Abstract
Weight loss after bariatric surgery is associated with reduction in adverse cardiovascular outcomes; however, the impact of obstructive sleep apnea (OSA) on reduction of cardiovascular outcomes after bariatric surgery in morbidly obese patients is not known. We retrospectively assessed differences in cardiovascular events after laparoscopic adjustable gastric banding (LAGB)–induced weight loss in patients with and without OSA before and after propensity score matching for age, sex, body mass index (BMI), and major comorbidities between the two groups and determined predictors of poor outcomes. OSA was present in 222 out of 830 patients (27 per cent) who underwent LAGB between 2001 and 2011. Despite a similar reduction in BMI (20.0 and 20.8 per cent), a significantly higher percentage of cardiovascular events were observed in patients with than without OSA (35.6 vs 6.9 per cent; p < 0.001) at 3 years (mean follow-up 6.0 ± 3.2; range: 0.5 to 13 years). The differences in the cumulative endpoint of new onset stroke, heart failure, myocardial infarction, venous thrombosis, and pulmonary embolism between the OSA and non-OSA groups were maintained after propensity matching. Patients with OSA treated with continuous positive airway pressure (CPAP) during sleep [n = 66] had lower cardiovascular event rates at 30 months compared with those not treated (p < 0.041). OSA (hazard ratio: 6.92, 95% CI: 3.39–14.13, p < 0.001) remained an independent predictor of cardiovascular events after multivariate analysis. Thus, patients with OSA, despite a similar initial weight loss after LAGB, had a higher incidence of cardiovascular events compared with a propensity-matched group without OSA. Treatment with CPAP appears to reduce such events.
Keywords: obesity, obstructive sleep apnea, bariatric surgery, cardiovascular, weight reduction
Statement of Significance
The higher cardiovascular event rates in those with obstructive sleep apnea (OSA) than without OSA—despite a similar weight reduction after laparoscopic gastric banding surgery—reported in this study highlight the importance of recognizing and correcting factors associated with OSA that limit the full benefit of bariatric surgery. This is of particular significance because of the high prevalence of OSA in patients referred for bariatric surgery that could attenuate the maximum benefit of weight reduction. The apparent beneficial impact of positive airway pressure devices for the treatment of OSA on cardiovascular outcomes in patients undergoing bariatric surgery is not fully defined and needs further investigation.
Introduction
Obesity is a major epidemic in the United States, with massive weight gain countering efforts to reduce overall and cardiovascular disease (CVD)–related morbidity and mortality [1, 2]. The increasing prevalence of morbid obesity, a major risk factor for CVD [3], undermines the last two decades of gains in prevention of CVD burden, which may lead to a decline of the life expectancy [1–4]. Approximately 85.6 million Americans suffer from some form of CVD, which directly and indirectly adds up to a more than $315 billion cost to the economy [3]. This alarming figure, paralleled by the obesity epidemic, raises serious public health concerns regarding an increase in CVD burden and health care cost implications [2, 3, 5, 6]. Weight loss reverses many aspects of abnormal cardiovascular function, which over the long term results in reduction of CVD risk factors, improvement in morbidity, quality of life, and a survival benefit [7, 8]. Multiple studies and meta-analyses published in the past decade highlight the benefits of bariatric surgery for weight reduction in morbidly obese individuals who were otherwise resistant to medical approaches to weight reduction and thus able to reduce risk factors for CVD, adverse cardiovascular events, and mortality [9–12]. Obesity also predisposes to the development and progression of obstructive sleep apnea (OSA), which further contributes to adverse cardiovascular outcomes [13–15]. Although weight loss results in improvement in cardiovascular outcomes [2], it is not known if the presence of OSA counteracts the beneficial effect of weight loss on reduction in cardiovascular events after bariatric surgery. We hypothesized that the benefit of weight loss on cardiovascular outcomes after bariatric surgery for morbid obesity would be attenuated in patients with OSA when compared with those without OSA. Therefore, the goal of this study was to assess the effect of weight loss after laparoscopic adjustable gastric banding (LAGB) surgery in morbidly obese patients with or without OSA on new onset stroke, heart failure (HF), myocardial infarction (MI), venous thrombosis, and pulmonary embolism and mortality, as well as to determine the impact of OSA treatment with continuous positive airway pressure (CPAP) therapy during sleep on vascular events and to determine whether OSA is an independent predictor of poor cardiovascular outcomes in this population.
Methods
Study design and methods
This was a retrospective study of a longitudinally collected dataset of 853 morbidly obese patients [body mass index (BMI) ≥ 40 kg/m2 or ≥35 kg/m2 with at least one obesity-associated comorbidity] [16] who underwent LAGB in a Bariatric Surgery Center of Excellence in Milwaukee, Wisconsin, from February 2001 to February 2011. The procedures were performed by a single bariatric surgeon (T.Y.C.) in a comprehensive, multidisciplinary program setting. Patients were eligible for LAGB after failure of conservative treatment for weight loss by a multidisciplinary team composed of an internist, psychologist, and nutritionist. Patients who failed conservative treatment for weight loss underwent further educational sessions in seminars organized by the bariatric surgery center and candidates for bariatric surgery selected from the pool of morbidly obese patients who met the published inclusion and exclusion criteria for bariatric surgery [16]. Approximately 480 patients per year (40 patients per month) attend the seminars and approximately 25 per cent of these patients per year were eligible and opted for LAGB surgery. During the study period (2001–2011), of all bariatric surgery procedures available, 50 per cent of patients wanted LAGB, because it was very popular at the time. The remaining 50 per cent opted for other bariatric surgical procedures, including Roux-en-Y bypass and sleeve gastrectomy. Roux-en-Y bypass surgery was usually selected for patients with extreme BMI (>50 kg/m2), diabetes mellitus, and severe symptomatic gastro-esophageal reflux disease. All patients underwent preoperative assessment to identify risk for cardiovascular events. The presence of OSA before surgery was determined based on the clinical information provided by the referring primary care physician to the bariatric surgery clinic and by history obtained from the patient by the clinic staff. Patients who were switched to another bariatric procedure, died in the perioperative period, or did not have at least 6 months follow-up data available were excluded from this analysis. Patients with prior MI or HF were excluded. There was no operative mortality, but two patients (0.002 per cent) died after surgery within 10 days after discharge from the hospital. Data were extracted from Exemplo eMD (Exemplo Medical, Oldsmar, FL) database, clinical charts, and electronic medical records (Epic Systems, Verona, WI). The electronic medical record of all patients with OSA who were followed at Aurora Health Care system was assessed and those treated with CPAP therapy identified. Baseline demographic, clinical, and follow-up information including change in body weight, BMI, surgical complications, and incidence of cardiovascular events was obtained. Patients were regularly followed up, in-person at their annual surgery clinic visits, and by phone calls or additional clinic visits, if any change in clinical status was reported. Calculations of BMI were performed based on weight and height (weight in kilograms divided by height in meters squared) assessed at yearly intervals after surgery. Excess body weight (EBW) was calculated by subtracting ideal body weight (derived from Metropolitan Life Insurance Company’s 1983 Height and Weight tables) [17] from the weight at surgery. The percent excess body weight loss (%EBWL) was then calculated by subtracting the weight at each time point from the weight at surgery divided by EBW and expressed as a percentage. Percent Excess Body Weight Loss was defined as good (>50%EBWL), fair (25–50%EBWL), or failure EBWL (<25 per cent). The time to adverse cardiovascular event was determined from the date of LAGB.
Differences in CVD outcomes after LAGB between patients with and without OSA were determined in the overall population and after one-to-one propensity score matching between the OSA and non-OSA groups for risk factors for CVD including age, sex, hypertension, diabetes, and dyslipidemia. The primary end point was a composite of cardiovascular events, including stroke, HF, MI, venous thrombosis, and pulmonary embolism in patients with and without OSA. Secondary cardiovascular end points included the individual components of the primary composite end point and overall mortality. Death information was obtained from the patients’ medical record system and confirmed in the Social Security Death Index. The study was approved by the Aurora Health Care Institutional Review Board and complied with Health Insurance Portability and Accountability Act (HIPAA) rules.
Statistical analysis
Characteristics of the study population were described by frequency (percentage) and mean (±standard deviation). Data were analyzed based on demographics including age, sex, race, comorbidities, and year of surgery. Propensity score matching was performed to balance the differences in baseline characteristics. Each patient with OSA was matched to a patient without OSA on the basis of his or her propensity score using the greedy matching protocol. After matching, the balance within the matched pairs was assessed using the standardized difference in covariate means and proportions. Comparisons between groups were made by Student’s t-test for continuous variables and chi-square and/or Fisher’s exact test wherever appropriate for categorical data. Time-to-event analyses were performed using Kaplan–Meier methods with the event being one of the combined or individual five cardiovascular events (stroke, HF, MI, venous thrombosis, and pulmonary embolism) or mortality and event-free survival curves were compared using the log-rank test. The parameters included age, sex, race, BMI, hypertension, hyperlipidemia, depression, degenerative joint disease, asthma, and OSA. Multivariate time-to-event analyses were performed using Cox proportional-hazards regression methods. A p-value of <0.05 was considered significant. The analysis was performed using SAS Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study population
Out of 853 patients, follow-up information was not available in 23 patients (2.7 per cent), leaving 830 (97.3 per cent) patients for analysis. Overall, the majority of patients undergoing LAGB were women (84 per cent), of European descent (75 per cent), and had a mean age of 44.1 ± 11.4 (median 44 years, range: 18 to 72 years) years and mean baseline BMI of 48.5 ± 8.09 kg/m2. OSA was present in 222 patients (27 per cent). When compared with the non-OSA patients, the group with OSA comprised older patients, more men, and patients with greater BMI and had a higher prevalence of hypertension, diabetes mellitus, dyslipidemia, and depression. The baseline characteristics of the overall group and those with and without OSA are summarized in Table 1.
Table 1.
Baseline characteristics of the entire cohort and propensity-matched patients with and without OSA undergoing LAGB surgery for morbid obesity
| Characteristics | Entire cohort (830) | Propensity matched (396) | ||||
|---|---|---|---|---|---|---|
| OSA | Non-OSA | P | OSA | Non-OSA | P | |
| N | (222) | (608) | (198) | (198) | ||
| Age (years) | 48.1 ± 10.8 | 42.7 ± 11.3 | <0.001 | 47.9 ± 10.9 | 46.7 ± 11.3 | 0.299 |
| Male N (%) | 72 (32.4) | 64 (10.5) | <0.001 | 52 (26.3) | 52 (26.3) | 0.999 |
| Height (m) | 1.68 ± 0.1 | 1.66 ± 0.1 | 0.001 | 1.60 ± 0.0 | 1.60 ± 0.0 | 0.999 |
| Weight (kg) | 143 ± 30.2 | 133 ± 25.5 | <0.001 | 141 ± 29.0 | 139 ± 26.3 | 0.491 |
| BMI (kg/m2) | 49.9 ± 8.3 | 48.1 ± 8.0 | 0.003 | 49.1 ± 7.6 | 49.8 ± 9.1 | 0.428 |
| Dyslipidemia | 154 (69.4) | 253 (41.7) | <0.001 | 132 (66.6) | 116 (58.6) | 0.096 |
| Hypertension | 171 (77.0) | 319 (52.5) | <0.001 | 148 (74.7) | 147 (74.2) | 0.908 |
| Diabetes | 114 (51.3) | 169 (27.8) | <0.001 | 93 (47.0) | 88 (44.4) | 0.614 |
| Depression | 62 (27.9) | 126 (20.8) | 0.029 | 54 (27.3) | 39 (19.7) | 0.075 |
| DJD | 187 (84.2) | 509 (83.9) | 0.895 | 168 (84.8) | 166 (83.8) | 0.782 |
| Asthma | 101 (45.5) | 290 (47.8) | 0.560 | 87 (43.9) | 92 (46.5) | 0.613 |
BMI = body mass index; DJD = degenerative joint disease; OSA = obstructive sleep apnea.
Matched population
Because marked baseline differences were present between the two groups, separate analysis was performed between the two groups after propensity matching for age, sex, BMI, and major comorbidities (dyslipidemia, hypertension, and diabetes mellitus), yielding 198 patients in each group, OSA and non-OSA, with matched baseline characteristics as summarized in Table 1.
Weight reduction after LAGB
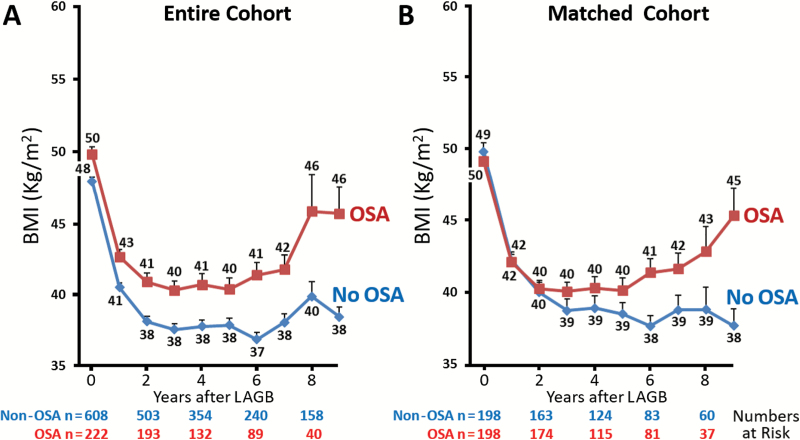
After LAGB, gradual weight loss was observed with a mean reduction of 27 kg and 29 kg, respectively, for the OSA and non-OSA groups at 3 years, which translates into a 19 and 22 per cent total body weight loss and a BMI reduction of 10 kg/m2 at 3 years for both groups, from 50 to 40 kg/m2 (20 per cent reduction in BMI) in the OSA group, and from 48 to 38 kg/m2 in the non-OSA group (20.8 per cent reduction, Figure 1). The overall weight reduction after LAGB was similar between those with and without OSA (p = 0.60). There were no difference in the percentage of good (28.9 vs 22.3), fair (42.8 vs 42.3), and failed (28.4 vs 35.4) %EBWL at 3 years, between OSA and non-OSA groups, respectively (p = 0.39). Over the long term, weight loss was maintained for up to 6 years in both the non-OSA and OSA groups followed by a gradual weight gain, and increase in BMI was then observed only in the OSA group in the 7th, 8th, and 9th year with maintained weight and BMI in the non-OSA group (Figure 1). Similar findings were observed in unmatched cohort (Figure 1A) and propensity-matched populations (Figure 1B) with or without OSA.
Figure 1.
Yearly mean BMI reduction after LAGB surgery for morbidly obese patients with and without OSA. (A) Entire cohort and (B) propensity-matched population.
Cardiovascular events after LAGB
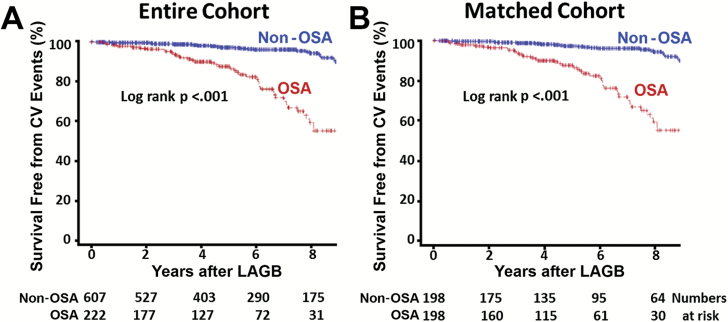
Over the mean follow-up of 6.0 ± 3.2 (median 5.1 years; range: 0.5 to 13 years) years after LAGB, there were 121 (14.6 per cent) cardiovascular events in the overall population of 830 patients undergoing bariatric surgery, with a significantly higher percentage of events in patients with OSA than non-OSA patients [35.5 per cent (79 out of 222 patients) vs 6.9 per cent (42 out of 608); p < 0.001]. Figure 2A summarizes the cumulative cardiovascular event rate as a Kaplan–Meier survival plot over 9 years in patients with and without OSA, indicating a higher annual combined cardiovascular event rate in patients with OSA than in the non-OSA group (4.0 vs 0.7 per cent at year 2, 10.2 vs. 2.2 per cent at year 4, 19.0 vs 4.3 per cent at year 6, and 43.0 vs 5.8 per cent at year 8; log-rank p-value < 0.001).
Figure 2.
Survival free of CV events in patients with or without OSA after LAGB surgery for morbid obesity. Kaplan–Meier survival plot for (A) entire cohort and (B) propensity-matched group.
Matched population
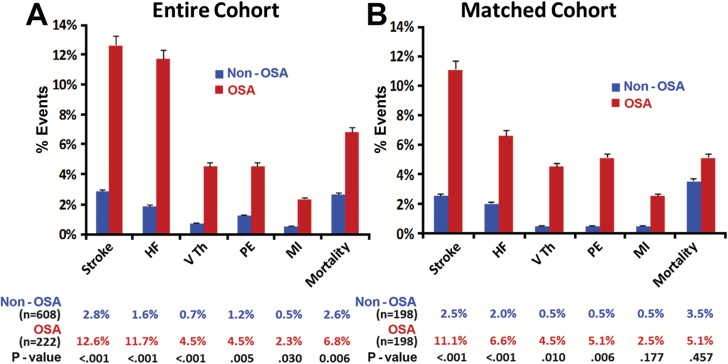
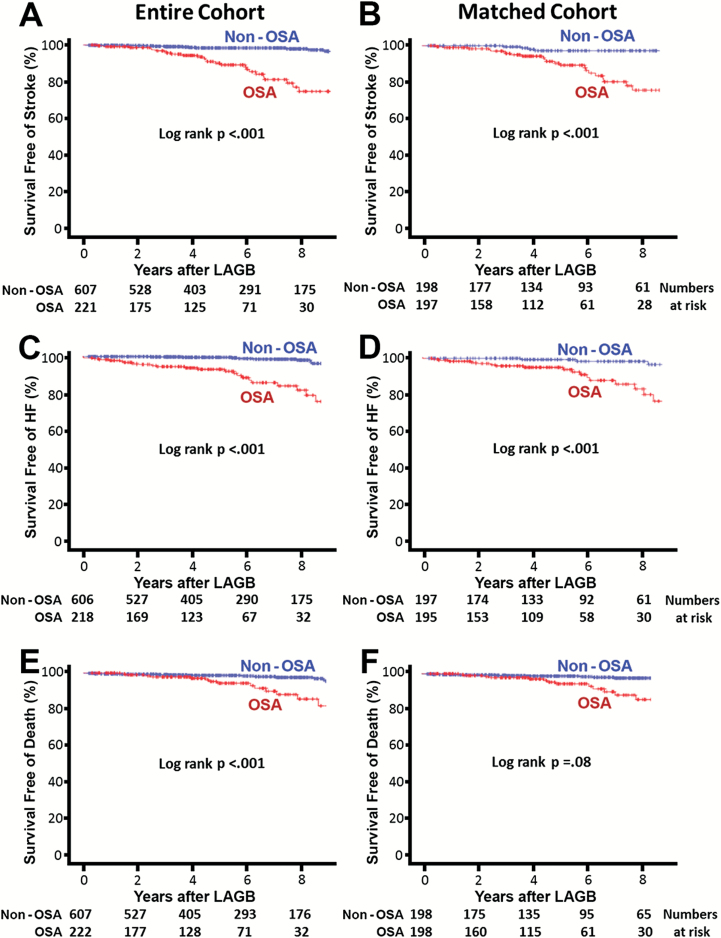
The differences in combined cardiovascular events between the two groups persisted even after propensity matching for baseline differences (Figure 2B, log-rank p < 0.001). The majority of adverse cardiovascular events were stroke, HF, venous thrombosis, and pulmonary embolism in both groups, with the incidence significantly higher in the OSA group than the non-OSA group, as illustrated in Figure 3B. The Kaplan–Meier survival plots for HF and stroke in patients with and without OSA in the entire cohort and the propensity-matched groups are displayed in Figure 4A–D. A significant difference in stroke events, new onset HF, MI, and thromboembolism was present between patients with and without OSA in the entire cohort (Figure 3A) that persisted for all cardiovascular events except MI after propensity matching for baseline differences (Figure 3B). The overall cardiovascular events per year in the non-OSA and OSA groups after LAGB were 0.7 vs 3.3 per cent for stroke, 0.9 vs 2.2 per cent for HF, 0.18 vs 0.87 per cent for venous thrombosis, 0.18 vs 0.34 per cent for pulmonary embolism, and 0.18 vs 0.34 per cent for MI, respectively.
Figure 3.
Cardiovascular events during follow-up after LAGB surgery for morbidly obese in patients with and without OSA. Percent (%) event during follow-up for each of the listed outcomes is depicted as a bar graph with % event on y-axis. (A) Entire cohort and (B) propensity-matched population. HF = heart failure; MI = myocardial infarction; PE = pulmonary embolism; VTH = venous thrombosis.
Figure 4.
Survival free of stroke, heart failure, and death in patients with or without history of OSA after LAGB surgery for morbid obesity. Kaplan–Meier plot for survival free of stroke [(A) entire cohort; (B) propensity-matched population], HF [(C) entire cohort; (D) propensity-matched population], and death [(E) entire cohort; (F) propensity-matched population].
Cumulative mortality
There was no operative mortality, but two patients (0.002 per cent) died after surgery within 10 days after discharge from the hospital, both with history of obstructive sleep apnea. Patient 1 was a 55-year-old male with history of depression and neuropathic pain treated with gabapentin and fentanyl who died at home of unclear cause, 9 days after the surgery. The second patient was a 54-year-old male with multiple comorbidities, including diabetes, hypertension, degenerative joint disease, and chronic renal disease who died of pulmonary embolism 9 days after the surgery. These two patients with postoperative deaths within 10 days were not included in the long-term analysis. Over the mean follow-up of 6.0 ± 3.2 years after LAGB, 31 more patients died, 15 out of 222 (6.8 per cent) in the OSA group and 16 out of 608 in the non-OSA group (2.6 per cent, p < 0.006). The overall survival probability was significantly worse for the OSA group than the non-OSA patients in the overall cohort (log-rank p < 0.001, Figure 4E). This difference between OSA and non-OSA patients diminished in the propensity-matched group that demonstrated a trend toward worse survival in OSA group that did not reach statistical significance (log-rank p = 0.08; Figure 4F).
Effect of continuous positive airway pressure therapy on reduction of cardiovascular events
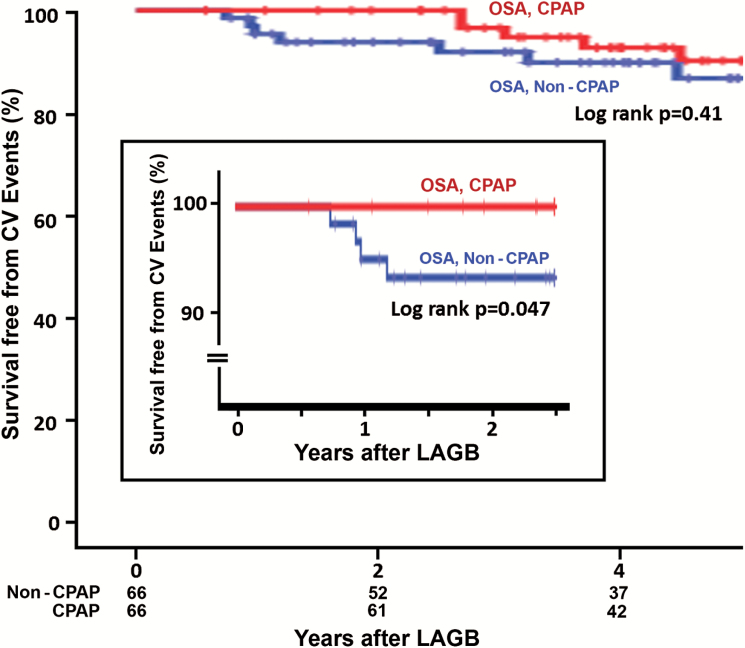
In the entire cohort, there were 68 patients with OSA who were treated with CPAP therapy, identified from the review of the electronic medical record system of all patients with OSA followed at our institution. The cardiovascular event-free survival probability within 30 months after LAGB in patients with OSA treated with CPAP therapy showed a trend toward better outcomes than those not on CPAP therapy, but the difference (0 events versus 8 events, respectively) did not reach statistical significance (log-rank p = 0.051).
Matched population
During 1:1 propensity-matching of CPAP-treated patients for baseline demographic and comorbidities with OSA patients not treated with CPAP, two CPAP-treated patients had to be removed making a matched pair of 66 patients who were compared for cardiovascular outcomes. A significantly higher cardiovascular event-free survival probability was observed in the CPAP-treated patients during the initial 30-month follow-up (log-rank p = 0.047; Figure 5, inset), which diminished with longer follow-up over the mean 6.0 ± 3.2 years (log-rank p = 0.41, Figure 5).
Figure 5.
Survival free of CV events after LAGB for morbid obesity in propensity matched patients with OSA treated or not treated with CPAP device. Inset: CV event rates within the first 30 months after LAGB.
Predictors of adverse cardiovascular events
Univariate predictors of cardiovascular events in the overall cohort undergoing LAGB with hazard ratio (HR) and 95% confidence interval (CI) are summarized in Table 2. Presence of OSA was a strong predictor of adverse cardiovascular events after LAGB with a HR of 5.89 (95% CI 3.75–9.25, p < 0.001). Age, male sex, history of hypertension, diabetes, dyslipidemia, and depression were other univariate predictors (Table 2). The variables with significant differences at p = 0.05 in the univariate analysis were included in the multivariate Cox regression model to determine independent predictors of cardiovascular events. OSA continued to be an independent predictor with a HR of 4.51 for cardiovascular events (95% CI 2.80–7.27, p < 0.001). A history of hypertension and depression were other independent predictors for cardiovascular events (Table 3).
Table 2.
Univariate analysis for predicting cardiovascular events in the entire cohort and propensity-matched patients undergoing LAGB surgery for morbid obesity
| Covariates | Entire cohort | Propensity-matched cohort | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.04 (1.02–1.06) | <0.001 | 1.04 (1.01–1.07) | 0.010 |
| Male | 2.10 (1.25–3.52) | 0.005 | 1.28 (0.69–2.37) | 0.427 |
| BMI | 0.99 (0.97–1.02) | 0.812 | 0.99 (0.96–1.02) | 0.562 |
| OSA | 5.89 (3.75–9.25) | <0.001 | 6.82 (3.38–13.74) | <0.001 |
| Dyslipidemia | 2.20 (1.39–3.48) | <0.001 | 2.17 (1.17–4.02) | 0.014 |
| Hypertension | 2.81 (1.66–4.75) | <0.001 | 2.05 (0.99–4.22) | 0.051 |
| Diabetes | 2.11 (1.36–3.28) | <0.001 | 1.57 (0.90–2.73) | 0.108 |
| Depression | 1.73 (1.08–2.76) | 0.021 | 1.47 (0.80–2.68) | 0.210 |
| DJD | 0.86 (0.47–1.57) | 0.634 | 0.84 (0.39–1.79) | 0.658 |
| Asthma | 1.02 (0.65–1.58) | 0.924 | 0.77 (0.44–1.34) | 0.365 |
BMI = body mass index; DJD = degenerative joint disease; OSA = obstructive sleep apnea.
Table 3.
Multivariate Cox regression analysis for predicting cardiovascular events in the entire cohort and propensity-matched patients after LAGB surgery for morbid obesity
| Covariates | Entire cohort | Propensity-matched cohort | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.01 (0.99–1.04) | 0.143 | 1.01 (0.99–1.05) | 0.236 |
| Male | 1.11 (0.65–1.89) | 0.702 | 1.25 (0.67–2.35) | 0.473 |
| OSA | 4.51 (2.80–7.27) | <0.001 | 6.92 (3.39–14.13) | <0.001 |
| Dyslipidemia | 1.33 (0.82–2.15) | 0.239 | 1.67 (0.88–3.15) | 0.114 |
| Hypertension | 1.81 (1.02–3.22) | 0.041 | 1.79 (0.82–3.90) | 0.041 |
| Diabetes | 1.20 (0.74–1.93) | 0.447 | 1.19 (0.66–2.15) | 0.554 |
| Depression | 1.68 (1.04–2.71) | 0.031 | 1.35 (0.72–2.52) | 0.342 |
OSA = obstructive sleep apnea.
Matched population
Both univariate and multivariate analyses were performed in the propensity-matched OSA and non-OSA patients with results summarized in Table 2. At the univariate level, OSA was a strong predictor of a cardiovascular event with a HR of 6.82 (95% CI 3.38–13.74, p < 0.001), along with age (HR 1.04, 95% CI 1.01–1.07, p = 0.01), and dyslipidemia (HR 2.17, 95% CI 1.17–4.02, p < 0.014; Table 2), but on multivariate analysis only OSA remained an independent predictor of a cardiovascular event with an HR of 6.92 (95% CI 3.39–14.13, p < 0.001; Table 3).
Discussion
The main findings of our study are that cardiovascular event rates after LAGB surgery for morbid obesity are significantly higher in patients who have OSA than patients without OSA, despite achieving a similar degree of weight loss and BMI reduction. The cardiovascular events were mainly driven by a higher incidence of new onset stroke, HF, and venous thromboembolism resulting in a trend toward a higher mortality in patients with OSA. After adjusting for baseline differences in risk factors for CVD, OSA remained an independent predictor of cardiovascular events with a 6.9-fold higher risk of adverse cardiovascular outcome. Patients with OSA treated with CPAP had lower cardiovascular event rates over a 30 month follow-up after LAGB compared with those not treated with CPAP. To the best of our knowledge, this is the first study to document the association of OSA with attenuation of the beneficial effect of weight reduction on cardiovascular events after bariatric surgery in a propensity-matched population of patients with and without OSA. The results highlight the importance of treating OSA after LAGB surgery to gain maximum benefit of weight reduction by this procedure.
All patients in this study were morbidly obese with a BMI ≥ 40 kg/m2 or ≥35 kg/m2 with at least one obesity-associated comorbidity [2, 16] and selected for LABG surgery after failure to lose weight by medical therapy under the supervision of a multidisciplinary team of physicians, nutritionists, and psychologists. The degree and timing of weight loss with the absolute reduction in body weight of 27–29 kg and the mean decrease in BMI of 20 per cent after LAGB in both the OSA and non-OSA groups with up to 10 kg/m2 decrease in BMI from baseline at 2–3 years are similar to what has been reported by other investigators [2, 18–20]. The initial weight loss was maintained in both the groups for up to 6 years and only thereafter a gradual weight gain was noted in the group with OSA, but not in those without OSA. The reason for this late weight gain in the OSA group is not clear and may or may not be related to OSA or its treatment or nonadherence with CPAP therapy, previously recognized as one of the factors associated with weight gain [21].
The desired benefit of bariatric surgery and weight loss is on reduction in cardiovascular events and overall mortality related to obesity that is reported to result in a reduction in life expectancy of 5–7 years in nonsmokers and up to 13.7 years in smokers [22]. For every 5-unit increase in BMI over 25 kg/m2, a 30 per cent increase in mortality was demonstrated in the Prospective Studies Collaboration, which analyzed 900000 adults [23]. The enhanced mortality with morbid obesity principally results from cardiovascular events [23, 24], which are favorably influenced by successful weight reduction following bariatric surgery [25, 26]. Although no sufficiently powered randomized control trial of the effect of bariatric surgery versus a nonsurgical approach to weight reduction on long-term survival or cardiovascular events has been conducted, the overall improvement in obesity-related CVD risk factors after weight loss is expected to reduce cardiovascular events and related mortality [18], as has been demonstrated in observational studies and their meta-analyses [3, 18]. In the Swedish Obese Subjects study, a nonrandomized, prospective, controlled study of 2010 obese patients who underwent bariatric surgery and 2037 matched obese controls treated conventionally, the overall and cardiovascular death and fatal or nonfatal cardiovascular events (MI or stroke) after a median follow-up of 14.7 years was reduced in the surgery group [8, 25]. Information about the impact of OSA on cardiovascular outcomes after bariatric surgery is, however, unknown. Obesity and OSA both are associated with abnormal cardiac remodeling with a higher prevalence of ventricular hypertrophy and HF [27]. A relationship between increasing BMI and OSA also exists, so for each standard deviation increase in BMI the risk for OSA is reported to increase by threefold [3, 15]. Weight loss and reduction in BMI attenuate the negative impact of obesity on cardiovascular risk factors and also reduce the severity of OSA, without, however, completely eliminating it [28]. A reduction in major modifiable risk factors for CVD [2, 29] and cardiovascular events [5, 18] in morbidly obese patients after weight loss with bariatric surgery has been reported by several investigators that appears to reach a nadir when BMI is reduced by 10 kg/m2 from baseline [18, 19], the degree of weight loss also accomplished in our patients with and without OSA. In the non-OSA group, the overall cardiovascular event rate and mortality after LAGB during 6.0 ± 3.2 years follow-up was low, similar to what has been reported in the literature comparing different bariatric surgery procedures and medical approaches to weight loss [18]. The effect of OSA on cardiovascular outcomes with weight reduction after bariatric surgery, however, has not been previously reported. Therefore, the results of our study demonstrating attenuation of the beneficial effect of weight reduction on cardiovascular events compared with historical control [8, 18, 25], with two- to threefold higher cardiovascular event rates in OSA patients compared with the non-OSA group, are of relevance for optimum management of these patients. The risk of stroke after bariatric surgery was significantly higher in patients with OSA compared with those without OSA, an association consistent with previous reports demonstrating an increased risk of stroke, worse functional outcomes, and post-stroke mortality in patients with OSA [30–32] that has been postulated to result from multiple factors, including hypoxia-induced activation of the sympathetic nervous system, higher prevalence of hypertension resulting in left ventricular hypertrophy, development and progression of atrial fibrillation, and other direct vascular effects [33]. In addition, a proinflammatory milieu with high levels of inflammatory markers and cytokines such as tumor necrosis factor-α and interleukin-6 promoting a procoagulant state further contributes to the risk for thromboembolic complications [33, 34].
Whether OSA itself increases the risk or is a marker for other factors that predispose to poor cardiovascular outcomes is not clear. To answer this, we adjusted for baseline differences in CVD risk factors and also performed a multivariate analysis to assess the independent effect of OSA on cardiovascular events. In the overall cohort, patients with OSA were more likely to have risk factors for CVD, including higher prevalence of hypertension, diabetes, dyslipidemia, older age, higher proportion of men, and a higher BMI compared with the non-OSA group, which could underlie differences in outcomes in the entire cohort. The difference in cardiovascular events observed in the overall nonmatched population, however, persisted after propensity matching the two groups for CVD risk factors, indicating that the higher residual cardiovascular events and mortality in the matched population were likely related to the effect of OSA or to other undefined factors that could not be adjusted. Since the timing of cardiovascular events was not related to the late weight gain in the OSA group, with most events occurring within 6 years after surgery when the weight loss was maintained (Figures 1 and 2), the difference in cardiovascular events could not be attributed to the late weight gain in patients with OSA.
The higher cardiovascular event rates in those with OSA than without OSA—despite a similar weight reduction—indicate the importance of recognizing and correcting factors associated with OSA that limit the full benefit of bariatric surgery. This is particularly of significance because OSA is present with a high prevalence (varying from 30 to 70 per cent) in those referred for bariatric surgery [35, 36]. OSA is associated with several pathophysiological effects, including metabolic (hypoxemia, hypercapnia), neurohumoral (sympathetic stimulation, renin–angiotensin–aldosterone pathway activation), inflammation, and mechanical (alteration in intrathoracic pressure) that contribute to hemodynamic stress and increased susceptibility to cardiovascular events. Improvements in the severity of OSA and metabolic milieu after weight loss induced by bariatric surgery have been reported [28, 37]; however, the impact on the cardiovascular events has not been well characterized. Whether preventing apneic episodes with the use of positive airway pressure devices such as CPAP, bi-level positive airway pressure (BIPAP), or upper airways surgery can help improve clinical outcomes in this population is unknown.
In the limited number of patients, where data regarding use of CPAP devices for OSA in our study were available, a reduction in cardiovascular events within the first 3 years after LAGB was observed, indicating the beneficial effect of controlling OSA after LAGB on cardiovascular outcomes. The difference in cardiovascular event rate on longer follow-up was, however, diminished and no longer statistically significant. Since data on long-term compliance with CPAP therapy were not available, we could not assess whether the reduction in initial event rates and the subsequent loss of this apparent protective effect when compared with the OSA patients who were not treated by CPAP at the time of the surgery (Figure 5) was related to nonadherence with CPAP therapy or other factors, such as the untreated patients may have been treated after the surgery, thus narrowing the difference in outcomes over the long term and thus require prospective assessment in a randomized fashion with closer monitoring for compliance and proper use of CPAP.
Whether compliance with the use of CPAP therapy after LAGB to prevent intermittent hypoxemia, sympathetic activation, sleep fragmentation, blood pressure elevation, inflammation, oxidative stress, and metabolic alterations would have kept the stroke and other cardiovascular event rates low is an important question in this population that has not been adequately addressed and requires further investigation [3, 21, 38, 39]. In a recent randomized, parallel-group, open-label secondary prevention trial, the use of CPAP in 45- to 75-year-old, predominantly Asian (64 per cent) men (81 per cent) with moderate-to-severe OSA and established CVD did not lower the risk of cardiovascular events (death from cardiovascular causes, MI, stroke, or hospitalization for unstable angina, HF, or transient ischemic attack) when compared with the usual care (composite endpoint 17.0 vs 15.4 per cent) over the mean 3.7-year follow-up [40]. The patients were predominantly nonobese (mean BMI 28.8 ± 4.6 kg/m2) with a mean duration of adherence to CPAP therapy of only 3.5 hr per night, which helped with the subjective outcomes, such as mood, health-related quality of life, and daytime sleepiness, but not the hard cardiovascular end points. The importance of adherence to CPAP has been demonstrated with improved outcomes reported in those compliant with CPAP (≥4 hr per night) therapy [41–43]. Whether better outcomes after bariatric surgery and weight loss could be expected with the use of CPAP over the long term in morbidly obese patients with OSA and relatively free of CVD has not been previously addressed and the hypothesis-generating findings of this retrospective study need to be assessed prospectively.
Limitations
Despite the main limitation of our study with an observational design that cannot prove causation, it is the only study with long-term follow-up of patients undergoing LAGB that points to the association of OSA to higher cardiovascular event rates compared with patients without OSA after LAGB. The limitations imposed by the retrospective nature of the study could not exclude selection bias or undetermined confounding factors that may not have been fully controlled even with adjusting for the baseline differences associated with increased mortality, including age, sex, and risk factors for CVD using a comprehensive propensity matching between those with and without OSA. Obesity was defined based on BMI, a function of weight and height that correlates well with percentage of body fat as measured by dual x-ray absorptiometry [44], but adiposity distribution information such as waist circumference or waist-to-hip ratio, measures of central obesity that are strongly associated with mortality and CVD [45], was not available in all patients. These and other anthropometric or imaging measures could have added more information, but BMI by itself being a strong predictor of overall mortality [23], particularly for patients with BMI > 35 kg/m2 (class II or III obesity), would not have affected the relationship to cardiovascular outcomes [2, 46]. Polysomnography was not routinely performed at baseline or during follow-up in our patient population, and objective information about the severity of OSA and its alleviation with weight loss or use of CPAP therapy or its compliance was not available. OSA may have been under-recognized and is one of the limitations of the study. However, the data on patients who were known to have OSA referred by the primary care physicians and determined by initial history by the bariatric clinic staff were relied on to identify the group, which may have more severe OSA. To truly understand the magnitude of the effect of OSA and its severity in the bariatric surgery group, a routine assessment of OSA by polysomnography before surgery will need to be performed to determine the distribution of the OSA severity and its impact on cardiovascular outcomes, which was not the practice at the bariatric clinic. A prospective study assessing the underlying sleep disturbance and the impact of its treatment with CPAP is needed to fully address this issue in this population. The information about CPAP therapy was obtained retrospectively from the review of medical records, and data regarding compliance with CPAP therapy or duration of use at night were not available. The possibility that noncompliance with CPAP in OSA patients contributed to the development of cardiovascular events, weight gain [13], or resistance to weight loss mediated by changes in energy intake, lifestyle, and energy expenditure needs to be further investigated. Despite these limitations, our results suggest a beneficial effect of CPAP therapy after bariatric surgery in reducing cardiovascular events, and this hypothesis-generating observation needs to be confirmed in a larger, multicenter study of morbidly obese patients undergoing bariatric surgery, whereby compliance with CPAP and the duration of therapy could be better controlled. In data reported from the Michigan Bariatric Surgery Collaborative, in patients with OSA who underwent bariatric procedures, a 30–80 per cent self-reported remission of sleep apnea symptoms was reported at 1 year [47], dependent on the amount of weight loss and the type of bariatric surgery performed, a finding also reported by the American College of Surgeons, Bariatric Surgery Center Network [48] and a systemic review of 69 studies with 13900 patients [37].
Conclusions
Our findings that despite a similar weight reduction after bariatric surgery, cardiovascular outcomes were significantly higher in those with OSA than in a propensity-matched group of patients without OSA is of high clinical significance, as OSA is frequently present [49] but under-recognized and under-treated in patients undergoing bariatric surgery [50]. With an increase in the prevalence of morbid obesity [1, 3], lack of efficacy of medical therapy to accomplish successful weight reduction, and improved safety of surgical approaches, the number of bariatric procedures is increasing. It is therefore imperative that factors such as OSA that reduce the beneficial effect of weight loss on decreasing obesity-related complications be completely defined. Although weight loss after bariatric surgery reduces the severity of OSA, it is not completely eliminated [28] and thus could attenuate the beneficial effect of weight reduction on prevention of cardiovascular events. A close monitoring for OSA and its treatment should be considered in patients undergoing bariatric surgery for morbid obesity. In addition, investigation into mechanisms underlying poor outcomes in patients with OSA, along with prospective studies to determine the impact of interventions to counter OSA that otherwise appear to offset the benefit of weight reduction on reducing morbidity, cost, and mortality associated with morbid obesity is warranted.
Funding
Part of Dr. Jahangir’s effort was supported by National Heart, Lung, and Blood Institute and National Institutes of Health grant support (R01-HL101240) and Intramural Research Awards.
Acknowledgments
The authors are grateful to Jennifer Pfaff and Susan Nord of Aurora Cardiovascular Services for editorial preparation of the manuscript and Aurora Research Institute and Wisconsin bariatric surgery unit staff, particularly the contribution by Sara Roloff.
Notes
Conflict of interest statement. None declared.
Work Performed: Aurora Cardiovascular Services, Aurora Health Care, Milwaukee, WI
References
- 1. Flegal KM, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensen MD, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benjamin EJ, et al. ; Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez-Jimenez F, et al. Trends in 10-year predicted risk of cardiovascular disease in the United States, 1976 to 2004. Circ Cardiovasc Qual Outcomes. 2009;2:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garvey WT, et al. ; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines American association of clinical endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22 (Suppl 3):1–203. [DOI] [PubMed] [Google Scholar]
- 6. Lightwood J, et al. Forecasting the future economic burden of current adolescent overweight: an estimate of the coronary heart disease policy model. Am J Public Health. 2009;99(12):2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Apovian CM, et al. Obesity and cardiovascular disease. Circulation. 2012;125(9):1178–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjöström L, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. [DOI] [PubMed] [Google Scholar]
- 9. Ashrafian H, et al. Effects of bariatric surgery on cardiovascular function. Circulation. 2008;118(20):2091–2102. [DOI] [PubMed] [Google Scholar]
- 10. Pontiroli AE, et al. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg. 2011;253(3):484–487. [DOI] [PubMed] [Google Scholar]
- 11. Kwok CS, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20–28. [DOI] [PubMed] [Google Scholar]
- 12. Neylan CJ, et al. The surgical management of obesity. Gastroenterol Clin North Am. 2016;45(4):689–703. [DOI] [PubMed] [Google Scholar]
- 13. Romero-Corral A, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dudley KA, et al. Interest in bariatric surgery among obese patients with obstructive sleep apnea. Surg Obes Relat Dis. 2015;11(5):1146–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. [DOI] [PubMed] [Google Scholar]
- 16. Mechanick JI, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery. Obesity (Silver Spring). 2013;21 (Suppl 1):S1–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. 1983 metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 18. Wolfe BM, et al. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016;118(11):1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ricci C, et al. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25(3):397–405. [DOI] [PubMed] [Google Scholar]
- 20. Courcoulas AP, et al. ; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collen J, et al. Postoperative CPAP use impacts long-term weight loss following bariatric surgery. J Clin Sleep Med. 2015;11(3):213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peeters A, et al. ; NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138(1):24–32. [DOI] [PubMed] [Google Scholar]
- 23. Whitlock G, et al. ; Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flegal KM, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037. [DOI] [PubMed] [Google Scholar]
- 25. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]
- 26. Adams TD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. [DOI] [PubMed] [Google Scholar]
- 27. Korcarz CE, et al. Effects of obstructive sleep apnea and obesity on cardiac remodeling: the Wisconsin sleep cohort study. Sleep. 2016;39(6):1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenburg DL, et al. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–542. [DOI] [PubMed] [Google Scholar]
- 29. Batsis JA, et al. Effect of bariatric surgery on cardiometabolic risk in elderly patients: a population-based study. Geriatr Gerontol Int. 2016;16(5):618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Somers VK, et al. ; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080–1111. [DOI] [PubMed] [Google Scholar]
- 31. Redline S, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loke YK, et al. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. [DOI] [PubMed] [Google Scholar]
- 33. Xie J, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5(8):e003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kardassis D, et al. Sleep apnea modifies the long-term impact of surgically induced weight loss on cardiac function and inflammation. Obesity (Silver Spring). 2013;21(4):698–704. [DOI] [PubMed] [Google Scholar]
- 35. Reed K, et al. Screening for sleep-disordered breathing in a bariatric population. J Thorac Dis. 2016;8(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flum DR, et al. ; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarkhosh K, et al. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obes Surg. 2013;23(3):414–423. [DOI] [PubMed] [Google Scholar]
- 38. Kim Y, et al. Can continuous positive airway pressure reduce the risk of stroke in obstructive sleep apnea patients? A systematic review and meta-analysis. PLoS One. 2016;11(1):e0146317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo J, et al. Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2016;20(3):965–974. [DOI] [PubMed] [Google Scholar]
- 40. McEvoy RD, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. [DOI] [PubMed] [Google Scholar]
- 41. Martínez-García MA, et al. ; Spanish Sleep Network Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. [DOI] [PubMed] [Google Scholar]
- 42. Peker Y, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. [DOI] [PubMed] [Google Scholar]
- 43. Barbé F, et al. ; Spanish Sleep and Breathing Network Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. [DOI] [PubMed] [Google Scholar]
- 44. Flegal KM, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cornier MA, et al. ; American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124(18):1996–2019. [DOI] [PubMed] [Google Scholar]
- 46. Ortega FB, et al. Obesity and cardiovascular disease. Circ Res. 2016;118(11):1752–1770. [DOI] [PubMed] [Google Scholar]
- 47. Nagendran M, et al. ; Michigan Bariatric Surgery Collaborative Self-reported remission of obstructive sleep apnea following bariatric surgery: cohort study. Surg Obes Relat Dis. 2015;11(3):697–703. [DOI] [PubMed] [Google Scholar]
- 48. Hutter MM, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lopez PP, et al. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74(9):834–838. [PubMed] [Google Scholar]
- 50. Ravesloot MJ, et al. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur Arch Otorhinolaryngol. 2012;269(7):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]