95.0 (18.5) |
| Mean number of visits per person | 48.1 (21.4) |
| Randomization, % | |
| Low BP | 49.4 |
| Moderate BP | 50.6 |
| Ramipril | 39.9 |
| Metoprolol | 40.3 |
| Amlodipine | 19.8 |
Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; BP, blood pressure; DBP, diastolic BP; SBP, systolic BP.
A drop of at least 20 mm Hg in SBP on standing was significantly associated with higher odds of dizziness (OR 1.87; 95% CI: 1.45, 2.40), and light-headedness (OR 2.03; 1.57, 2.62), but not syncope (Table 2). In contrast, a drop of at least 10 mm Hg in DBP was significantly associated with higher odds of syncope (OR 3.37; 1.25, 9.13), dizziness (OR 1.90; 95% CI: 1.40, 2.58), and light-headedness (OR 2.03; 95% CI: 1.52, 2.72). Meanwhile, an increase in HR of at least 20 bpm was associated with higher odds of dizziness (OR 1.28; 95% CI: 1.07, 1.52) and marginally associated with light-headedness (OR 1.22; 95% CI: 1.00, 1.49), but not syncope. The consensus definition (based on either SBP or DBP) was associated with all 3 symptoms, while a composite definition based on any of the 3 definitions was only associated with dizziness and light-headedness. These analyses were repeated in strata of BP target and findings were mildly attenuated but similar (Supplementary Table S2). In the subgroup with concurrent medication use data, results were also attenuated but similar (Supplementary Table S3).
Table 2.
Association of orthostatic hypotension definitions with participant symptoms in the AASK trial, odds ratio (95% CI)
| Definition of orthostatic hypotension | |||||
|---|---|---|---|---|---|
| Drop in SBP of ≥20 mm Hg, N = 715a | Drop in DBP of ≥10 mm Hg, N = 491a | Rise in HR of ≥20 beats per minute, N = 1,868a | Consensus definition: either BP criteria, N = 1,002a | Any criteria (SBP, DBP, or HR), N = 2,810a | |
| Syncope, N = 137 | 2.30 (0.88, 6.01) | 3.37 (1.25, 9.13) | 1.13 (0.46, 2.76) | 2.49 (1.13, 5.51) | 1.65 (0.90, 3.03) |
| Dizzy, N = 3,711 | 1.87 (1.45, 2.40) | 1.90 (1.40, 2.58) | 1.28 (1.07, 1.52) | 1.89 (1.53, 2.33) | 1.51 (1.31, 1.73) |
| Light-headedness, N = 4,146 | 2.03 (1.57, 2.62) | 2.03 (1.52, 2.72) | 1.22 (1.00, 1.49)* | 1.88 (1.50, 2.35) | 1.43 (1.22, 1.67) |
| Any one of the above symptoms, N = 5,723 | 1.90 (1.51, 2.39) | 1.94 (1.50, 2.50) | 1.29 (1.09, 1.52) | 1.84 (1.52, 2.23) | 1.47 (1.30, 1.68) |
All models are adjusted for age, sex, blood pressure goal, and medication assignment. *P = 0.053. Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
a N represents number of visits out of a total of 52,636.
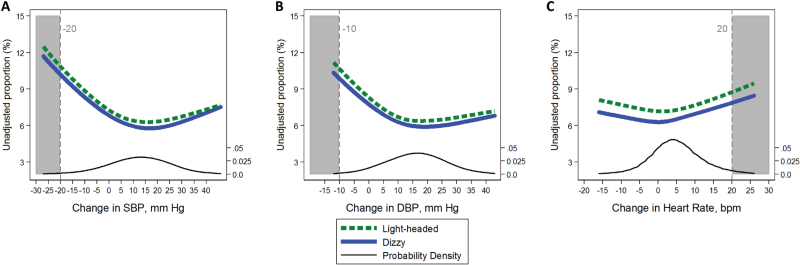
The consensus definition corresponded to the 1st–2nd percentile of changes in SBP and <1st percentile of changes in DBP. The clinical HR definition corresponded to the 97th percentile of changes in HR (Supplementary Table S4). When plotted continuously, there was generally no natural threshold corresponding to the definitions with regards to participant symptoms (Figures 1 & 2). In fact, the proportion reporting syncope, dizziness, or light-headedness trended higher at a positive increase in SBP of about 5 mm Hg, a positive increase in DBP of about 5 mm Hg, and a positive increase in HR of as little as 5 bpm. One exception was HR and syncope which plateaued at 10 bpm suggesting a threshold effect at a value lower than the clinical definition.
Figure 1.
Association of postural change in (a) systolic blood pressure (SBP), (b) diastolic blood pressure (DBP), and (c) heart rate (HR) with the unadjusted proportion (%) of participants, who report syncope. Relationships were modeled via generalized estimating equations to account for repeated assessments. Dashed vertical lines (gray shade) represent conventional cut points for orthostatic hypotension. Black line represents the probability density.
Figure 2.
Association of postural change in (a) systolic blood pressure (SBP), (b) diastolic blood pressure (DBP), and (c) heart rate (HR) with the unadjusted proportion (%) of participants, who report feeling light-headedness (dashed line) or dizzy (solid line). Relationships were modeled via generalized estimating equations to account for repeated assessments. Dashed vertical lines (gray shade) represent conventional cut points for orthostatic hypotension. Black line represents the probability density.
We compared the sensitivity and specificity of each of the consensus cut points with participant symptoms (Table 3). Each of the 3 components had a low sensitivity (1–5%), but were highly specific for symptoms (>96%). Composite definitions, i.e., the consensus definition (based on either BP criteria) or any of the three components (SBP or DBP or HR), increased sensitivity, but decreased specificity. We identified cut points corresponding to the 1st/99th percentile, a specificity of 99%, or the largest magnitude of association with symptoms based on a rolling average (3 adjacent cut points). All approaches yielded similar values: SBP of −21 or −22, DBP of −8 or −9, and an HR of 24 to 27 (Table 4). Comparison of all cut points for each symptom individually or overall may be found in the Supplementary Tables S5–S8.
Table 3.
Diagnostic performance of existing cut points
| Syncope | Dizziness | Light-headedness | |||||
|---|---|---|---|---|---|---|---|
| Cut point | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| SBP, mm Hg | Drop in SBP ≥20 | 2.9 | 98.7 | 2.6 | 98.8 | 2.9 | 98.8 |
| DBP, mm Hg | Drop in DBP ≥10 | 2.9 | 99.1 | 1.6 | 99.1 | 1.8 | 99.1 |
| HR, beats per minute | Rise in HR ≥20 | 4.5 | 96.5 | 5.2 | 96.6 | 5.3 | 96.6 |
| Consensus definition: SBP or DBP | Drop in SBP ≥20 or drop in DBP ≥10 | 4.4 | 98.1 | 3.5 | 98.2 | 3.6 | 98.3 |
| Any of the 3 components | Drop in SBP ≥20 or drop in DBP ≥10 or rise in HR ≥20 beats per minute | 8.9 | 94.7 | 8.6 | 94.9 | 8.5 | 94.9 |
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Table 4.
Comparison of cut points selected for the same percentile, the same specificity (99%), and largest magnitude of association with any of 3 symptoms, syncope, dizzy, and light-headedness
| 1st/99th percentile | 99% specificity | Largest statistically significant magnitude of association (moving average of 3)a | Consensus or clinical definition | |
|---|---|---|---|---|
| Systolic blood pressure, mm Hg | −22 | −21 | −21 | −20 |
| Diastolic blood pressure, mm Hg | −9 | −9 | −8 | −10 |
| Heart rate, beats per minute | 24 | 26 | 27 | 20 |
aBased on an average of odds ratios above and below each cut point (see Supplementary Table S5 for details). Odds ratios were determined via generalized estimating equations adjusted for age, sex, blood pressure goal assignment, and blood pressure medication assignment.
DISCUSSION
In this secondary analysis of the AASK trial, we examined the relationship of both the consensus and clinical definitions for OH with patient symptoms. While both definitions for SBP and DBP were associated with syncope, dizziness, or light-headedness, the clinical definition for HR was not consistently associated with these symptoms. None of the definitions reflected natural symptom thresholds, but rather they demonstrated low sensitivity and high specificity. These findings suggest that while the presence of OH based on the consensus or clinical definitions may effectively be used to confirm orthostatic symptoms, its absence does not exclude them. This has important implications for the use of OH in practice to evaluate clinical events.
OH was originally established by consensus in 1996 (a drop in SBP of 20 mm Hg or DBP of 10 mm Hg upon standing) based on several small physiology studies as well as pragmatic considerations.7 OH as defined by this consensus definition has been associated with multiple clinical outcomes in observational studies, including falls and motor vehicle accidents.1,16 Thus, the consensus definition for OH has established utility in identifying persons at risk for adverse health events. While not formally included in the consensus definition, HR is used clinically to represent OH given the role of HR in maintaining BP with standing.9,10 However, comparison of SBP, DBP, and HR cut points with regards to clinical symptoms has not been reported.
Syncope, dizziness, and light-headedness are among the most commonly cited clinical manifestations of OH.17,18 These symptoms are thought to result from transient cerebral hypoperfusion in the presence of low BP upon standing19,20 and may mediate the relationship of OH with falls long-term. Patient-reported symptoms with standing or clinical events like falls are among the primary motivations for the assessment of OH in clinical practice settings.13 The identification of OH often triggers interventions focused on volume resuscitation or BP augmentation (e.g., compression stockings, increased fluid intake, cessation of antihypertensive agents, and administration of mineralocorticoids).13
We observed evidence of a U-shaped relationship between the proportion of symptoms and postural change in SBP, DBP, or HR. Such a relationship has been described with regards to end-organ damage.21 Whether a similar association is observed for the development of long-term events such as falls or cardiovascular disease should be explored in future studies. A higher risk of adverse events among those with increases in SBP and DBP upon standing could result in null findings in studies where OH is simply considered a dichotomous variable.
Our study demonstrated that while the existing cut points are fairly consistent in terms of corresponding to similar percentiles as well as being at points that maximize their associations with symptoms, the definitions are highly specific with poor sensitivity. Thus, OH assessments performed in response to a fall event among a population similar to our study (African Americans with hypertensive kidney disease) have the potential to miss many cases with underlying orthostatic symptoms. This has important implications for the use of OH in clinical practice in evaluation of clinical events like falls or loss of consciousness where the presence of orthostatic symptoms is unknown (poor medical history, unknown circumstances surrounding the clinical event). While a positive test is useful for confirming orthostatic symptoms, a negative OH test is potentially misleading since it does not exclude orthostatic symptoms. Based on this observation, our findings do not support a higher threshold for OH among adults with hypertension, i.e., a drop in SBP ≥30 mm Hg as advocated in an update of the consensus guideline,8,22 as this would make the test even less sensitive with minimal improvement in specificity.
This study has limitations that warrant discussion. First, the AASK trial enrolled African American adults with chronic kidney disease attributed to hypertension. Most were middle-aged. While hypertension is strongly associated with OH,23 individuals with other common conditions related to OH such as Parkinson’s disease or diabetes, were excluded from this trial. These exclusions may limit the generalizability of our findings to broader populations as well as to institutionalized or more elderly populations. Second, we did not have information on subsequent clinical events such as falls or fainting episodes. Long-term outcomes play a central role in determining the prognostic value of different cut points and represent an important area for further research as to the definition of OH.24 Third, symptom assessments were based on self-reported history between visits and while assessed at the same visit as OH measures, they do not reflect symptoms occurring during the active process of the standing protocol. Fourth, participants went from sitting to standing, rather than supine to standing. While this may be a safer protocol25 and arguably more reflective of daily activity, it is possible that OH was underascertained.26 Fifth, OH was assessed after 2:45 minutes of standing, consistent with the consensus recommendation that OH be assessed within 3 minutes of standing. While this affords the opportunity to evaluate the consensus definition, we recently showed that earlier assessments of OH within 1 minute of standing are more highly associated with dizziness.24 Timing is a controversial aspect of OH assessments that warrant further investigation.27–29 Future research is necessary to evaluate thresholds at earlier time points. Sixth, the etiology of symptoms in this study was not investigated. For example, whether syncope symptoms were caused by an arrhythmia was not assessed as part of this study. Finally, given our observational treatment of the AASK trial data, residual confounding is always a potential concern.
Despite these limitations, our study has a number of strengths. First, the study was conducted in a population with a higher prevalence of OH, i.e., adults with treated hypertension. Second, we have 52,636 paired symptoms and OH assessments, representing one of the largest studies of OH and symptoms, which allows us to comprehensively compare the OH measured definition with its clinical presentation. Syncope, light-headedness, and dizziness are among the most important manifestations of OH, causally related to adverse events with profound impact on quality of day-to-day life. Third, OH was assessed by experienced research staff who were rigorously trained to follow a standardized protocol, which minimized bias as well as imprecision.
In conclusion, components of the OH consensus definition are highly specific for orthostatic symptoms, but also extremely insensitive. Health professionals applying the consensus definition to evaluate clinical events should be aware that the absence of OH does not rule out underlying orthostatic symptoms. Further work is needed to evaluate alternative definitions of OH in relation to long-term outcomes.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension online.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
S.P.J. is supported by a National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases T32DK007732-20 Renal Disease Epidemiology Training Grant and Error! Bookmark not defined. NHLBI 7K23HL135273-02. The original AASK study was supported by grants to each clinical center and the coordinating center from the National Institute of Diabetes and Digestive and Kidney Diseases; by the Office of Research in Minority Health (now the National Center on Minority Health and Health Disparities); by institutional grants from the National Institutes of Health (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, and DK 2818-02); by King Pharmaceuticals, which provided monetary support and antihypertensive medications to each clinical center; and by Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn, which donated antihypertensive medications. The authors thank the staff and participants of the AASK study for their important contributions.
REFERENCES
- 1. Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic hypotension in middle-age and risk of falls. Am J Hypertens 2017; 30:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Hypertension 1992; 19:508–519. [DOI] [PubMed] [Google Scholar]
- 3. Yatsuya H, Folsom AR, Alonso A, Gottesman RF, Rose KM; ARIC Study Investigators Postural changes in blood pressure and incidence of ischemic stroke subtypes: the ARIC study. Hypertension 2011; 57:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fedorowski A, Stavenow L, Hedblad B, Berglund G, Nilsson PM, Melander O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur Heart J 2010; 31:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens 2015; 29:599–603. [DOI] [PubMed] [Google Scholar]
- 6. Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens 2015. doi:10.1097/HJH.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 7. Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res 1996; 6:125–126. [DOI] [PubMed] [Google Scholar]
- 8. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21:69–72. [DOI] [PubMed] [Google Scholar]
- 9. Emergency Nurses Association. Clinical Practice Guideline: Orthostatic Vital Signs, 2015. [Google Scholar]
- 10. Fuchs SM, Jaffe DM. Evaluation of the “tilt test” in children. Ann Emerg Med 1987; 16:386–390. [DOI] [PubMed] [Google Scholar]
- 11. Moyer VA; U.S. Preventive Services Task Force Prevention of falls in community-dwelling older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157:197–204. [DOI] [PubMed] [Google Scholar]
- 12. Hypertension in adults: diagnosis and management | Guidance and guidelines | NICE. <https://www.nice.org.uk/guidance/cg127/chapter/1-guidance> Accessed 30 October 2017. [Google Scholar]
- 13. Lanier JB, Mote MB, Clay EC. Evaluation and management of orthostatic hypotension. Am Fam Physician 2011; 84:527–536. [PubMed] [Google Scholar]
- 14. Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X; AASK Collaborative Research Group Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010; 363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, Glassock R. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int 1997; 51:244–252. [DOI] [PubMed] [Google Scholar]
- 16. Ricci F, Manzoli L, Sutton R, Melander O, Flacco ME, Gallina S, De Caterina R, Fedorowski A. Hospital admissions for orthostatic hypotension and syncope in later life: insights from the Malmö Preventive Project. J Hypertens 2017; 35:776–783. [DOI] [PubMed] [Google Scholar]
- 17. Atkins D, Hanusa B, Sefcik T, Kapoor W. Syncope and orthostatic hypotension. Am J Med 1991; 91:179–185. [DOI] [PubMed] [Google Scholar]
- 18. Kaufmann H, Malamut R, Norcliffe-Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res 2012; 22:79–90. [DOI] [PubMed] [Google Scholar]
- 19. Novak P. Cerebral blood flow, heart rate, and blood pressure patterns during the tilt test in common orthostatic syndromes. Neurosci J 2016; 2016:6127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 1998; 29:1876–1881. [DOI] [PubMed] [Google Scholar]
- 21. Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, Shimada K. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol 2002; 40:133–141. [DOI] [PubMed] [Google Scholar]
- 22. Singer W, Low PA. Early orthostatic hypotension and orthostatic intolerance-more than an observation or annoyance. JAMA Intern Med 2017; 177:1324–1325. [DOI] [PubMed] [Google Scholar]
- 23. Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc 2011; 59:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER 3rd, Windham BG, Griswold ME, Heiss G, Selvin E. Association of history of dizziness and long-term adverse outcomes with arly vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med 2017; 177:1316–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koziol-McLain J, Lowenstein SR, Fuller B. Orthostatic vital signs in emergency department patients. Ann Emerg Med 1991; 20:606–610. [DOI] [PubMed] [Google Scholar]
- 26. Cooke J, Carew S, O’Connor M, Costelloe A, Sheehy T, Lyons D. Sitting and standing blood pressure measurements are not accurate for the diagnosis of orthostatic hypotension. QJM 2009; 102:335–339. [DOI] [PubMed] [Google Scholar]
- 27. Maurer MS, Cohen S, Cheng H. The degree and timing of orthostatic blood pressure changes in relation to falls in nursing home residents. J Am Med Dir Assoc 2004; 5:233–238. [DOI] [PubMed] [Google Scholar]
- 28. Braam EA, Verbakel D, Adiyaman A, Thien T. Orthostatic hypotension: revision of the definition is needed. J Hypertens 2009; 27:2119–2120; author reply 2120. [DOI] [PubMed] [Google Scholar]
- 29. Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc 2011; 59:655–665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.