Abstract
Randomized controlled trials of blood pressure (BP) lowering and antihypertensive medication use on cognitive outcomes have often been disappointing, reporting mixed findings and small effect sizes. We evaluate the extent to which cognitive assessment protocols used in these trials approach state-of-the-art. Overall, we find that a primary focus on cognition and the systematic selection of cognitive outcomes across trials take a backseat to other trial goals. Twelve trials investigating change in cognitive functioning were examined and none met criteria for state-of-the-art assessment, including use of at least 4 tests indexing 2 cognitive domains. Four trials investigating incident dementia were also examined. Each trial used state-of-the-art diagnostic criteria to assess dementia, although follow-up periods were relatively short, with only 2 trials lasting for at least 3 years. Weaknesses in each trial may act to obscure or weaken the positive effects of BP lowering on cognitive functioning. Improving trial designs in terms of cognitive outcomes selected and length of follow-up periods employed could lead to more promising findings. We offer logical steps to achieve state-of-the-art assessment protocols, with examples, in hopes of improving future trials.
Keywords: antihypertensive medication, blood pressure, cognitive assessment, cognitive function, dementia, hypertension, randomized controlled trial, systematic review
The number of Americans aged 65 years and older living with Alzheimer’s disease (AD) is projected to increase from 5.1 million in 2015 to 13.5 million in 2050,1 and many more will develop other forms of dementia, including vascular and mixed dementia. Sperling et al.2 have estimated that a hypothetical intervention delaying the onset of AD by 5 years would reduce the number of AD patients by 57% and reduce associated Medicare costs by approximately $283B.
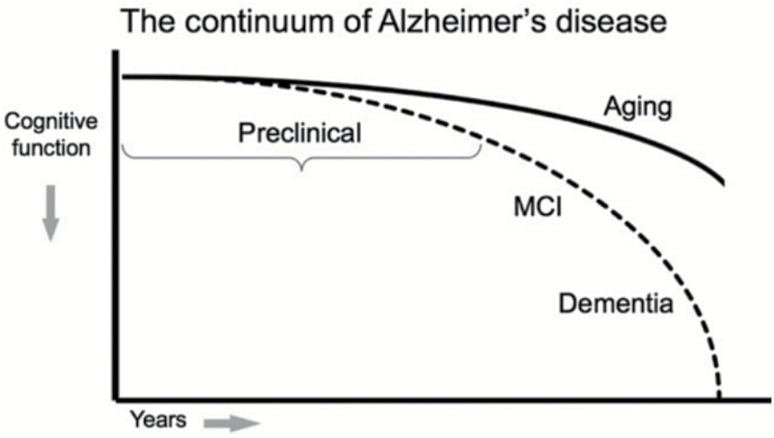
Arterial hypertension is a well-established risk factor for cognitive decline and development of dementia.3–7 Reduction of blood pressure (BP) with the goal of slowing these processes has been investigated in randomized controlled trials (RCTs). Some trials are devoted to examining cognitive performance in persons who have not been diagnosed with dementia, but who may ultimately succumb to the disease. Others attempt to intervene in the long latency period or prodromal phase of dementia (Figure 1) with the goal of slowing or halting disease progression.2
Figure 1.
Model of the clinical trajectory of Alzheimer’s disease (AD). Reused from Sperling et al.2 with permission.
Findings in RCTs of BP lowering and antihypertensive medication use have been inconsistent and overall effect sizes have been disappointingly small.8,9 In 2011, Staessen et al.10 conducted a meta-analysis involving 8 major trials of BP lowering for the prevention of dementia. Nonsignificant findings were observed for all trials combined, although subanalyses of specific antihypertensive medications revealed that risk for dementia was reduced by 18% in trials involving a diuretic or dihydropyridine calcium channel blocker as part of active treatment (P < 0.05). This level of protection is not high and is not surprising, as small effect sizes are common when we average over many trials.4,8–10
A recent decision by the pharmaceutical company Pfizer not to continue with research efforts to discover new medications for the treatment of AD11 may in part reflect the results of previous trials such as these. We raise the possibility that more promising trial results could be achieved if state-of-the-art protocols for the assessment of cognitive outcomes were adopted. In this review, we create a set of criteria for state-of-the-art assessment of cognitive function and dementia and apply these criteria to previous RCTs of BP lowering and antihypertensive medication use. We conclude by providing models for state-of-the-art cognitive test batteries to use in future RCTs.
CRITERIA FOR STATE-OF-THE-ART ASSESSMENT
Cognitive function
The concepts of state-of-the-art and “done well” are not the same. State-of-the-art methods are those that are highly developed and result from common methodologies employed at a given point in time. We based our judgment of state-of-the art on the evolution of cognitive theories and what has been done in an increasing number of observational studies of hypertension, antihypertensive medication use, and cognitive function.3,4,12 State-of-the-art assessment of cognitive function is characterized by 2 fundamental features: (i) assessment of multiple cognitive abilities and (ii) arrangement of these abilities in a systematic, hierarchical way that is theoretically important and clinically relevant.
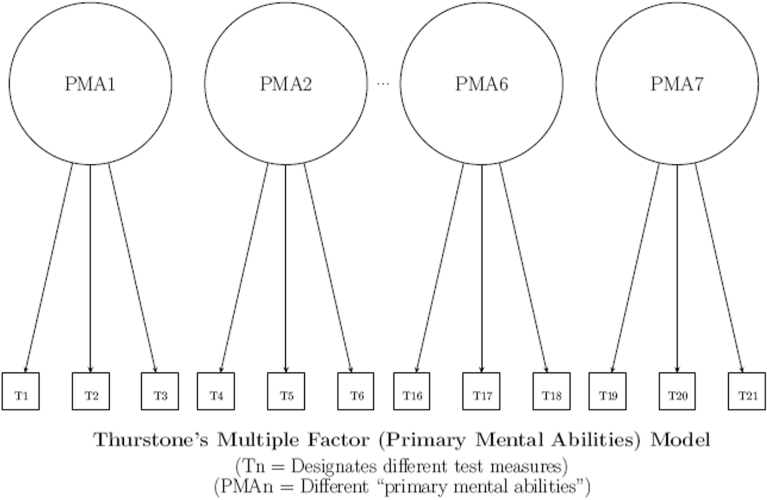
To understand our emphasis on hierarchies, it is important to first understand the structure of cognitive abilities. Spearman13 and Spearman and Jones14 have provided evidence for a general or global human ability (g) and a subset of specific abilities that are correlated with g (Figure 2). More recent theories recognize a complex hierarchical relationship among primary mental abilities, also referred to as cognitive domains, and the subservient specific abilities that index them (Figure 3).15,16 Cognitively healthy persons who exhibit higher global performance tend to have greater functioning in specific domains. However, different aspects of cognition are affected by disease processes and brain injury in different ways. State-of-the-art test batteries must assess multiple cognitive domains in order to accurately capture this variability, but how many tests are necessary?
Figure 2.
Spearman’s general factor model.
Figure 3.
Thurstone’s multiple factor model.
Thurstone15 identified Word Fluency, Verbal Comprehension, Spatial Visualization, Number Facility, Associative Memory, Reasoning and Perceptual speed as the 7 cognitive domains. On this basis, we could recommend a minimum of 7 cognitive tests for a state-of-the-art RCT. However, many other domains have been identified and clinical trials are limited by cost, time, and subject burden in terms of the number of outcome measures that can be employed. The Spearman–Brown prophecy formula was created to solve for a desired reliability or required number of tests to use in a research study.17,18 Applying this formula, 4 cognitive tests, each with a reliability of 0.5, can be employed to achieve an overall reliability of 0.8. If each of the 4 cognitive tests have a reliability of 0.65, the overall reliability will be increased to 0.9. A clinical trial with 4 tests thus meets a minimal criterion for state-of-the-art cognitive assessment. However, the ideal design should also involve multiple cognitive domains. We recommend that state-of-the-art assessment protocols include a minimum of 2 cognitive domains, each indexed by at least 2 cognitive tests that are both reliable and valid. The argument for using at least 2 tests to index each domain may be found in the paper by Elias et al.4
A good example of a hierarchical arrangement of tests to index higher order cognitive domains is provided by the Wechsler Adult Intelligence Scale (WAIS)-IV (Figure 4).19 Use of the complete WAIS IV may be impractical, but one can, at a minimum, select 2 tests that define each domains of interest. Some RCTs have taken advantage of these tests, although none have employed a hierarchical design in which important theoretical or clinical domains are examined as well.
Figure 4.
Wechsler Adult Intelligence Scale hierarchy of cognitive domains, abilities and tests.
In summary, to be state-of-the-art, an assessment protocol must include multiple measures of cognitive abilities and use these to index higher order domains. There are no set rules for the number of tests used or domains examined. However, we recommend the examination of at least 2 domains, each indexed by a minimum of 2 tests. A seminal text by Lezak et al.20 is recommended for investigators who wish to obtain more information about cognitive testing. Additional texts are recommended for investigators who wish to obtain a better understanding of test construction and interpretation: Kline,21 Embretson,22 Cronbach,23 and Guilford.24
Dementia
Achieving state-of-the-art assessment of dementia requires a different set of more clinically oriented considerations. The most important criterion is the use of well-established diagnostic criteria, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Statistical Classification of Diseases and Related Health Problems (ICD), 10th edition, criteria. Definitions of dementia based on the Mini Mental State Examination (MMSE), a screening test or dementia rating scale alone are not sufficient. All-cause dementia is most often used as the primary study outcome in RCTs of BP lowering and antihypertensive medication use. Separating all-cause dementia into AD, vascular dementia, and mixed dementia would improve RCTs.25–27 However, given time constraints and the high cost of obtaining the data needed to make more specific diagnoses, this is generally not feasible. Instead of criteria for state-of-the-art assessment, we thus consider this to be a gap in knowledge.
Clinical trial length is an important consideration when dementia is an outcome. The question of how long a trial aiming to slow the progression of dementia should be is not easily answered. Five and 10 years are often given as “off the top of the head” answers among those who perform longitudinal research on aging, and these time spans are supported by many studies.6,28–30 In a small study by Tscharnz et al.31 (n = 129), 3-year rates of conversion to dementia were 46% among those with cognitive impairment at baseline and 3.3% among those with no impairment. We suggest that a state-of-the-art trial with dementia outcomes include a follow-up period of at least 3 years, although longer periods are always better.
In the following section, we describe the search methods used to identify RCTs of antihypertensive medication use and cognitive outcomes, including cognitive function and dementia. We subsequently provide a description of each trial and rate it with respect to the state-of-the-art criteria discussed.
ARE PREVIOUS RCTS STATE-OF-THE-ART?
Search methods
Online databases and search engines that were used to identify RCTs of antihypertensive medication use and cognitive outcomes included: PubMed, Google Scholar, EBSCOhost, PsychINFO, Medline, ScienceDirect, Web of Science, and the Cochrane library. The following requirements were used to select studies: (i) needed to be a RCT; (ii) participants needed to have high BP at baseline; (iii) participants needed to be randomized to a treatment arm including antihypertensive medications or a placebo; (iv) cognitive performance, cognitive impairment, and/or dementia needed to be included as outcome measures and; (v) assessment procedures needed to be adequately described.
Search terms related to the study design were: RCT, placebo-controlled trial, and clinical trial. Search terms related to outcome measures were: cognition, cognitive performance, cognitive functioning, cognitive impairment, cognitive dysfunction, dementia, and AD. Search terms related to the predictor were: hypertension, high BP, antihypertensive medication, antihypertensive drug, and antihypertensive treatment. An ongoing RCT of BP lowering was recommended to us by a reviewer and is evaluated in the following section. More information on the search procedures used in this review can be found in Supplement 1.
RCTs of antihypertensive medication use and cognitive function
Twelve RCTs of antihypertensive medication use and cognitive function met criteria for inclusion in the review. Each trial is briefly summarized in Table 1. Eight RCTs included comparison of multiple active treatment groups and 5 RCTs included a placebo condition, a current recommendation in clinical trial design. Significant improvement in cognitive function or reduction in risk for cognitive decline was found in 5 RCTs, albeit effect sizes were modest.
Table 1.
Summary of clinical trials examining the effects of antihypertensive medication use on cognitive function (reverse chronological order)
| Study | Study goal | Study design | Cognitive measures | Change in BP across the study period | Change in cognition across the study period |
|---|---|---|---|---|---|
| AVEC Trial, 201232 | Examine different therapeutic options for executive dysfunction in hypertensives | Treatment arms: ACEI (lisinopril) vs. ARB (candesartan) vs. diuretic (HCTZ) Study length = 1.0 year n = 53 |
MMSE, Trail Making Test A/B, Hopkins Verbal Learning Test—Revised, Digit Span Test | Systolic BP reductions were similar in all treatment groups (range 21 ± 5 to 28 ± 5 mm Hg) | Participants in the ARB group showed the greatest improvement on the Trail Making Test and Hopkins Verbal Learning Test |
| ONTARGET, 201133 | Examine the effects of renin–angiotensin system blockade on cognitive function | Treatment arms: ACEI (ramipril) vs. ARB (telmisartan) vs. combination Study length = 4.7 years n = 25,620 |
MMSE | Systolic/diastolic BP fell more in the combination and ARB groups than in the ACEI group (2.4/1.4 mm Hg and 0.9/0.6 mm Hg differences, respectively) | No differences in cognitive function between groups |
| TRANSCEND Trial, 201133 | Examine the effects of renin–angiotensin system blockade on cognitive function | Treatment arms: ARB (telmisartan) vs. placebo Study length = 4.7 years n = 5,926 |
MMSE | Systolic/diastolic BP fell more in the ARB group than in the placebo group (4.0/2.2 mm Hg difference) | No differences in cognitive function between groups |
| PRoFESS Trial, 200834 | Examine the neuroprotective effects of ARB use after an ischemic stroke | Treatment arms: ARB (telmisartan) vs. placebo; participants also took clopidogrel or aspirin + dipyridamole Study length = 2.4 years n = 20,332 |
MMSE | Systolic BP fell more in the ARB group than the placebo group (5.4 mm Hg difference at 1 month) | No difference in cognitive function between groups |
| PROBE Study, 200635 | Examine the effects of antihypertensive medication use on ambulatory BP and cognitive function | Treatment arms: ARB (telmisartan) + diuretic (HCTZ) vs. ACEI (lisinopril) + diuretic (HCTZ) Study length = 24 weeks n = 160 |
1-minute recall of animal names, modified Boston Naming test, word-list memory test, word-list recall test, word-list recognition, Trail Making Test B | Mean decreases in 24-hour, daytime and night time systolic/ diastolic BP were greater in the ARB group compared with the ACEI group | Participants in the ARB group had improved scores on word-list memory (17.1% increase at 12 weeks and 15.7% at 24 weeks), word- list recall (13.5% increase at 12 weeks and 16.9% at 24 weeks) and Trail Making Test B (33% increase at 12 weeks and 30.5% at 24 weeks). The ACEI did not affect cognition |
| PROBE Study, 200436 | Examine the effects of antihypertensive medication use on BP and cognitive function | Treatment arms: ARB (valsartan) vs. ACEI (enalapril) Study length = 16 weeks n = 144 |
1-minute recall of animal names, modified Boston Naming Test, word-list memory test, word-list recall test, word-list recognition | Systolic/diastolic BP fell more in the ARB group than the ACEI group (3/2.8 mm Hg differences) | Participants in the ARB group had improved scores on word-list memory (11.8% increase) and word- list recall tests (18.7% increase). The ACEI did not affect cognition |
| PROGRESS, 200337 | Examine the effects of antihypertensive medication use on risk for dementia and cognitive decline | Treatment arms: ACEI (perindopril) ± diuretic (indapamide) vs. placebo Study length =3.9 years n = 6,105 |
MMSE | Systolic/diastolic BP fell more in the ACEI group than in the placebo group (9/5 mm Hg difference) | Participants in the ACEI group had a 19% reduction in risk for cognitive decline compared with the placebo group |
| MRC Treatment Trial of Hypertension in Older Adults, 199638 | Examine the effects of antihypertensive medication use on cognitive function | Treatment arms: diuretic (HCTZ + amiloride) vs. beta blocker (atenolol) vs. placebo Study length = 4.5 years n = 2,584 |
Paired Associate Learning Test, Trail Making Test A, New Adult Reading Test, Raven’s matrices | Systolic BP fell more in the diuretic and beta blocker groups than in the placebo group (24.6 mm Hg and 16.8 mm Hg differences, respectively) | No differences in cognitive function between groups |
| HOPE Study, 199639 | Examine the effects of antihypertensive medication use on cognition function | Treatment arms: diuretic (bendrofluazide) vs. ACEI (captopril) Study length = 24 weeks n = 81 |
MMSE, Logical Memory test, Paired Associates test, Raven’s Colored Progressive Matrices, Anomalous Sentence Repetition Test, Trail Making Test A | Systolic/diastolic BP reductions were similar between ACEI (39/14 mm Hg) and diuretic (37/13 mm Hg) groups | No difference in cognition between groups |
| SHEP Study, 199440 | Examine the effects of antihypertensive medication use on quality of life, including cognitive function | Treatment arms: diuretic (chlorthalidone) ± beta blocker (atenolol) or rauwolfia alkaloid (reserpine) vs. placebo Study length = 5.0 years n = 4,736 |
Digit Symbol Substitution, Boston Naming Test, Delayed Recognition Span Test, Addition test, Finding A’s Test, Letter Sets Test | Not reported | No differences in cognitive function between groups |
| Croog et al., 199441 | Examine the effects of antihypertensive medication use on quality of life, including cognitive function | Treatment arms: beta blocker (atenolol) vs. ACEI (enalapril) vs. CCB (isradipine). Open-label HCTZ added if needed Study length = 22 weeks n = 309 |
Digit Span Test, Digit Symbol Substitution, Trail Making Test A/B | Systolic BP fell more in the ACEI group than the beta blocker group (6.1 mm Hg difference). Diastolic BP reduction was similar between groups (range 15.1 ± 0.9 to 16.2 ± 0.7 mm Hg) | No differences in cognitive function between groups |
| Croog et al., 198642 | Examine the effects of antihypertensive medication use on quality of life, including cognitive function | Treatment arms: ACEI (captopril) vs. alpha blocker (methyldopa) vs. beta blocker (propranolol). Open-label HCTZ added if needed Study length = 24 weeks n = 626 |
Trail Making Test A/B, Wechsler Memory Scale (visual-memory assessment) | Diastolic BP was similar between groups at follow-up (range 86.1 ± 0.65 to 87.7 ± 0.72 mm Hg). Information on systolic BP at follow-up was not reported | Trail Making B scores improved for all groups. Participants taking the ACEI improved more than those taking the alpha blocker |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AVEC, Antihypertensives and Vascular, Endothelial and Cognitive function; BP, blood pressure; HCTZ, hydrochlorothiazide; HOPE, Hypertensive Old People in Edinburgh; MMSE, Mini Mental State Examination; MRC, Medical Research Council; ONTARGET, Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial; PROBE, Parallel-group, prospective, Randomized, Open-label, Blinded-Endpoint; PRoFESS, Prevention Regimen For Effectively avoiding Second Strokes; PROGRESS, Perindopril Protection Against Recurrent Stroke Study; SHEP, Systolic Hypertension in the Elderly Program.; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease Trial.
Understanding the mixed findings reported across trials is made difficult by several factors. Sample sizes, trial lengths, and antihypertensive medications selected for active treatment were variable among RCTs. Sample size ranged from 53 participants in the AVEC trial32 to 20,332 participants in the PRoFESS trial,34 with a mean of 5,556 participants across all trials. Trial length ranged from 16 weeks in the PROBE study36 to 5 years in the SHEP study40 with a mean of 2.3 years across all trials. Medication classes examined were angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretics, beta blockers, alpha blockers, and rauwolfia alkaloids. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were the only medication classes examined in at least 5 RCTs, although specific medications used within these classes were not consistent.
Measurement of cognitive function also varied between trials in terms of tests used and abilities measured (see Supplement 2, Table 1s for full test descriptions). Each battery contained between 1 and 6 tests. However, only 3 of 22 tests (the MMSE, Trail Making Test parts A and B and the Modified Boston Naming test) were used in >2 RCTs. None of the test batteries used met our criteria for state-of-the-art assessment. However, failure to meet these criteria does not imply that the trials did not provide useful information.
Even RCTs with a single outcome provide some information, as many tests are highly correlated with each other (range r = 0.20 to r = 0.80 among subtests of the WAIS43). Six of 12 RCTs used 4 or more tests and findings reported by these trials are considerably more informative than trials using 1 or a few tests. Not a single RCT identified domains of interest and indexed them with multiple tests of ability. Six of 12 RCTs used the MMSE, which is often promoted and used as a measure of global cognitive function. However, this test is actually a low ceiling measure of mental status that, though valuable when screening for dementia, is not suitable for evaluating normal cognitive function.44–47 Some argue that there are more useful tests of cognitive status, such as the Montreal Cognitive Assessment (MoCA).48 However, even this test is not a substitute for assessing global cognitive function. Global cognitive function should only be assessed by aggregating scores from multiple cognitive measures.
RCTs of antihypertensive medication use and dementia
Four RCTs of antihypertensive medication use and incident dementia met criteria for inclusion and are summarized in Table 2. All RCTs included a placebo condition, although primary active treatment differed for each trial. Significant reduction in risk for dementia was found in 2 of 4 trials, with a 12% reduction in risk for participants taking an angiotensin-converting enzyme inhibitor in the PROGRESS trial37 and a 50% reduction in risk for participants taking a calcium channel blocker in the Sys-Eur trial.51
Table 2.
Summary of clinical trials examining the effects of antihypertensive medication use on risk for dementia (reverse chronological order)
| Study | Study design | Dementia assessment | Change in BP across the study period | Dementia risk |
|---|---|---|---|---|
| HYVET-COG, 200849 | Treatment arms: diuretic (indapamide) ± ACEI (perindopril) vs. placebo Study length = 2.2 years n = 3,336 |
DSM-IV criteria and images from a cranial CT scan or the Hachinski ischemic score | Systolic/diastolic BP fell more in the diuretic group than the placebo group (15/5.9 mm Hg differences) | No difference in dementia risk between groups |
| SCOPE, 200550 |
Treatment arms: ARB (candesartan) ± diuretic (HCTZ) vs. placebo Study length = 3.7 years n = 4,937 |
ICD-10 criteria and images from a cranial CT scan or MRI if available | Systolic/diastolic BP fell more in the ARB group than the placebo group (2.5/1.9 mm Hg differences in participants with low cognitive function and 3.3/1.5 mm Hg differences in participants with high cognitive function at baseline) | No differences in dementia risk between medication groups |
| PROGRESS, 200337 | Treatment arms: ACEI (perindopril) ± diuretic (indapamide) vs. placebo Study length = 3.9 years n = 6,105 |
DSM-IV criteria | Systolic/diastolic BP fell more in the ACEI group than in the placebo group (9/5 mm Hg difference) | Participants in the ACEI group had a 12% reduction in risk for dementia compared with the placebo group |
| Sys-Eur Trial, 199851 | Treatment arms: CCB (nitrendipine) ± ACEI (enalapril) ± open-label HCTZ vs. placebo Study length = 2.0 years n = 2,418 |
DSM-III-R criteria and the modified ischemic score (including cranial CT scan) or the Hachinski score | Systolic/diastolic BP fell more in the CCB group than the placebo group (8.3/3.8 mm Hg differences) | Participants in the CCB group had a 50% reduction in risk for dementia compared with the placebo group |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CT, computed tomography; DSM, Diagnostic and Statistical Manual of Mental Disorders; HCTZ, hydrochlorothiazide; ICD, International Classification of Diseases; MRI, magnetic resonance imaging; PROGRESS, Perindopril Protection Against Recurrent Stroke Study; HYVET, Hypertension in the Very Elderly Trial; SCOPE, Study on Cognition and Prognosis in the Elderly; Sys-Eur, Systolic Hypertension in Europe.
Sample size and trial length ranged from 2,418 participants followed for 2.0 years in the Sys-Eur trial51 to 6,105 participants followed for 3.9 years in the PROGRESS trial.37 The MMSE was used to screen for cognitive impairment and identify participants in need of further evaluation. Dementia was diagnosed in 1 trial using the ICD-10 criteria, in 2 trials using the DSM-IV criteria and in 1 trial using the DSM-III-Revised criteria. Assessment of dementia also included cranial imaging in 3 trials and the Hachinski ischemic score in 2 trials, when records of these were available. All 4 trials met our criteria for state-of-the-art assessment based on using standard criteria for diagnosing dementia. However, only 2 (i.e., PROGRESS and SCOPE) were conducted for at least 3 years. It is regrettable that we have no dementia trials extending at least 5 years. Length of follow-up time could be increased if more trials focused on dementia as a primary instead of a secondary study outcome. From a qualitative perspective, we would prefer more detailed reporting with regard to how the various criteria for dementia were met. Specifically, we encourage adopting a standard, comprehensive systematic case review approach to diagnosis as illustrated by previous studies relating BP to dementia (e.g., Elias4 and Qui52). More information on methodological issues in the design of dementia studies can be found in the paper by Kennelly et al.53
Study priorities and the assessment of cognitive outcomes
One could argue that our criteria for state-of-the-art assessment of cognitive outcomes are impossible to meet. Inclusion of multiple cognitive measures and long follow-up periods may be difficult in terms of participant burden, time, and budget constraints, but these limitations are self-imposed and determined by study priorities. Table 3 displays the primary and secondary outcomes of each trial identified in this review. Out of 12 RCTs examining change in cognitive function, only 5 included cognition as a primary study outcome. Three of 4 RCTs examining dementia included dementia as a primary study outcome, although 1 of these studies was terminated early. Greater focus on cognition and dementia as primary study outcomes would allow more time and resources to be devoted to state-of-the-art assessment.
Table 3.
Outcomes and early termination in RCTs of cognitive function and incident dementia
| Study | Primary outcome | Secondary outcome | Early termination |
|---|---|---|---|
| AVEC Trial, 201232 | Cognitive function | None listed | No |
| ONTARGET, 201133 | CV composite (CV death, MI, stroke, heart failure) All-cause mortality |
Incident heart failure Revascularization procedures Incident diabetes Nephropathy Incident atrial fibrillation Cognitive impairment Cognitive decline |
No |
| TRANSCEND Trial, 201133 | CV composite (CV death, MI, stroke, heart failure) All-cause mortality |
Incident heart failure Revascularization procedures Incident diabetes Nephropathy Incident atrial fibrillation Cognitive impairment Cognitive decline |
No |
| HYVET-COG, 200849 | Dementia | All-cause mortality Heart failure Stroke |
Yes (The main trial was stopped early because a substantial reduction in total mortality and stroke was established at the second preplanned interim analysis. Not all patients who entered the HYVET trial reached at least 1 year of follow-up, as required for the assessment of any change in cognitive function.) |
| PRoFESS Trial, 200834 | Recurrent stroke | Disability following recurrent stroke Any CV event (stroke, MI, CV death) Cognitive function |
No |
| PROBE Study, 200635 | Ambulatory BP (SBP, DBP, HR, trough to peak ratio) | Cognitive function | No |
| SCOPE, 200550 | Major CV events (MI, stroke, CV death) Dementia |
None listed | No |
| PROBE Study, 200436 | BP (SBP, DBP) HR |
Cognitive function | No |
| PROGRESS, 200337 | Dementia Cognitive decline |
Dementia with recurrent stroke Other dementia Cognitive decline with recurrent stroke Other cognitive decline |
No |
| Sys-Eur Trial, 199851 | Stroke | Dementia | No |
| MRC Treatment Trial of Hypertension in Older Adults, 199638 | Stroke Coronary events All CV events All-cause mortality |
Cognitive function | No |
| HOPE Study, 199639 | Cognitive function | None listed | No |
| SHEP Study, 199440 | Stroke | Physical, emotional and cognitive function Leisure activities |
No |
| Croog et al., 199441 | Quality of life (well-being, physical status, emotional state, cognitive function, social roles) | None listed | No |
| Croog et al., 198642 | Quality of life (well-being, physical status, emotional state, cognitive function, social roles) | None listed | No |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CV, cardiovascular; DBP, diastolic blood pressure; DSM, Diagnostic and Statistical Manual of Mental Disorders; HCTZ, hydrochlorothiazide; HR, heart rate; ICD, International Classification of Diseases; MI, myocardial infarction; MRC, Medical Research Council; PROGRESS, Perindopril Protection Against Recurrent Stroke Study; HYVET, Hypertension in the Very Elderly Trial; SCOPE, Study on Cognition and Prognosis in the Elderly; Sys-Eur, Systolic Hypertension in Europe; SBP, systolic blood pressure.
A STATE-OF-THE-ART TRIAL IN PROGRESS
The Memory and cognition IN Decreased hypertension (MIND) component of the Systolic blood PRessure Intervention Trial (SPRINT) is an ongoing RCT that meets our criteria for state-of-the-art assessment of cognitive outcomes. Set to be completed in 2019,54 the SPRINT MIND’s primary goal is to determine whether aggressively lowering systolic BP to <120 mm Hg as opposed to the conventional <140 mm Hg reduces: (i) incidence of all-cause dementia; (ii) rate of decline in global and domain-specific cognitive function; and (iii) volume of small vessel ischemic disease. Table 4 provides a brief overview of the SPRINT MIND as it is described in the most recent SPRINT protocol.55
Table 4.
Overview of the SPRINT MIND
| Studies | Length and sample | Assessment protocol |
|---|---|---|
| Study 1. Determine whether lowering systolic BP to <120 mm Hg will reduce the incidence of all-cause dementia more than lowering systolic BP to <140 mm Hg | 5 years n = 9,250; all SPRINT participants |
Possible cases of dementia will be identified using a brief Cognitive Screening Batterya administered at baseline, 2 years, 4 years, and closeout. Participants scoring below a cut-point for impairment will be administered the Extended Cognitive Assessment Batteryb. All test and questionnaire data will be submitted to a panel of experts who will diagnose probable dementia using DSM-IV criteria |
| Study 2. Determine whether function in global cognition and key cognitive domains will decline less when systolic BP is lowered to <120 mm Hg compared with <140 mm Hg | 4 years n = 2,800; subsample of SPRINT participants |
All participants will complete the Cognitive Screening Battery and Extended Cognitive Assessment Battery at baseline, 2 years and 4 years. Tests will be used to create composite scores for two cognitive domains, Memory and Processing Speed, which will be used in addition to individual test scores |
| Study 3. Determine whether magnetic resonance imaging-derived changes in brain structure differ when systolic BP is lowered to <120 mm Hg compared with <140 mm Hg | 4 years n = approx. 640; subsample of Study 2 participants |
Magnetic resonance imaging will be performed at baseline and 4 years to obtain small vessel ischemic disease and total brain volumes |
Abbreviations: BP, blood pressure; DSM, Diagnostic and Statistical Manual of Mental Disorders; MIND, Memory and cognition IN Decreased hypertension; SPRINT, Systolic blood PRessure Intervention Trial.
aThe Cognitive Screening Battery includes: the Montreal Cognitive Assessment, Digit Symbol Coding test and Logical Memory test.
bThe Extended Cognitive Assessment Battery includes: the Hopkins Verbal Learning Test, Trail Making Test: Parts A and B, Digit Span test, Boston Naming Test, Modified Rey-Osterrieth Complex Figure and Category Fluency-Animals.
Study 1 meets criteria for state-of-the-art dementia assessment, identifying cases of all-cause dementia over the course of 5 years using a comprehensive systematic case review approach and well-established diagnostic criteria. Study 2 is the first trial to be discussed in this review that meets both criteria for state-of-the-art cognitive assessment. Nine tests are used to assess multiple cognitive abilities and SPRINT MIND investigators plan to use several tests to create two domain scores: Hopkins Verbal Learning Test, Logical Memory and Modified Rey-Osterrieth Complex Figure will be used to index Memory and; Digit Symbol Coding and Trail Making Test parts A and B will be used to index Processing Speed. Study 3 uses magnetic resonance imaging to investigate small vessel ischemic disease and total brain volumes longitudinally, a valuable addition to any study of BP and cognition.4
Though the Study 2 protocol indicates that 2 composite scores will be created, it is also possible to index the Language domain using the Boston Naming Test and Category Fluency. We note that the investigators identify the MoCA as a reliable instrument for characterizing global cognitive functioning.55 While the MoCA is a useful measure of cognitive status, we recommend assessing global cognitive function by aggregating scores from multiple cognitive measures. The SPRINT MIND provides one possible model for cognitive assessment in future trials. Other approaches are discussed in the following section.
MODELS FOR STATE-OF-THE-ART COGNITIVE TEST BATTERIES
The major issue with RCTs of antihypertensive medication use and cognitive function, with the exception of the ongoing SPRINT MIND, is that there seem to be no guiding principles for test selection. Batteries varied in size and the cognitive tests selected, making it difficult to compare outcomes between trials. Six of the 12 RCTs examined used test batteries containing 4 or more tests, meeting our criteria for state-of-the-art cognitive assessment. However, 4 RCTs used only 1 test, 1 RCT used 2 tests, and 1 RCT used 3 tests. Considering consistency in test selection between trials, 18 of 22 tests examined were used in <3 trials and the most commonly used test, the MMSE, was used in only 6 trials. No RCTs employed a hierarchical design in which tests were used to assess higher order cognitive domains. It is also of interest to note that many of the tests used assess abilities that are not involved in the progression from normal cognitive function to dementia.
There are many ways to design better cognitive test batteries for use in RCTs. One way is to consult the literature and choose cognitive tests that are commonly used in BP and cognition research. Test batteries used to examine the original Framingham Cohort and Framingham Offspring Cohort56 are short and have been used in many studies of BP, stroke, cardiovascular disease, and dementia.4 For example, a study of the original Framingham Cohort conducted by Elias et al.57 found that lower functioning on 4 of 9 cognitive measures (Logical Memory, Similarities, Paired Associate Learning, and a Learning and Immediate Recall composite) at baseline predicted increased risk of dementia over a 22-year follow-up period (odds ratios ranging from 1.31 to 1.57). These tests could be useful in future RCTs and similar, well-established measures can be found with a thorough search of the literature.
With regard to domain selection, the WAIS IV (Figure 4) includes 4 cognitive domains and provides multiple tests with which to index them.19 With a generous allotment of time for a RCT, one could use the full WAIS IV, possibly with the addition of measures of Executive Function. If time is restricted, we have selected 2 tests to index each domain: Information and Similarities to index Verbal Comprehension; Digit Span and Letter Number to index Working Memory; Picture Completion and Block Design to index Perceptual Organization and; Digit Symbol Coding and Symbol Search to index Processing Speed. Many other test batteries based on theoretical frameworks are also available and would be appropriate for use in a clinical trial.
Taking a more clinical approach, the most state-of-the-art assessment protocol would select domains that have been previously associated with conversion to dementia. These domains and the 2 tests we have selected to index them are as follows: Hopkins Verbal Learning Test58 and Logical Memory Test59 to index Episodic Memory; Boston Naming Test60 and Pyramid and Palm Trees Test61 to index Semantic Memory; Letter-Number Sequencing20 and Paced Auditory Serial Addition Test20 to index Working Memory; Trail Making Test Part B59 and Controlled Oral Word Association Test20 to index Executive Functioning; Brief Test of Attention59 and Digit Span Forward59 to index Attention and; Verbal Fluency Test59 and Token Test20 to index Language. An excellent chapter on the diagnosis of dementia written by Rainville et al.62 discusses how each domain is affected by the disease. Other tests that could be used to index these domains are provided in Supplement 2, Table 2s. Final selection of tests and domains is up to the investigator and should be based on the specific goals of the study. The examples provided here show how one might select domains and the tests used to index them.
Electronic-based cognitive testing
Our review does not consider technological advances in state-of-the-art assessment criteria. However, it is important to note that recent advances have begun to shift cognitive assessment away from the traditional pencil-and-paper paradigm to one which is electronic in nature. Computerized adaptations of established paper-based cognitive measures are now widely available, saving time, and improving accuracy through more consistent administration, adaptive presentation of items and automated scoring.63 However, both forms of assessment utilize tasks that have few real-world counterparts and have thus been criticized as having limited ability to predict everyday functional performance and impairment.64 Virtual reality-based measures have been proposed as an alternative way to assess cognition through immersion in an interactive, 3-dimensional environment that contains tasks embedded in ecologically valid scenarios.64,65
A recent meta-analysis of 18 studies conducted by Negut et al.65 indicates that virtual reality-based measures can detect impairment in Executive Function, Memory, and Visuospatial Abilities with moderate sensitivity. However, comparison of these findings with those of other meta-analyses indicates that sensitivity for detecting impairment is similar for virtual reality-based, paper-and-pencil, and computerized measures. As virtual reality-based assessment does not currently offer an advantage over more traditional measures of cognitive assessment and has several substantial drawbacks (e.g., substantial costs, simulator sickness, and need for special technological skills),64 it is unnecessary for most cognitive research and clinical assessment. We recommend a paper by Au et al.66 that discusses how technological advances will play a role in the future of state-of-the-art cognitive testing.
LIMITATIONS
Due to the volume of the literature, we cannot be certain that we have retrieved all RCTs meeting criteria for the review. However, we feel that a reasonable sample has been obtained and that all relevant trials meeting our criteria have been included.
SUMMARY AND CLINICAL RELEVANCE
We have reviewed RCTs with respect to state-of-the-art assessment of cognitive function and dementia. All previous trials of dementia met our state-of-the-art criteria for use of well-established diagnostic criteria, but only 2 met criteria for trial length. No previous trials of cognitive function met state-of-the-art criteria, as they did not include multiple tests of ability that index multiple cognitive domains. The diversity of cognitive tests used across all RCTs and the frequent use of a single test to assess cognitive function are problematic and represent an unguided approach to test selection. However, construction of a state-of-the-art test battery based on a theoretical framework that includes clinically relevant cognitive domains is possible and important in RCTs of BP lowering and antihypertensive medication use. The argument that our recommendations are unrealistic can be countered by the argument that, though time and cost limitations exist, finding ways to prevent or reduce cognitive decline is extremely important and must not take a back seat to other trial goals.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
DISCLOSURE
Merrill Elias was a paid consultant of the Glaxo Pharmaceutical Corporation (1990–1992) regarding the conduct and design of a clinical trial. None of discussion or conclusions in this review represent a conflict of interest related to this paid consultancy. Other authors declared no conflict of interest.
ACKNOWLEDGMENTS
Adam Davey is supported by the National Cancer Institute, National Institutes of Health (FundRef ID: http://dx.doi.org/10.13039/100000054; award number R01CA194178). The content in this review does not necessarily reflect the official views of the National Institutes of Health.
REFERENCES
- 1. Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars. Alzheimer’s Association: Chicago, IL, 2015. [Google Scholar]
- 2. Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elias MF, Goodell AL, Robbins MA. Blood pressure and cognitive functioning: longitudinal studies, treatment and new directions. In Waldstein S, Elias MF (eds), Neuropsychology of Cardiovascular Disease, 2nd edn Taylor & Francis: New York, NY, 2015. [Google Scholar]
- 4. Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: a perspective in historical context. Hypertension 2012; 60:260–268. [DOI] [PubMed] [Google Scholar]
- 5. Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol 1993; 138:353–364. [DOI] [PubMed] [Google Scholar]
- 6. Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 2005; 45:374–379. [DOI] [PubMed] [Google Scholar]
- 7. Waldstein SR, Katzel LI. Hypertension and cognitive function. In Waldstein DR, Elias MF (eds), Neuropsychology of Cardiovascular Disease. Lawrende Erbaum Associates: Mahwah, NJ, 2001. [Google Scholar]
- 8. Williams B. Recent hypertension trials: implications and controversies. J Am Coll Cardiol 2005; 45:813–827. [DOI] [PubMed] [Google Scholar]
- 9. Obisesan TO. Hypertension and cognitive function. Clin Geriatr Med 2009; 25:259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Staessen JA, Thijs L, Richart T, Odili AN, Birkenhäger WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension 2011; 57:e6–e7. [DOI] [PubMed] [Google Scholar]
- 11. Dockrill P. One of the world’s biggest drug companies just abandoned Alzheimer’s and Parkinson’s research <https://www.sciencealert.com/one-world-s-biggest-drug-companies-abandoned-alzheimer-s-parkinson-s-disease-pfizer> 2018. Accessed 26 January 2018.
- 12. Waldstein SR. The relation of hypertension to cognitive function. Curr Dir Psychol Sci 2003; 12:9–12. [Google Scholar]
- 13. Spearman CE. “General intelligence,” objectively determined and measured. Am J Psychol 1904; 15:201–293. [Google Scholar]
- 14. Spearman C, Jones LW.. Human Ability. Macmillan: London, UK, 1950. [Google Scholar]
- 15. Thurstone LL. The Nature of Intelligence. Routledge: London, UK, 1973. [Google Scholar]
- 16. McGrew KS. The Cattell-Horn-Carroll theory of cognitive abilities. In Flanagan DP, Harrison PL (eds), Contemporary Intellectual Assessment: Theories, Tests and Issues. 2nd edn Guilford Press: New York, NY, 2005. [Google Scholar]
- 17. Spearman CC. Correlation calculated from faulty data. Br J Psychol 2010; 3:271–295. [Google Scholar]
- 18. Brown W. Some experimental results in the correlation of mental abilities. Br J Psychol 1910; 3:296–322. [Google Scholar]
- 19. Petermann F. (ed). Wechsler Adult Intelligence Scale, 4th Edn Pearson: New York, NY, 2008. [Google Scholar]
- 20. Lezak MD, Howieson DB, Bigler ED, Tranel D.. Neuropsychological Assessment. 5th edn Oxford University Press: New York, NY, 2012. [Google Scholar]
- 21. Kline P. A Handbook of Test Construction (Psychological Revivals): Introduction to Psychometric Design. Routledge: London, UK, 2015. [Google Scholar]
- 22. Embretson SE.(ed). Test Design: Developments in Psychology and Psychometrics. Academic Press: London, UK, 2013. [Google Scholar]
- 23. Cronbach LJ. Essentials of Psychological Testing. Harper & Row: New York, NY, 1969. [Google Scholar]
- 24. Guilford JP. Psychometric Methods. 2nd edn McGraw-Hill: New York, NY, 1954. [Google Scholar]
- 25. Elias MF, Torres RV, Davey A. Parameters of left ventricular mass and dementia: moving the literature forward. Hypertension 2018; 71:411–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sullivan KJ, Elias MF. Vascular dementia. In Whitebourne KS. (ed), The Encyclopedia of Adulthood and Aging. Wiley-Blackwell: New York, NY, 2016. [Google Scholar]
- 27. Bennett D. Public health importance of vascular dementia and Alzheimer’s disease with cerebrovascular disease. Int J Clin Pract Suppl 2001; 120:41–48. [PubMed] [Google Scholar]
- 28. Hogan DB, Ebly EM. Predicting who will develop dementia in a cohort of Canadian seniors. Can J Neurol Sci 2000; 27:18–24. [DOI] [PubMed] [Google Scholar]
- 29. Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference?Hypertension 2004; 44:631–636. [DOI] [PubMed] [Google Scholar]
- 30. Elias MF, Robbins MA, Elias PK, Streeten DH. A longitudinal study of blood pressure in relation to performance on the Wechsler Adult Intelligence Scale. Health Psychol 1998; 17:486–493. [DOI] [PubMed] [Google Scholar]
- 31. Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, Norton MC, Zandi PP, Toone L, West NA, Breitner JC; Cache County Investigators Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology 2006; 67:229–234. [DOI] [PubMed] [Google Scholar]
- 32. Hajjar I, Hart M, Chen YL, Mack W, Milberg W, Chui H, Lipsitz L. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double-blind randomized clinical trial. Arch Intern Med 2012; 172:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, Commerford P, Dyal L, Schumacher H, Pogue J, Paolasso E, Holwerda N, Chazova I, Binbrek A, Young J, Yusuf S; ONTARGET and TRANSCEND Investigators Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol 2011; 10:43–53. [DOI] [PubMed] [Google Scholar]
- 34. Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW; Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study group Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008; 7:875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fogari R, Mugellini A, Zoppi A, Lazzari P, Destro M, Rinaldi A, Preti P. Effect of telmisartan/hydrochlorothiazide vs lisinopril/hydrochlorothiazide combination on ambulatory blood pressure and cognitive function in elderly hypertensive patients. J Hum Hypertens 2006; 20:177–185. [DOI] [PubMed] [Google Scholar]
- 36. Fogari R, Mugellini A, Zoppi A, Marasi G, Pasotti C, Poletti L, Rinaldi A, Preti P. Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur J Clin Pharmacol 2004; 59:863–868. [DOI] [PubMed] [Google Scholar]
- 37. Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J; PROGRESS Collaborative Group Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163:1069–1075. [DOI] [PubMed] [Google Scholar]
- 38. Prince MJ, Bird AS, Blizard RA, Mann AH. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council’s trial of hypertension in older adults. BMJ 1996; 312:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Starr JM, Whalley LJ, Deary IJ. The effects of antihypertensive treatment on cognitive function: results from the HOPE study. J Am Geriatr Soc 1996; 44:411–415. [DOI] [PubMed] [Google Scholar]
- 40. Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, Greenlick MR, Hadley E, Moye L, Perry HM Jr. Impact of the treatment of isolated systolic hypertension on behavioral variables. Results from the systolic hypertension in the elderly program. Arch Intern Med 1994; 154:2154–2160. [PubMed] [Google Scholar]
- 41. Croog SH, Elias MF, Colton T, Baume RM, Leiblum SR, Jenkins CD, Perry HM, Hall WD. Effects of antihypertensive medications on quality of life in elderly hypertensive women. Am J Hypertens 1994; 7:329–339. [DOI] [PubMed] [Google Scholar]
- 42. Croog SH, Levine S, Testa MA, Brown B, Bulpitt CJ, Jenkins CD, Klerman GL, Williams GH. The effects of antihypertensive therapy on the quality of life. N Engl J Med 1986; 314:1657–1664. [DOI] [PubMed] [Google Scholar]
- 43. Robbins J. The Neuropsychological Application of the WAIS-IV Over the WAIS-III <http://nsuworks.nova.edu/cps_stuetd/91> 2014. Accessed 15 February 2018.
- 44. Baek MJ, Kim K, Park YH, Kim S. The validity and reliability of the mini-mental state examination-2 for detecting mild cognitive impairment and Alzheimer’s disease in a Korean population. PLoS One 2016; 11:e0163792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc 1992; 40:922–935. [DOI] [PubMed] [Google Scholar]
- 46. Carnero-Pardo C. Should the mini-mental state examination be retired?Neurologia 2014; 29:473–481. [DOI] [PubMed] [Google Scholar]
- 47. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 48. Godefroy O, Fickl A, Roussel M, Auribault C, Bugnicourt JM, Lamy C, Canaple S, Petitnicolas G. Is the Montreal Cognitive Assessment superior to the mini-mental state examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke 2011; 42:1712–1716. [DOI] [PubMed] [Google Scholar]
- 49. Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C; HYVET Investigators Incident dementia and blood pressure lowering in the hypertension in the very elderly trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008; 7:683–689. [DOI] [PubMed] [Google Scholar]
- 50. Skoog I, Lithell H, Hansson L, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A; SCOPE Study Group Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: study on cognition and prognosis in the elderly (SCOPE). Am J Hypertens 2005; 18:1052–1059. [DOI] [PubMed] [Google Scholar]
- 51. Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 52. Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol 2003; 60:223–228. [DOI] [PubMed] [Google Scholar]
- 53. Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord 2009; 2:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. National Institutes of Health. Systolic Blood Pressure Intervention Trial (SPRINT) Overview <https://www.nhlbi.nih.gov/news/systolic-blood-pressure-intervention-trial-sprint-overview> 2017. Accessed 15 February 2018.
- 55. Systolic Blood Pressure Intervention Trial. Systolic Blood Pressure Intervention Trial (SPRINT): Protocol Version 4.0 <https://www.sprinttrial.org/public/Protocol_Current.pdf> 2012. Accessed 15 February 2018.
- 56. Au R, Seshadri S, Wolf PA, Elias M, Elias P, Sullivan L, Beiser A, D’Agostino RB. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp Aging Res 2004; 30:333–358. [DOI] [PubMed] [Google Scholar]
- 57. Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol 2000; 57:808–813. [DOI] [PubMed] [Google Scholar]
- 58. Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK, Grant I; HIV Neurobehavioral Research Center (HNRC) Group Construct validity of Hopkins verbal Learning test-revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol 2005; 20:1061–1071. [DOI] [PubMed] [Google Scholar]
- 59. Rebok GW, Parisi JM, Gross AL, Spira AP. Assessment of cognitive training. In Lichtenberg PA. (ed), Handbook of Assessment in Clinical Gerontology, 2nd edn Academic Press: London, UK, 2010. [Google Scholar]
- 60. Willers IF, Feldman ML, Allegri RF. Subclinical naming errors in mild cognitive impairment: a semantic deficit?Dement Neuropsychol 2008; 2:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klein LA, Buchanan JA. Psychometric properties of the pyramids and palm trees test. J Clin Exp Neuropsychol 2009; 31:803–808. [DOI] [PubMed] [Google Scholar]
- 62. Rainville C, Caza N, Belleville S, Gillbert B. Neuropsychological assessment. In Gauthier S. (ed), Clinical Diagnosis and Management of Alzheimer’s Disease, 3rd edn Informa Healthcare: New York, NY, 2008. [Google Scholar]
- 63. Silverberg NB, Ryan LM, Carrillo MC, Sperling R, Petersen RC, Posner HB, Snyder PJ, Hilsabeck R, Gallagher M, Raber J, Rizzo A, Possin K, King J, Kaye J, Ott BR, Albert MS, Wagster MV, Schinka JA, Cullum CM, Farias ST, Balota D, Rao S, Loewenstein D, Budson AE, Brandt J, Manly JJ, Barnes L, Strutt A, Gollan TH, Ganguli M, Babcock D, Litvan I, Kramer JH, Ferman TJ. Assessment of cognition in early dementia. Alzheimers Dement 2011; 7:e60–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parsons TD. Virtual reality for enhanced ecological validity and experimental control in the clinical, affective and social neurosciences. Front Hum Neurosci 2015; 9:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Neguț A, Matu SA, Sava FA, David D. Virtual reality measures in neuropsychological assessment: a meta-analytic review. Clin Neuropsychol 2016; 30:165–184. [DOI] [PubMed] [Google Scholar]
- 66. Au R, Piers RJ, Devine S. How technology is reshaping cognitive assessment: lessons from the Framingham Heart Study. Neuropsychology 2017; 31:846–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.