To the Editor:
High-mobility group box 1 (HMGB1) is a multifunctional protein. It is a nonhistone chromatin-binding protein that regulates chromatin structure and transcription, and after cellular release, it functions as a damage-associated molecular pattern to activate and sustain inflammatory responses. HMGB1 is increased in the BAL and sputum of patients with chronic respiratory illnesses such as chronic obstructive pulmonary disease (1) and cystic fibrosis (CF) (2), and functions as a biomarker (2, 3) for lung disease severity in patients with CF. HMGB1 binds and activates Toll-like receptor 2 (TLR-2), TLR-4, and TLR-9 (4), and receptor for advanced glycation end products (RAGE) (5). In murine models of Pseudomonas aeruginosa pneumonia, airway HMGB1 levels are increased and inhibition of HMGB1 rescues impaired phagocytic function and improves mouse survival (6). Intratracheal administration of HMGB1 upregulates IL-1β, TNF-α, and macrophage inflammatory protein 2, resulting in increased lung neutrophilic inflammation (7). Thus, HMGB1 is not only a biomarker but also a mediator of airway neutrophilic inflammation.
Previously, we reported that neutrophil elastase (NE), a major protease in the airways of patients with CF, induces the release of HMGB1 both in vivo and in vitro (8). NE also upregulates expression of MUC5AC (9), one of the major secreted airway mucins. Therefore, we hypothesized that an HMGB1 interaction with RAGE may have a previously unrecognized activity in the lung, i.e., upregulating the expression of two major airway mucins: MUC5AC and MUC5B. To test this hypothesis, we grew primary normal human bronchial epithelial (NHBE) cell cultures at the air–liquid interface and exposed them to recombinant HMGB1 in the presence or absence of a RAGE inhibitor. NHBE cells from at least two different deidentified donors were obtained from tracheal remnants after lung transplantation according to an institutional review board–approved protocol. After one passage for expansion, the cells were cultured in defined, serum-free media on Transwell inserts under submerged conditions until they reached confluency and then under air–liquid interface conditions for 9–11 days as previously described (10). The cells were stimulated with HMGB1 (10 or 100 ng/ml) or control vehicle in the apical and basolateral compartments for 24 or 48 hours. The apical media were collected and used in microtiter plate assays (11, 12) (5–50 μl) to determine the relative quantities of secreted MUC5AC protein (anti-MUC5AC monoclonal antibody: 45M1; GTX23659; GeneTex) and secreted MUC5B protein (anti-MUC5B rabbit polyclonal antibody: H-300; SCBT). The cells were lysed to harvest total RNA for qRT-PCR to quantitate MUC5AC mRNA, MUC5B mRNA, and AGER (RAGE gene) mRNA. Total cellular lysate protein (50 μg) was evaluated by Western blot analysis for RAGE expression (anti-RAGE rabbit polyclonal antibody: PA1-075; Thermo Fisher Scientific), normalized to β-actin, and band densities were expressed as a percentage of control treatment conditions. Statistical analyses for mRNA and relative protein expression were performed using ANOVA with post hoc comparisons. P < 0.05 was considered a statistically significant difference between treatment conditions.
In a separate set of experiments, RAGE was inhibited using FPS-ZMI (13) (553030; Millipore-EMD Biosciences), which was added to the apical and basolateral media 1 hour before stimulation with HMGB1 or vehicle control for 24 hours. Total cell RNA was isolated for qRT-PCR to evaluate the effect of RAGE inhibition on HMGB1-induced MUC5AC and MUC5B mRNA expression. For details regarding the methods used, please refer to the data supplement.
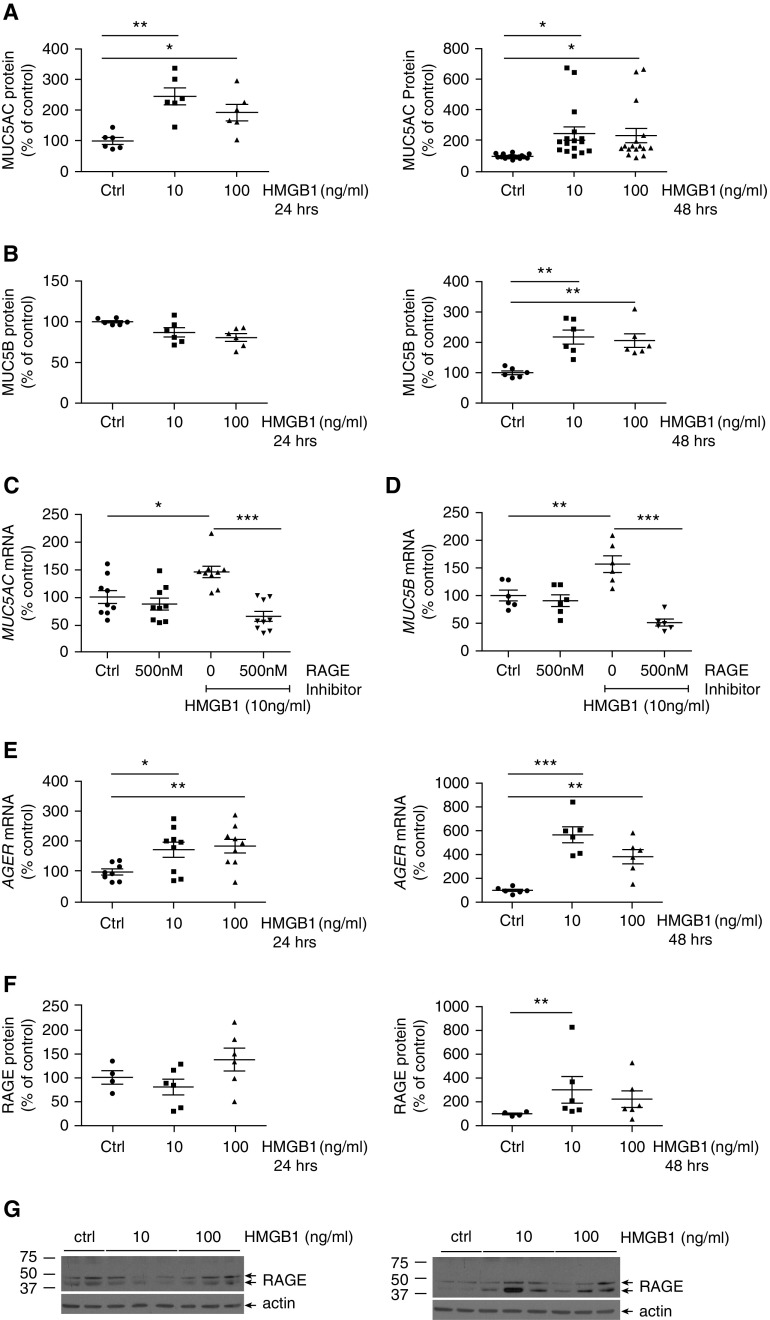
Here, we demonstrated that HMGB1 increased both MUC5AC and MUC5B mRNA and secreted protein in NHBE cells (Figures 1A–1D). The concentration of HMGB1 required to increase mucin expression is within the range detected in CF sputum (3). Inhibiting RAGE with a pharmacologic inhibitor was sufficient to block HMGB1-induced MUC5AC and MUC5B expression (Figures 1C–1D), supporting the idea that RAGE activation is necessary for mucin gene regulation. Interestingly, HMGB1 upregulated AGER mRNA (RAGE gene) and protein levels in NHBE cells (Figures 1E–1G), consistent with a mechanism of sustained HMGB1-RAGE activation in the airway. Given the strong association between airway HMGB1 and the risk for pulmonary exacerbations and lung disease progression in patients with CF, we speculate that HMGB1-induced mucin production and secretion may contribute to increased airway mucin concentrations during CF pulmonary exacerbations.
Figure 1.
High-mobility group box1 (HMGB1) upregulated MUC5AC and MUC5B protein expression, and gene regulation was blocked by a receptor for advanced glycation end products (RAGE) inhibitor. Normal human bronchial epithelial (NHBE) cells were cultured at the air–liquid interface, treated with HMGB1 (10 or 100 ng/ml) for 24 or 48 hours, and analyzed for relative MUC5AC (A) and MUC5B (B) protein levels in the apical media by microtiter plate assay (see the data supplement). Involvement of the RAGE receptor in HMGB1-induced MUC5AC (C) and MUC5B (D) mRNA expression was tested by pretreatment with a RAGE inhibitor, FPS-ZMI (500 nM), for 1 hour, followed by administration of HMGB1 (10 ng/ml, 24 h). Expression of MUC5AC and MUC5B mRNA was determined by qRT-PCR as described in the data supplement. HMGB1 (10 or 100 ng/ml; 24–48 h) increased AGER (RAGE) mRNA expression (E) and RAGE protein levels as shown by densitometric analysis (F) of Western blots in well-differentiated, primary NHBE cells. A representative Western blot for RAGE is shown in G. RAGE mRNA was determined by qRT-PCR, and RAGE protein levels were compared with vehicle control–treated cells by Western blot, as described in the data supplement. Data are expressed as mean ± SEM. P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001 comparisons between conditions are marked by lines). Two to five independent experiments with six or more replicates per experimental treatment condition were performed using NHBE cells obtained from two to five different donors. Ctrl = control.
Our results are contrary to previous observations made by Kim and colleagues using the lung cancer cell line NCI-H292 (14). The authors reported that HMGB1 upregulated MUC8; slightly upregulated MUC2, MUC5AC, MUC6, and MUC7; and did not regulate MUC5B expression. In contrast to their study, we used primary airway epithelial cells from at least two different donors instead of a lung cancer cell line, and incubated the cells for 24–48 hours, versus 12 hours in the previous study. One limitation of our report is that we have yet to examine the signaling mechanisms or kinetics of exposure required for upregulation of MUC5AC and MUC5B expression by HMGB1. HMGB1 upregulates MUC8 mRNA via increased phosphorylation of ERK and JNK (14). In NHBE cells, HMGB1 promotes wound healing via MAPK and Smad-2 signaling (15). These findings support the notion that MAPK and TGF-β signaling intermediates are triggered by an interaction between HMGB1 and RAGE/TLR4. We predict that activation of NF-κB by HMGB1 ligation of RAGE may play a role in MUC5AC and MUC5B upregulation (16). The time dependence of HMGB1 regulation of mucin gene expression also requires further examination, as a delayed effect on mRNA upregulation suggests the requirement of an HMGB1-regulated secondary signal to activate mucin gene expression.
In summary, this report highlights a novel function for HMGB1 in the airways: upregulation of MUC5AC and MUC5B mRNA expression and protein secretion. Because NE stimulates the release of HMGB1 into murine airways or culture media (8), it is possible that HMGB1 is required for NE-induced MUC5AC expression. Alternatively, HMGB1 ligation of RAGE may be an additional, nonredundant mechanism for upregulating airway mucin expression in chronic lung diseases such as chronic obstructive pulmonary disease and CF.
Footnotes
Supported by VA Commonwealth Health Research Board grant 236-14-14 (J.A.V.) and Cystic Fibrosis Foundation grant VOYNOW15I0 (J.A.V.).
Author Contributions: Conception and design: A.B.K., S.Z., J.L., and J.A.V. Performed experiments and analyzed data: A.B.K. and S.Z. Interpreted data and drafted the manuscript: A.B.K., S.Z., S.K., and J.A.V. Edited the manuscript: J.A.V. and J.L.
This letter has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. 2010;181:917–927. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 2.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C, et al. Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: High-mobility group box 1 (hmgb1) between inflammation and infection. 2015;21:368.e1-9. doi: 10.1016/j.cmi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. 2009;86:655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 5.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 6.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 8.Griffin KL, Fischer BM, Kummarapurugu AB, Zheng S, Kennedy TP, Rao NV, et al. 2-O, 3-O-desulfated heparin inhibits neutrophil elastase-induced HMGB-1 secretion and airway inflammation. 2014;50:684–689. doi: 10.1165/rcmb.2013-0338RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fischer BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. 1999;276:L835–L843. doi: 10.1152/ajplung.1999.276.5.L835. [DOI] [PubMed] [Google Scholar]
- 10.Zheng S, Byrd AS, Fischer BM, Grover AR, Ghio AJ, Voynow JA. Regulation of MUC5AC expression by NAD(P)H:quinone oxidoreductase 1. 2007;42:1398–1408. doi: 10.1016/j.freeradbiomed.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanabe T, Kanoh S, Tsushima K, Yamazaki Y, Kubo K, Rubin BK. Clarithromycin inhibits interleukin-13-induced goblet cell hyperplasia in human airway cells. 2011;45:1075–1083. doi: 10.1165/rcmb.2010-0327OC. [DOI] [PubMed] [Google Scholar]
- 12.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. 2009;40:277–285. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H, Park JR, Kim WJ, Sundar IK, Rahman I, Park SM, et al. Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signaling. 2017;31:2076–2089. doi: 10.1096/fj.201601155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DE, Min KJ, Kim JS, Kwon TK. High-mobility group box-1 protein induces mucin 8 expression through the activation of the JNK and PI3K/Akt signal pathways in human airway epithelial cells. 2012;421:436–441. doi: 10.1016/j.bbrc.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 15.Ojo OO, Ryu MH, Jha A, Unruh H, Halayko AJ. High-mobility group box 1 promotes extracellular matrix synthesis and wound repair in human bronchial epithelial cells. 2015;309:L1354–L1366. doi: 10.1152/ajplung.00054.2015. [DOI] [PubMed] [Google Scholar]
- 16.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]