Abstract
Study Objectives
Slow oscillations (SO) during sleep contribute to the consolidation of learned material. How the encoding of declarative memories during subsequent wakefulness might benefit from their enhancement during sleep is less clear. In this study, we investigated the impact of acoustically enhanced SO during a nap on subsequent encoding of declarative material.
Methods
Thirty-seven healthy young adults were studied under two conditions: stimulation (STIM) and no stimulation (SHAM), in counter-balanced order following a night of sleep restriction (4 hr time-in-bed [TIB]). In the STIM condition, auditory tones were phase-locked to the SO up-state during a 90 min nap opportunity. In the SHAM condition, corresponding time points were marked but tones were not presented. Thirty minutes after awakening, participants encoded pictures while undergoing fMRI. Picture recognition was tested 60 min later.
Results
Acoustic stimulation augmented SO across the group, but there was no group level benefit on memory. However, the magnitude of SO enhancement correlated with greater recollection. SO enhancement was also positively correlated with hippocampal activation at encoding. Although spindle activity increased, this did not correlate with memory benefit or shift in hippocampal signal.
Conclusions
Acoustic stimulation during a nap can benefit encoding of declarative memories. Hippocampal activation positively correlated with SO augmentation.
Keywords: acoustic stimulation, slow oscillations, encoding, hippocampus
Statement of Significance
Slow oscillations (SO) during sleep have been reported to support both the consolidation and encoding of new memories. Although multiple studies examined their role in sleep-related memory consolidation, few studies have explored their possible facilitation of post-sleep encoding. We used phase locked acoustic stimulation to enhance these oscillations during an afternoon nap and tested encoding performance in the subsequent waking period. We found positive correlations between the magnitude of SO enhancement and stronger memory encoding, as well as encoding-related hippocampal activity. These findings suggest that nap-based acoustic stimulation can benefit hippocampus–related declarative memory encoding.
Introduction
Manipulations performed during sleep to augment memory performance provide novel avenues for cognitive enhancement as well as understanding how sleep contributes to memory. Two key neurophysiological features of sleep—0.5 to 4 Hz slow wave activity (SWA) including the <1 Hz slow oscillations (SO), and sleep spindles, are important contributors to memory consolidation [1], and their enhancement has been shown to boost declarative [2–6] and motor [7] memories in humans. Efforts to enhance sleep slow waves pharmacologically [8–10], through direct [11] or alternating current stimulation [12], or acoustic stimulation [3–6], suggest that the last of these approaches is the most promising for boosting declarative memory. However, there remain gaps in our understanding of the physiology underlying how acoustic stimulation influences memory.
The first issue we explored was whether acoustic stimulation during sleep can also benefit encoding during subsequent wakefulness. Prior experiments using this method have targeted memory consolidation during overnight sleep [3, 5, 6] or a nap [4]. Boosting encoding following sleep is well motivated. Apart from encoding failures attributable to briefly falling asleep, sustained wakefulness can negatively affect hippocampal engagement [13] as well as the quality of encoded memoranda captured in the parahippocampal cortex [14]. Slow-wave sleep might restore encoding capacity by driving the redistribution of hippocampus-dependent memories to neocortical sites [1, 15, 16], downscaling “over-saturated” synapses [17], or restoring brain energetics [18]. To date, only two studies, we know of, have examined how manipulating SO during sleep can affect memory encoding upon waking. One documented the deleterious effects of SWA suppression [19], while the other demonstrated the benefit of SWA enhancement through rhythmical electrical stimulation [12] on the encoding of subsequent declarative memoranda. As elaborated on later, acoustic and electrical stimulation appear to have different effects on brain physiology.
Secondly, the neural substrate(s) linking SWA enhancement to improvement in declarative memory in a dose-dependent manner have yet to be elucidated. Reduced hippocampal activation during picture encoding has been observed after a night of total sleep deprivation [13] as well as following selective slow-wave sleep interruption [19]. However, it remains to be shown whether slow-wave augmentation in healthy persons results in greater hippocampal activation at encoding.
The third issue we explore here is the variability of responses to acoustic stimulation and its physiological correlates. Studies to-date demonstrating the benefit of acoustic stimulation have involved small samples of healthy participants [3, 4, 6] and even then, significant variability in responses has been observed. Inter-individual variation in amplitude and number of endogenous slow waves available for stimulation could be important determinants of a positive behavioral outcome. In addition, spindle activity (9–15 Hz), while not the primary target, increases following stimulation targeted at SO [3–5]. How increased spindles would affect memory following a stimulated nap is unclear.
To address these gaps, we used real-time sleep staging to facilitate closed-loop acoustic stimulation locked to the SO up-state, as healthy young adults napped. Upon awakening, they underwent a picture encoding task. Performance on this task has been shown to be enhanced via transcranial SO augmentation [12] and conversely impaired by reductions in slow-wave activity [19]. Recognition was tested an hour later. We anticipated significant variation in electrophysiological and behavioral responses to acoustic stimulation but that recognition would benefit according to degree of SO enhancement. We also hypothesized that individuals with larger SO enhancement would also show greater hippocampal activation for successfully encoded trials.
Materials and Methods
Participants
Forty-two healthy young, undergraduates from the National University of Singapore participated in this study. They were recruited from respondents to a web-based questionnaire who reported that they (1) had English as a first language, (2) were nonsmokers, (3) had no history of psychiatric, neurological, or sleep disorders, (4) consumed no more than two caffeinated drinks per day, (5) had good habitual sleep between 6 and 9 hr daily, (6) were not of an extreme chronotype as assessed on the Horne–Östberg Morningness–Eveningness questionnaire [20], (7) were not color-blind, and (8) were right-handed. All participants provided informed consent in compliance with a protocol approved by the National University of Singapore Institutional Review Board and were paid for their involvement. Following recruitment, two participants voluntarily terminated participation in the study as they were unable to comply with the enforced sleep schedule. Three additional participants were dropped as their first 90 min nap session did not contain non-rapid eye movement (NREM) 2 or 3 sleep. A total of 37 healthy, young undergraduates (mean ± SD: 22.5 ± 2.3 years; 18 males) completed the entire study comprising one briefing and two nap sessions.
Study procedure
Participants visited the laboratory three times. At the first visit, they were briefed on the study protocol, completed questionnaires and practice tasks, and collected a wrist actigraph (Actiwatch 2, Philips Respironics, USA). At the second and third visits, they underwent an experimental nap session: STIM or SHAM. The order of the nap sessions was counterbalanced across participants and separated by 1 week to reduce carry-over effects between sessions.
Participants were instructed to keep to their habitual sleep schedules 1 week prior to each experimental session. However, to increase sleep propensity, participants were required to sleep at 01:00 and wake at 05:00 the morning of each experimental session. Adherence to the sleep schedule was ensured via sleep diaries and wrist actigraphy. Strenuous physical activity, napping, and consumption of alcoholic or caffeinated beverages were also prohibited in the 24 hr preceding each sleep session.
STIM and SHAM nap sessions commenced at 14:00. Participants underwent preparation for polysomnographic (PSG) recordings and an auditory threshold test before taking a 90 min nap. In the STIM condition, acoustic stimulation was delivered at 16 dB above the wake hearing threshold via headband headphones (AcousticSheep LLC) throughout the entire nap period, whenever the participant was in N2 or N3 sleep. In the SHAM condition, identical procedures were carried out except that stimulation volume was muted. Following both nap sessions, a 30–45 min break was provided for participants to clean up and to minimize the possible effects of sleep inertia.
Polysomnographic recordings
Recordings were conducted using a BrainAmp MR amplifier (Brain Products GmbH, Munich, Germany) from seven electroencephalographic (EEG) channels (international 10–20 system, F3, F4, C3, C4, O1, O2, and A1) and two electrooculographic (EOG) channels (EOG1, EOG2) referenced to the right mastoid (A2). In addition, bipolar submental electromyography (EMG) measures were also obtained. Impedances were kept below 5 kΩ for EEG electrodes and below 10 kΩ for EOG and EMG electrodes. Signals were sampled at 500 Hz.
Automated detection of sleep stage and phase-locked acoustic stimulation
Real-time automated sleep stage detection and up-state targeting of SO during N2 and SWS were performed ([20]; https://z3score.com). Briefly, data from 3 EEG channels (F3-A2, C3-A2, and C4-A1) and two EOG channels (EOG1-A2 and EOG2-A1) were fed into a 30 s running buffer at 50 Hz. Due to the presence of large DC drifts, a DC-blocking filter with cutoff of 0.03 Hz was applied to the buffer. The running buffer was resampled at 100 Hz using polyphase finite impulse response (FIR) filters. Filters were applied in both temporal directions to avoid phase delays introduced by filtering. The running buffer was sleep scored at 1 Hz, whereas up-state targeting was carried out at 50 Hz using a predetermined voltage threshold and inflection point–based detection and targeting algorithm (Patanaik et al., under review). The target electrode for SO up-state detection was set at F3-A2 given the large amplitude and likelihood of SO typically originating from this region and due to the fact that SO preferentially propagate from anterior to posterior sites [22–24].
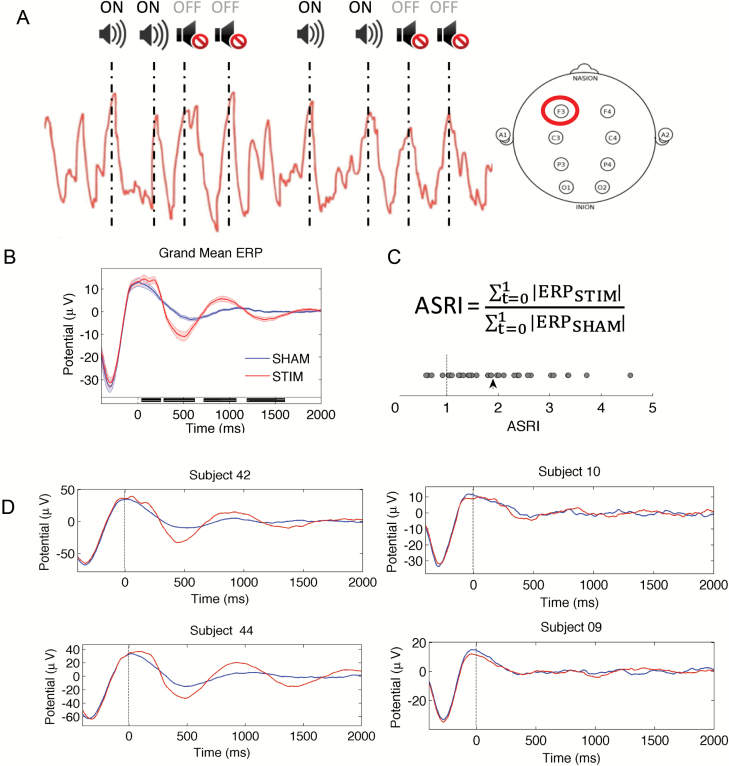
In the STIM condition, auditory tones (50 ms bursts of pink noise) locked to SO up-states were played in 2-ON, 2-OFF blocks. Tone presentation was halted if an arousal occurred or if voltage thresholds were not met. In the SHAM condition, SO up-state locations were marked in the EEG recordings but no tones were played (Figure 1A).
Figure 1.
Acoustic stimulation protocol and EEG measures. (A) Acoustic stimulation protocol. In the STIM condition, tones locked to the up-state of slow oscillations from electrode F3 were played in 2-ON, 2-OFF blocks. In the SHAM condition, corresponding ON and OFF blocks were marked in the EEG recordings but no tones were played. (B) Grand mean ERP waveforms of all participants locked to the start of each auditory tone/marker onset (t = 0) for each block during sleep. Black bars indicate time points where ERP significantly differed between conditions (p < 0.05, FDR-corrected). (C) Derivation of the ASRI and values for 36 participants. Values greater than 1 (dotted line) indicate greater responses in the STIM compared with the SHAM condition. The arrowhead denotes the mean ASRI across all participants. (D) Mean ERP waveforms of four individual participants locked to the onset of each tone/marker (t = 0).
Analyses of sleep measures
Sleep was autoscored in 30 s epochs using FASST-Z3Score toolbox (https://github.com/amiyapatanaik/FASST-Z3Score) and visually checked by a trained technician following criteria set by the AASM Manual for the Scoring of Sleep and Associated Events [25]. Total sleep time (TST) and time spent in different sleep stages were determined separately for both conditions.
EEG preprocessing and analyses
Functions from the EEGLAB toolbox, v13.2.2 (http://sccn.ucsd.edu/eeglab) along with custom scripts in MATLAB were used to preprocess and analyze the EEG data. Average event-related potentials (ERPs) were computed in both conditions using the EEG signal from F3 locked to all tones (or corresponding markers in the SHAM condition), bandpass filtered between 0.3 and 35 Hz, and averaged across trials. An index of responsiveness to the stimulation, termed the Acoustic Stimulation Response Index (ASRI), was computed based on the ratio of total absolute ERP in the 0–1 s time window following each tone (or marker) onset in the STIM compared with the SHAM condition (equation 1). Total absolute ERP was used as this could account simultaneously for both SO amplitude enhancements and phase-locking across trials. The ASRI is 1 when values are equal in both conditions.
To identify time-varying changes evoked by acoustic stimulation, event-related spectral perturbation (ERSP) plots were also computed locked to the onset of each 2-ON, 2-OFF block. To do this, the EEGLAB function “newtimef.m” was used to perform time-frequency analysis. This function employs a family of complex Morlet wavelets to decompose signals into time-frequency representations. The number of wavelet cycles used increased from 1 cycle at 0.8 Hz to 28 cycles at 25 Hz in 25 log-spaced frequencies corresponding to each frequency bin.
In addition, to investigate whether stimulation induced prolonged changes beyond the duration of the stimulation, we repeated ERP and time-frequency analyses locked to the onset of each 2-OFF block.
Power spectral density estimates for all artifact-free NREM epochs were also computed using Welch’s modified periodogram method [26] (Hamming window; 0.25 Hz bin resolution) and subsequently integrated using the trapezoidal rule for integral approximation for the following frequency bands: (1) SO (0.5–1 Hz), (2) SWA (0.5–4 Hz) (3) θ (4–8 Hz), (4) α (8–12 Hz), and (5) β (12–16 Hz), to obtain spectral power measures per epoch. Data from all NREM epochs were averaged to obtain values of mean power for each frequency band in both STIM and SHAM conditions.
Spindle detection was also performed on channel C3 using functions from the swa-matlab toolbox (https://github.com/Sinergia-BMZ/swa-matlab). Spindle duration, density, and count were computed for all NREM epochs in both STIM and SHAM conditions.
Experimental task
To assess whether SO enhancements affected episodic encoding, a subsequent memory paradigm was used [27]. Encoding occurred in-scanner while retrieval was tested 60 min later to identify brain activity at encoding associated with successful retrieval. Prior to both phases, participants rated their subjective sleepiness using the Karolinska Sleepiness Scales [28] to ensure that differences across STIM and SHAM conditions did not arise from differences in self-reported sleepiness.
Encoding phase
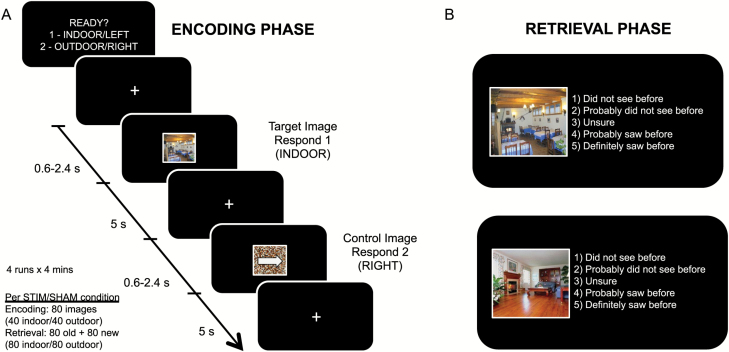
Participants encoded 40 indoor (Category: Living Room, Restaurant) and 40 outdoor (Category: Forest, Building) scene images from the LabelMe database (Figure 2A) [29]. They performed a short version of this task at the briefing session and were informed that their memory for studied items would be subsequently tested. To minimize differences in encoding strategy across participants, they were instructed to look for distinct objects in the scenes. These scenes alternated with a control image constructed using scrambled images of the same scenes to equate for overall luminance. A white arrow pointing to the left or right was superimposed on the control images to equate for motor execution. To confirm that participants were actively attending to these stimuli, they were required to make a button press (Current Designs, Inc., Philadelphia, USA) to indicate whether these were (1) indoor or outdoor scenes, or (2) right or left arrows.
Figure 2.
Experimental paradigm. (A) During the encoding phase, participants viewed 40 indoor and 40 outdoor scene images from the LabelMe database (Russell et al.). These scenes alternated with a control image constructed using scrambled images of the same scenes to equate the amount of perceptual information present. A white arrow pointing to the left or right was superimposed on the control images. To confirm that participants were actively attending to these stimuli, they were required to make a button press to indicate whether these were indoor or outdoor scenes, or if they contained a right or left arrow (control images). (B) During the retrieval phase, participants were presented with 160 images—80 were previously shown, and 80 were new. Participants were required to respond accordingly whether they had seen these images before, on a 5-point scale. Responses of “1” and “2” were pooled to indicate a “no” response, and responses of “4” and “5” were pooled to indicate a “yes” response. Performance was assessed by computing hit (proportion answered “yes” to old images) and false alarm (proportion answered “yes” to new images) rates for each participant. Only trials that were correctly judged as indoor/outdoor during the encoding phase were included in this analysis.
Stimuli were presented using the Psychophysics Toolbox v3.0.9 [30, 31] for MATLAB (R2009a, Mathworks; Natick, MA) and projected onto a screen located in the scanner bore. Stimuli were delivered in 4 runs of 20 scene and 20 control images each (278 s), administered in a randomized, counterbalanced order across participants. Each image (visual angle 6° × 6°) was presented for 5 s, separated by a variable jitter of 0.6–2.4 s.
Retrieval phase
Participants completed the psychomotor vigilance task (PVT) [32] and watched a documentary until it was time for the retrieval phase to commence (60 min following the end of the encoding phase).
In the retrieval phase (Figure 2B), participants were presented with 160 images—80 old and 80 new. The presentation order of these images was randomized across participants. Participants were required to respond on a 5-point scale: (1) Did not see before, (2) Probably did not see before, (3) Unsure, (4) Probably saw before, and (5) Definitely saw before. Responses of “1” and “2” were pooled to indicate a “no” response, and responses of “4” and “5” were pooled to indicate a “yes” response. Responses of “3” were excluded from further analyses. Performance was assessed by computing hit (proportion answered “yes” to old images) and false alarm (proportion answered “yes” to new images) rates for each participant. The A′ measure of discriminability that simultaneously takes into account hits and false alarm rates was also computed [33]. A′ ranges from 0 to 1, with 0.5 indicating performance at chance levels. Only trials that were correctly judged as indoor/outdoor during the encoding phase were included in these analyses.
Statistical analyses
For EEG and behavioral analyses, paired sample tests and correlational analyses were performed using SPSS version 24 (IBM Corp., Armonk, New York). Paired t-tests were used for normally distributed variables, and Wilcoxon signed-rank tests were used where assumptions of normality were violated (p < 0.05 on the Shapiro–Wilk test). For correlational analyses, Pearson’s product-moment correlation coefficients were computed for normally distributed variables, and Spearman’s rank correlation coefficients were computed for the other variables. All p-values reported use two-tailed hypothesis testing and the significance level was set at p = 0.05.
ERP and time-frequency analyses were also compared using paired sample t-tests for each time/time-frequency point and corrected using a false discovery rate (FDR) of q < 0.05. To investigate the hypothesis that differences in hippocampal activity between conditions would mediate the effect of the ASRI on encoding performance, a mediation analysis was conducted with the SPSS macro PROCESS [34]. The indirect effect was tested using 5000 bootstrap samples and a 95% confidence interval (bias-corrected).
Imaging procedure
Functional images were acquired on a 3T MRI scanner (MAGNETOM PrismaFit, Siemens Healthcare, Erlangen, Germany). A gradient echo-planar imaging (EPI) sequence (TR: 2000 ms; TE: 30 ms; FA: 90°; FOV: 192 × 192 mm; matrix size: 64 × 64; voxel size: 3.0 × 3.0 × 3.0 mm) was used. Thirty-six oblique axial slices (slice thickness: 3 mm) parallel to the AC-PC line were obtained. A total of 136 volumes were collected in each run. Structural images for co-registration and normalization were acquired using a T1-weighted magnetization–prepared rapid gradient-echo (MP-RAGE) sequence (TR: 2300 ms; TI: 900 ms; FA: 8°; BW: 200 Hz/pixel; FOV 256 × 240 mm; matrix size: 256 × 256; voxel size: 1.0 × 1.0 × 1.0 mm).
The functional imaging data underwent the following preprocessing steps: (1) slice-time correction with SPM2 (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Cognitive Neurology, London, UK) and (2) motion correction using rigid body translation and rotation parameters (FSL [35, 36]). Individual participants’ T1 scans were then reconstructed into surface representations using FreeSurfer (http://surfer.nmr.mgh.harvard.edu). Functional data were registered to structural images using the reconstructed cortical surfaces [37] (http://surfer.nmr.mgh.harvard.edu/fswiki/FsFast). The structural images were in turn nonlinearly registered to the MNI152 space [38, 39]. The resulting nonlinear deformations were used to warp the functional data into MNI152 space and smoothed with a 6 mm FWHM smoothing kernel. All time courses were normalized as percent signal change relative to the mean BOLD signal in each voxel.
Statistical analyses were performed in Brain Voyager QX 2.3 (Brain Innovation). Regressors for later remembered (R), later forgotten (F), unsure (U), control (C), and error trials were modeled using stick functions convolved with a double γ hemodynamic response function. Error trials were those that were incorrectly judged or not responded to at encoding. BOLD activity associated with successful encoding (R > C) was contrasted using a random-effects general linear model (GLM)-based analysis normalized to percent with an AR(1) model to correct for serial correlations. A voxel-level threshold of p < 0.001 (uncorrected) for t-maps was applied for comparisons of main effects. For correlational analyses, a threshold of p < 0.01 was adopted in view of the lower signal-to-noise ratio often observed in the anterior medial temporal lobe (MTL) [40–42]. In addition, to control for type I errors, the remaining voxels were then processed using an iterative cluster size thresholding procedure [43] that considered the spatial smoothness of functional imaging data when generating activation maps based on a corrected cluster threshold (p < 0.05).
Results
Data from 36 out of 37 participants (mean ± SD: 22.5 ± 2.4 years; 17 males) contributed to EEG and behavioral analyses. The excluded participant had an extremely high false alarm rate of 0.70 suggesting failure to comply with task instructions. Of the remaining 36 persons, 33 participants (mean ± SD: 22.6 ± 2.3 years; 17 males) contributed to the imaging analysis investigating encoding-related activity. Of the excluded participants, two had >1 mm within-run motion for more than one task run while one had insufficient brain coverage.
EEG measures
Participants received an average of 1016 stimulations (SD = 434) in the STIM condition, compared with 1054 marked SO up-states (SD = 324) in the SHAM condition. Although this difference was not significant (p = 0.88), there was almost a 10-fold variation in the number of SO available for stimulation across participants (range = 169–1663). Furthermore, the variation in SO counts across two nap opportunities was also large (STIM – SHAM SO count range = −1401 to 877) as evidenced by a relatively low concordance in SO counts across conditions within the same individual ( = 0.46, p = 0.005).
Across the 36 participants, acoustic stimulation resulted in significantly larger evoked responses compared with those in the SHAM condition (Figure 1B). However, there was a wide range in ASRI scores (range = 0.60–4.56, mean = 1.89, SD = 0.92, one-sample t-test from unity; t35 = 5.75, p < 0.001) across all participants (Figure 1C). Interestingly, a low SO count did not preclude a high ASRI; the difference in SO count between conditions was not significantly correlated with the ASRI (p = 0.21).
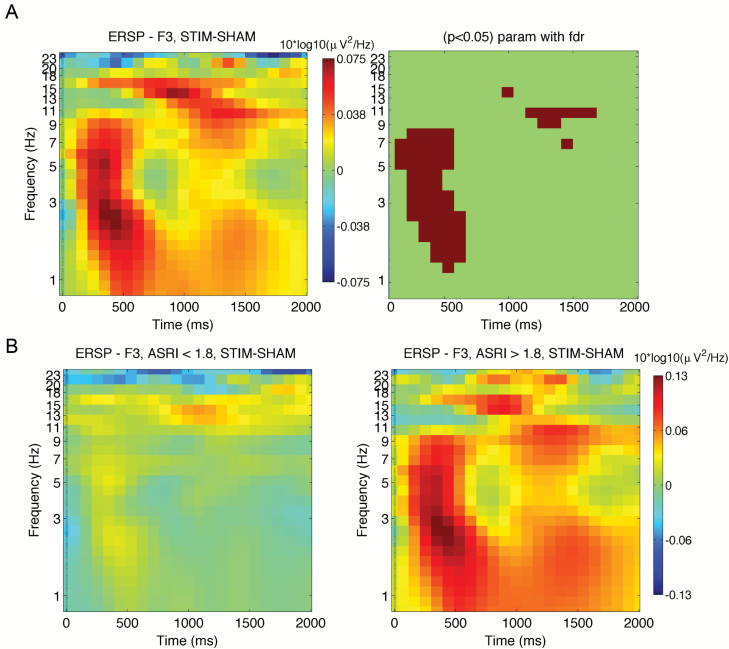
ERSP plots revealed an increase in SWA/θ power and spindle activity (9–15 Hz) approximately 150–600 ms and 1000–1600 ms, respectively, after tone onset (Figure 3A). Again, there was a range in response variability, as shown by the sub-group plots defined with a median split: individuals with ASRI < 1.80 and with ASRI > 1.80 (Figure 3B).
Figure 3.
EEG time-frequency plots. (A) STIM-SHAM ERSP plots at electrode F3 locked to the onset of each ON block (t = 0) for all participants (N = 36). Time-frequency points significantly different between the two conditions are shown on the right panel (p < 0.05, FDR-corrected). (B) ERSP plots for groups of individuals defined by a median split: with ASRI < 1.80 (N = 18; left panel), and with ASRI > 1.80 (N = 18; right panel).
ERP and time-frequency analyses locked to the onset of OFF blocks revealed no differences between STIM and SHAM conditions, suggesting that SO augmentation was acute rather than prolonged, i.e. limited to SOs targeted during the ON blocks.
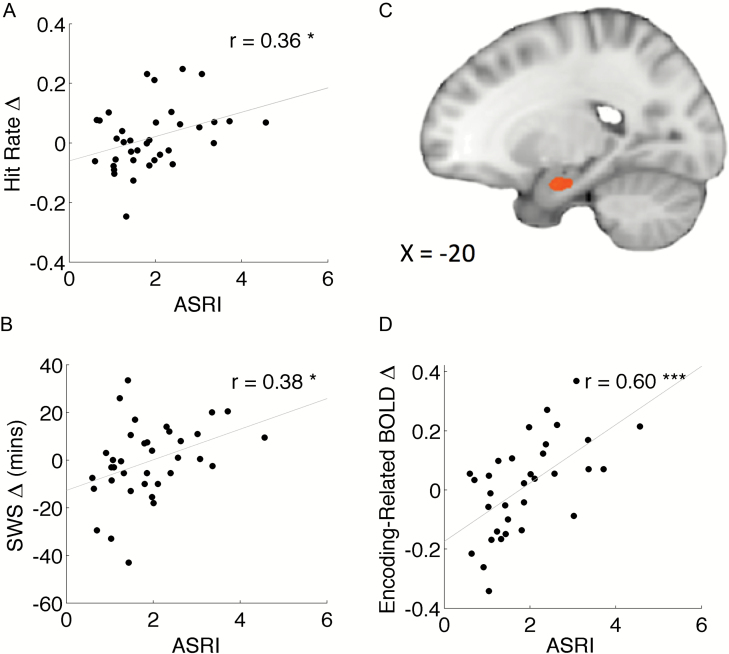
ASRI and differences in slow-wave sleep duration between conditions were positively correlated (r34 = 0.38, p = 0.024, Figure 4B) suggesting that stimulation of SOs increased the amount of SWS. ASRI did not correlate with duration alterations in other sleep stages (p’s > 0.09). Other facets of sleep architecture for the STIM and SHAM conditions are documented in Table 1.
Figure 4.
Associations between the ASRI and changes in behavior as well as brain activity in the STIM vs. SHAM conditions. (A) Correlation between ASRI and change in hit rate. Individuals who responded more to the stimulation had a higher hit rate in the STIM condition. (B) Correlation between ASRI and change in SWS. Individuals who showed a greater response to stimulation had longer SWS. (C) Correlation between the ASRI and change in encoding-related BOLD activity in the left anterior hippocampus (p < 0.01, cluster-corrected at p < 0.05; k ≥ 149 mm3). (D) Corresponding correlation plot from the peak voxel (x = −20, y = −5, z = −23).
Table 1.
Sleep architecture
| STIM | SHAM | t/Z | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Total sleep time (min) | 79.52 | 11.03 | 78.33 | 9.67 | 0.44 | 0.66 |
| N1 sleep (min) | 5.54 | 4.67 | 4.53 | 3.51 | 1.35 | 0.19 |
| N2 sleep (min) | 38.78 | 12.09 | 39.17 | 11.30 | 0.17 | 0.87 |
| SWS (min) | 28.69 | 17.40 | 29.26 | 13.55 | 0.21 | 0.83 |
| REM sleep (min) | 6.51 | 8.45 | 5.38 | 6.79 | 0.69 | 0.49 |
| NREM sleep (min) | 73.01 | 13.20 | 72.96 | 9.97 | 0.03 | 0.98 |
| N2 sleep latency (min) | 7.19 | 4.30 | 8.63 | 5.22 | 2.09 | 0.04 |
| WASO (min) | 3.82 | 5.67 | 3.43 | 5.45 | 0.41 | 0.68 |
| Sleep efficiency (%) | 87.74 | 9.28 | 86.66 | 9.35 | 1.48 | 0.14 |
Averages and standard deviations (in parentheses) of sleep parameters (in minutes) for the STIM and SHAM conditions (N = 36) are presented.
SD = standard deviation; N1 = stage 1; N2 = stage 2; SWS = slow-wave sleep; WASO = wake after sleep onset.
Across the whole nap period, ASRI was positively correlated with differences in mean NREM EEG power between the STIM and SHAM conditions for the lower frequencies but not the higher ones, suggesting that only the lower frequency bands were affected by the stimulation. In particular, ASRI correlated with changes in SO, SWA, and θ power ( = 0.40, p = 0.02, = 0.46, p = 0.005, and r34 = 0.36, p = 0.03, respectively), but not for changes in α or β power (p = 0.30 and 0.48, respectively). Hence, individuals more responsive to stimulation had larger spectral power increases at low EEG frequencies. Regarding spindle detection from channel C3, there was no difference between conditions for all measures considered (spindle density, count, and duration; p’s > 0.32).
Behavioral measures
Behavioral measures during picture encoding and retrieval in both STIM and SHAM conditions are summarized in Tables 2 and 3. Hit rate was computed by the proportion of “yes” responses to old images, whereas false alarm rate was the proportion of “yes” responses to new images. Only correct trials during the encoding phase (indoor/outdoor scene judgment) entered analyses.
Table 2.
Memory performance
| STIM | SHAM | t | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Hit rate | 0.68 | 0.15 | 0.67 | 0.15 | 0.96 | 0.34 |
| False alarm rate | 0.24 | 0.11 | 0.24 | 0.15 | 0.05 | 0.96 |
| A-prime | 0.80 | 0.09 | 0.79 | 0.11 | 0.62 | 0.54 |
| Number of correct trials at encoding (of 80) | 78.36 | 1.99 | 78 | 5.46 | 0.46 | 0.65 |
Averages and standard deviations (in parentheses) of hit and false alarm rates, together with the number of correct trials at encoding for the STIM and SHAM conditions (N = 36). Note that only trials that were correctly judged (indoor/outdoor) at encoding were included in calculations of hit rate (proportion answered “yes” to old images) and false alarm rate (proportion answered “yes” to new images) are shown.
SD = standard deviation.
Table 3.
Response times at encoding and retrieval
| STIM | SHAM | t | P | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Encoding phase | ||||||
| Hits (s) | 1.13 | 0.32 | 1.20 | 0.45 | 1.14 | 0.26 |
| Misses (s) | 1.13 | 0.34 | 1.24 | 0.43 | 2.01 | 0.05 |
| Retrieval phase | ||||||
| Hits (s) | 3.19 | 1.23 | 3.14 | 1.21 | 0.42 | 0.67 |
| Misses (s) | 3.64 | 1.48 | 3.60 | 1.34 | 0.22 | 0.83 |
| False alarms (s) | 3.56 | 1.62 | 3.56 | 1.54 | 0.39 | 0.70 |
| Correct rejections (s) | 3.74 | 1.64 | 3.70 | 1.33 | 0.20 | 0.85 |
Averages and standard deviations (in parentheses) of response time (in seconds) for each trial type during the encoding phase (indoor/outdoor judgment task) and retrieval phase (recognition task) in the STIM and SHAM conditions (N = 36) are shown.
SD = standard deviation.
There was no group-level memory difference between STIM and SHAM conditions. However, there was a significant positive correlation between the ASRI and the difference in hit rate across conditions (r34 = 0.36, p = 0.037, Figure 4A). Individuals who were more responsive to stimulation showed greater memory improvement in the STIM condition. This relationship was not observed with changes in false alarm rates (p = 0.44), A′ (p = 0.54), or with changes in the proportion of low confidence (“Unsure”) responses (p = 0.90). There was also no correlation between the ASRI and changes in response time during encoding or retrieval (p = 0.78 and p = 0.96, respectively) or with changes in self-reported sleepiness prior to encoding or retrieval (p = 0.14 and p = 0.82, respectively) that would suggest a more general effect of vigilance on performance.
Thus, there appears to be dissociation between the clear, significant effect of acoustic stimulation on SO enhancement and a more variable effect on boosting declarative memory that was correlated with degree of SO enhancement. The positive association with memory was specific for SO enhancement and not with spindle activity increase in the 1000–1600 ms window following tone onset (p = 0.67).
Imaging data
Overall, there was no difference in encoding-related activity between STIM and SHAM conditions anywhere in the brain. However, corresponding to the dissociation in EEG and behavioral effects of stimulation, individuals who showed greater SO enhancement following stimulation showed larger left anterior hippocampal BOLD responses for subsequently remembered compared with control items (peak voxel: x = −20, y = −5, z = −23, rmax = 0.60, p = 0.00025, cluster-corrected p < 0.05; Figure 4C and D). There was a significant positive correlation between ASRI and the between-condition difference in anterior hippocampal BOLD signal. The magnitude of hippocampal signal shift across conditions also correlated with memory benefit (r31 = 0.37, p = 0.04). Although both an increase in hippocampal activation and memory benefit were associated with stronger augmentation of SO by acoustic stimulation, memory benefit was not mediated by a larger shift in hippocampal signal (mediation analysis: nonsignificant indirect effect; 95% CI: [−0.017, 0.0516]).
Although spindles were also significantly augmented by acoustic stimulation, the extent to which they were boosted did not correlate with either memory improvement or hippocampal activation. For completeness, we also computed correlations with spindle counts, density, and duration (all p’s > 0.29).
Discussion
In the largest study of acoustic stimulation to augment SO in sleep to date, we found that averaged across all participants, phase-locked acoustic stimulation significantly enlarged and entrained SO in addition to increasing SWS duration. The magnitude of SO enhancement indexed by the ASRI, correlated with superior picture encoding. Hippocampal activation at encoding was positively correlated with ASRI. Although spindle band activity was also enhanced by SO, their degree of enhancement was not associated with behavioral improvement. In contrast to the overall efficacy of acoustic stimulation in enhancing SO, there was, on average, no group-level benefit on encoding, suggesting the need for further inquiry into factors contributing to interindividual differences in behavioral response to SO enhancement.
Benefit of SO augmentation on encoding and potential mechanisms
To date, most research on how slow wave sleep aid memory has focused on its role in the consolidation of declarative memory. Given this, it comes as no surprise that total sleep deprivation can impair encoding [13]. However, only a single study has reported that boosting SO with electrical stimulation enhances learning following a nap. An uncertainty surrounding this work is its use of nonphase-locked electrical stimulation. Beyond merely enhancing SO, phase locking of stimulation to the up phase of each oscillation appears to be important for the achievement of memory benefit [3, 44]. Furthermore, interference of the EEG signal during stimulation precludes evaluation of the extent to which augmentation of individual SOs contributes to behavioral improvement. The present work is the first to directly demonstrate that, in line with mechanistic predictions, endogenous SO augmentation by acoustic stimulation can modulate memory encoding.
Three mechanisms can explain how SO augmentation benefits memory encoding. According to the synaptic homeostasis hypothesis, synapses potentiated during wakefulness are downscaled by SO during sleep to restore encoding capacity in subsequent wakefulness [17, 45, 46]. Secondly, hippocampal-neocortical dialogue during sleep results in redistribution of newly encoded representations from the hippocampus to long-term storage sites in neocortical regions [47, 48]. This consolidation of memory representations serves to free up hippocampal capacity to encode new information in the following wake period [16]. Thirdly, an upward surge in ATP occurs in wake-active parts of the brain including the hippocampus during early NREM sleep and is delayed by sleep deprivation [18]. The surge correlates with NREM δ (0.5–4.5 Hz) activity and we conjecture that SO augmentation might thus boost brain ATP. Although the benefit of this surge has been attributed to enhancing synaptic plasticity during sleep and memory consolidation, it seems reasonable that the energetic benefit would extend to encompass improved encoding during subsequent wakefulness.
Hippocampal engagement following acoustic stimulation
The hippocampus is critical for the encoding and retrieval of declarative memories. Following a night of total sleep deprivation, poorer memory was associated with reduced hippocampal activation during encoding [13]. Although several studies have shown the benefit of a mid-afternoon nap in improving declarative memory consolidation [49–53], only one prior work has shown that a nap may benefit encoding [54]. The present study is the first to correlate the magnitude of SO augmentation (ASRI) with a shift in hippocampal activation, as well as with the benefit on encoding. The association between ASRI and shift in hippocampal activation helps alleviate a potential concern that better encoders are simply those with higher hippocampal activation for reasons unrelated to SO augmentation.
Spindle activity: augmented but not contributory to memory
Although spectral analysis indicated a boost in EEG power in the spindle frequency range, this increase was neither correlated with ASRI nor memory benefit. Although an association between spindle power and episodic memory was demonstrated in one study [55], another that used electrical stimulation to enhance SO reported no significant correlation between an increase in phase-coupling of spindle activity to SO and encoding benefit [12]. Given these mixed findings and the vast literature on the role of sleep spindles in aiding memory, we feel that it is premature to rule out the possibility that enhancing spindles might contribute to improvement in memory. In addition, memory improvement was not mediated by the increase in hippocampal activation, suggesting that there could be other factors not presently investigated that contributed to memory benefit and its variation across participants. Beyond the hippocampus, successful encoding requires the engagement of various neural substrates including perceptual processing regions. Variability in these other mnemonic-related regions could have diluted subsequent behavioral outcomes.
It is unclear why more participants did not evidence memory improvement despite successful SO augmentation. Illustrative of this conundrum is a recent finding of a negative association between SWA and cerebrospinal fluid (CSF) amyloid-β levels [54]. Despite SWA reduction after slow-wave disruption, there was no group-level effect on amyloid-β levels. It was reasoned that “non-responders” already had low levels of SWA in the control condition, making it difficult to reduce this further. Perhaps there is a threshold level of SO augmentation that is necessary for memory augmentation.
Large interindividual differences in the number of SOs available for stimulation were observed both between and within participants. Although participants adhered to a sleep schedule prior to each session, we were not able to monitor or control variations in sleep architecture or daytime activity of each participant throughout the week. These variations may have had an equal or larger impact than our manipulation itself during the experimental session, pointing to a need for further studies to systematically investigate these. For example, the amplitude/occurrence of slow waves have been shown to differ depending on prior learning experience [56], during a nap vs. nocturnal sleep [57], after sleep deprivation vs. after a normal night of sleep [58], and across different age-groups [59].
The use of a recognition task here could also have contributed to the lack of a group-level increase in performance. As recognition could be supported by neural substrates other than the hippocampus [60, 61], the SO enhancement benefit here could be weaker compared with recall-based tasks that rely mainly on the hippocampus.
Conclusion
In summary, this study suggests compelling links between the magnitude of SO enhancement, encoding performance on a hippocampal-dependent task, and increase in hippocampal activation. The high variability of SO occurrence and memory response across participants, as well as the absence of memory benefit of incidentally enhancing spindles are areas that merit further inquiry.
Funding
This work was supported by the National Medical Research Council, Singapore (NMRC/STaR/015/2013) and The Far East Organization.
Acknowledgments
The authors would like to thank Wanzheng Zhu for his help with algorithm development; Te Yang Lau, Shirley Koh, and Gloria Huan for their help with data collection; and James Cousins and June Lo for invaluable discussions. This work was approved by the Institutional Review Board of the National University of Singapore (B-15–116). All participants provided written informed consent.
Notes
Conflict of interest statement. None declared.
References
- 1. Diekelmann S, et al. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. [DOI] [PubMed] [Google Scholar]
- 2. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. [DOI] [PubMed] [Google Scholar]
- 3. Ngo HV, et al. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78: 545–553. [DOI] [PubMed] [Google Scholar]
- 4. Ong JL, et al. Effects of phase-locked acoustic stimulation during a nap on EEG spectra and declarative memory consolidation. Sleep Med. 2016;20:88–97. [DOI] [PubMed] [Google Scholar]
- 5. Papalambros NA, et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front Hum Neurosci 2017;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leminen MM, et al. Enhanced memory consolidation via automatic sound stimulation during non-REM sleep. Sleep. 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lustenberger C, et al. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Biol. 2016;26:2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh JK, et al. Tiagabine enhances slow wave sleep and sleep maintenance in primary insomnia. Sleep Med. 2006;7:155–161. [DOI] [PubMed] [Google Scholar]
- 9. Walsh JK, Snyder E et al. . Slow wave sleep enhancement with gaboxadol reduces daytime sleepiness during sleep restriction. Sleep. 2008;31:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feld GB, et al. Slow wave sleep induced by GABA agonist tiagabine fails to benefit memory consolidation. Sleep. 2013;36:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marshall L, et al. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antonenko D, et al. Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci. 2013;37:1142–1151. [DOI] [PubMed] [Google Scholar]
- 13. Yoo SS, et al. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. [DOI] [PubMed] [Google Scholar]
- 14. Poh JH, et al. Degradation of cortical representations during encoding following sleep deprivation. NeuroImage. 2017;153:131–138. [DOI] [PubMed] [Google Scholar]
- 15. Gais S, et al. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saletin JM, et al. Nocturnal mnemonics: sleep and hippocampal memory processing. Front Neurol. 2012;3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tononi G, et al. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. [DOI] [PubMed] [Google Scholar]
- 18. Dworak M, et al. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. [DOI] [PubMed] [Google Scholar]
- 20. Patanaik A, et al. An end-to-end framework for real-time automatic sleep stage classification. Sleep, 2018;41:zsy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 22. Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Massimini M, et al. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riedner BA, et al. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 26. Welch PD. The use of the fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE T Acoust Speech. 1967;15:70–73. [Google Scholar]
- 27. Paller KA, et al. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6:93–102. [DOI] [PubMed] [Google Scholar]
- 28. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. [DOI] [PubMed] [Google Scholar]
- 29. Russell B, et al. LabelMe: a database and web-based tool for image annotation. Int J Comput Vis. 2008;77:157–173. [Google Scholar]
- 30. Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 31. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- 32. Dinges DF, et al. Microcomputer analyses of performance of a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17: 652–655. [Google Scholar]
- 33. Stanislaw H, et al. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–149. [DOI] [PubMed] [Google Scholar]
- 34. Hayes AF. Introduction to Mediation, Moderation and Conditional Process Analysis. New York, NY, USA: The Guilford Press, 2013. [Google Scholar]
- 35. Jenkinson M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. [DOI] [PubMed] [Google Scholar]
- 36. Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 37. Greve DN, et al. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ojemann JG, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 1997;6:156–167. [DOI] [PubMed] [Google Scholar]
- 41. Olman CA, et al. Distortion and signal loss in medial temporal lobe. PLoS One. 2009;4:e8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Davachi L, et al. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. [DOI] [PubMed] [Google Scholar]
- 43. Goebel R, et al. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006;27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weigenand A, et al. Timing matters: open-loop stimulation does not improve overnight consolidation of word pairs in humans. Eur J Neurosci. 2016;44:2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tononi G, et al. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. [DOI] [PubMed] [Google Scholar]
- 46. de Vivo L, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355:507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takashima A, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sirota A, et al. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst. 2005;3:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lo JC, et al. Comparing the effects of nocturnal sleep and daytime napping on declarative memory consolidation. PLoS One. 2014;9:e108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tucker MA, et al. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tucker MA, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–247. [DOI] [PubMed] [Google Scholar]
- 52. Baran B, et al. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci. 2016;28:792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schabus M, et al. Influence of midday naps on declarative memory performance and motivation. Somnologie. 2005;9:148–153. [Google Scholar]
- 54. Mander BA, et al. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21:R183–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ju YS, et al. Slow wave sleep disuption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huber R, et al. Local sleep and learning. Nature.2004;430: 78–81. [DOI] [PubMed] [Google Scholar]
- 57. Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6–S15. [PMC free article] [PubMed] [Google Scholar]
- 58. Dijk DJ, et al. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–R661. [DOI] [PubMed] [Google Scholar]
- 59. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. [DOI] [PubMed] [Google Scholar]
- 60. Norman KA, et al. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. [DOI] [PubMed] [Google Scholar]
- 61. Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]