Abstract
Background and Aims
Studies have indicated that plant stomatal conductance (gs) decreases in response to elevated atmospheric CO2, a phenomenon of significance for the global hydrological cycle. However, gs increases across certain CO2 ranges have been predicted by optimization models. The aim of this work was to demonstrate that under certain environmental conditions, gs can increase in response to elevated CO2.
Methods
Using (1) an extensive, up-to-date synthesis of gs responses in free air CO2 enrichment (FACE)experiments, (2) in situ measurements across four biomes showing dynamic gs responses to a CO2 rise of ~50 ppm (characterizing the change in this greenhouse gas over the past three decades) and (3) a photosynthesis–stomatal conductance model, it is demonstrated that gs can in some cases increase in response to increasing atmospheric CO2.
Key Results
Field observations are corroborated by an extensive synthesis of gs responses in FACE experiments showing that 11.8 % of gs responses under experimentally elevated CO2 are positive. They are further supported by a strong data-model fit (r2 = 0.607) using a stomatal optimization model applied to the field gs dataset. A parameter space identified in the Farquhar–Ball–Berry photosynthesis–stomatal conductance model confirms field observations of increasing gs under elevated CO2 in hot dry conditions. Contrary to the general assumption, positive gs responses to elevated CO2, although relatively rare, are a feature of woody taxa adapted to warm, low-humidity conditions, and this response is also demonstrated in global simulations using the Community Land Model (CLM4).
Conclusions
The results contradict the over-simplistic notion that global vegetation always responds with decreasing gs to elevated CO2, a finding that has important implications for predicting future vegetation feedbacks on the hydrological cycle at the regional level.
Keywords: Stomata, stomatal conductance, climate change, CO2, hydrology, CLM, vegetation, run-off, drought, photosynthesis, temperature, VPD
INTRODUCTION
Water loss through plant stomata – small pores on the surface of leaves through which gas exchange between plants and the atmosphere takes place – is an unavoidable trade-off in the exchange for CO2, the substrate for photosynthesis. Decreased stomatal conductance (gs), via physiological (stomata responding dynamically to environmental stimuli) and/or morphological changes (via alteration in stomatal density and size) has been observed in elevated carbon dioxide (CO2) environments in both laboratory and free air CO2 enrichment (FACE) studies (Farquhar and Sharkey, 1982; Woodward, 1987; Drake et al., 1997; Ainsworth and Rogers, 2007; Leuzinger and Körner, 2007). However, recent studies suggest that rising atmospheric CO2-induced decreases in gs may be offset by contemporaneous increases in leaf area index (LAI) during the course of a growing season (Piao et al., 2007; Wu et al., 2012; Niu et al., 2013; Frank et al., 2015; Schymanski et al., 2015). Thus, despite significant improvements in our understanding of plant–atmosphere interactions in recent years, the net stomatal conductance response of the entire global vegetation system to rising anthropogenic CO2 remains unclear.
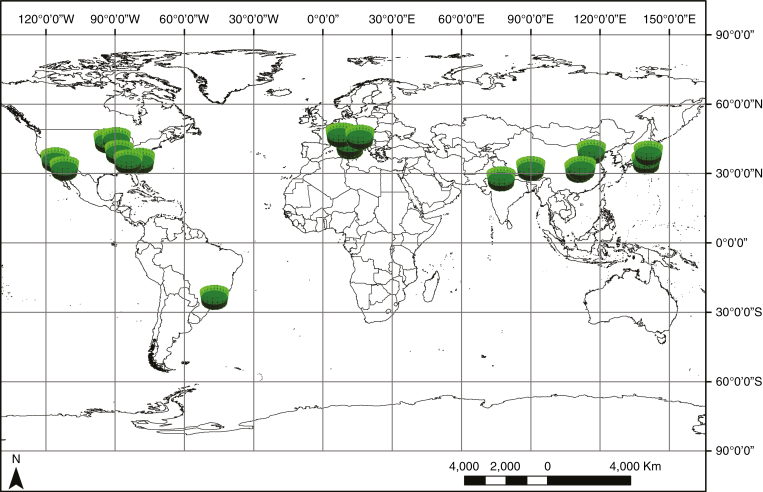
In addition, little is known regarding the physiological response of plants to increasing CO2 across multiple biomes, and in varying temperature and humidity regimes. For example, FACE studies are predominantly limited to the mid-latitudes of the northern hemisphere (Fig. 1), biasing our understanding of plant responses to these regions. Moreover, disparate vegetation responses in dry and drought-prone environments have been reported (Choat et al., 2012; Limousin et al., 2013; Zhou et al., 2013; De Kauwe et al., 2015; Mencuccini et al., 2015). It is therefore critical to improve our understanding of these responses to better predict future freshwater cycling, especially in regions vulnerable to drought and desertification in the 21st century (Lawrence et al., 2011).
Fig. 1.
The location of FACE studies included in our assessment. Fifty-one FACE studies are shown (most overlap on this scale). Most FACE studies are located in northern hemisphere locations between 30 and 60°N. FACE studies which did not, to our knowledge, document gs changes were not included. See Materials and Methods for all cited studies used.
Here we demonstrate that gs can in some cases increase in response to increasing atmospheric CO2. This is shown using (1) in situ measurements of 51 woody plant taxa across four biomes showing dynamic gs responses to a CO2 rise of ~50 ppm, which represents the change in this greenhouse gas over the past three decades, (2) an extensive, up-to-date, synthesis of gs responses in FACE experiments, (3) both the stand-alone and the Community Land Model version 4 (CLM4)-integrated application of the Farquhar–Ball–Berry (FBB) photosynthesis–stomatal conductance model and (4) the Medlyn et al. (2011) optimal stomatal model.
MATERIALS AND METHODS
Synthesis of free air CO2 enrichment (FACE) studies
A literature review was undertaken of studies that specifically focused on the effect of elevated CO2 on plant stomatal conductance (gs) in FACE experiments. A total of 51 studies were included in the database (in alphabetical order: Adachi et al., 2014; Ainsworth and Rogers, 2007; Ainsworth et al., 2003; Bader et al., 2010; Bhattacharya et al., 1994; Borjigidai et al., 2006; Bryant et al., 1998; Calfapietra et al., 2005; Chen et al., 2014; Ellsworth, 1999; Ellsworth et al., 1995, 2012; Garcia et al., 1998; Ghini et al., 2015; Grant et al., 1999; Gunderson et al., 2002; Hamerlynck et al., 2000, 2002; Hao et al., 2013; Hättenschwiler et al., 2002; Herrick et al., 2004; Herrick and Thomas, 1999, 2003; Hileman et al., 1992, 1994; Huxman and Smith, 2001; Ji et al., 2015; Keel et al., 2006; Leakey et al., 2006; Lee et al., 2001; Marchi et al., 2004; McElrone et al., 2005; Naumburg and Ellsworth, 2000; Naumburg et al., 2003, 2004; Neal et al., 2000; Nijs et al., 1997; Noormets et al., 2001; Nowak et al., 2001; Pataki et al., 2000; Pearson et al., 1995; Rogers et al., 2004; Ruhil et al., 2015; Shimono et al., 2010; Singsaas et al., 2000; Tricker et al., 2005; Wall et al., 2000, 2001; Wechsung et al., 2000; Wullschleger et al., 2002; Yoshimoto et al., 2005). The FACE synthesis was built on the original data set by Ainsworth and Rogers (2007). Values reported in tables and in the text were taken directly from publications, whereas results in graphs were digitized. Individual independent observations were obtained following the longest period of CO2 exposure reported in each study (independent = plant; repeated = species). Studies that examined multi-factorial designs could have contributed several observations for each response variable (drought, nitrogen enrichment, etc.). The mean, standard deviation (s.d.) and the effect size of the treatment (Ne) and of the relative control treatment (Na) were recorded. If standard error (s.e.) was reported we transformed these according to s.e. = s.d.*[(n − 1)/2]. Database records typically included the year and month the data were collected, GPS site locations, ambient CO2, elevated CO2, study organism (incl. varieties), plant functional type (PFT), photosynthetic pathway and other experimental treatments (e.g. nitrogen fertilization). Stomatal conductance measurements from 52 different species, within seven PFTs (C3 crops, C3 forbs, C3 grasses, C3 herbs, C3 shrubs, C3 conifer trees and C3 broadleaved trees) were included in the analysis. The ranges of ambient and elevated CO2 between studies were 350–411 and 538-680 ppm, respectively. A kernel density estimation was used to visualize the stomatal conductance data by estimating the unknown probability of the data, based on a sample of points taken from that distribution.
Dynamic gs responses to CO2 change (across four biomes)
Assessment of the dynamic stomatal responses to increasing CO2 across four different biomes (including a tropical seasonal biome which had been subjected to drought) was achieved during a 10-week scientific expedition to North and Central America in summer 2014. A total of 51 woody tree and shrub species were measured with a CIRAS-2 gas analyser (PP-Systems, Amesbury, MA, USA) attached to a PLC6 (U) cuvette fitted with a 1.7-cm2 measurement window and a red/white light LED unit.
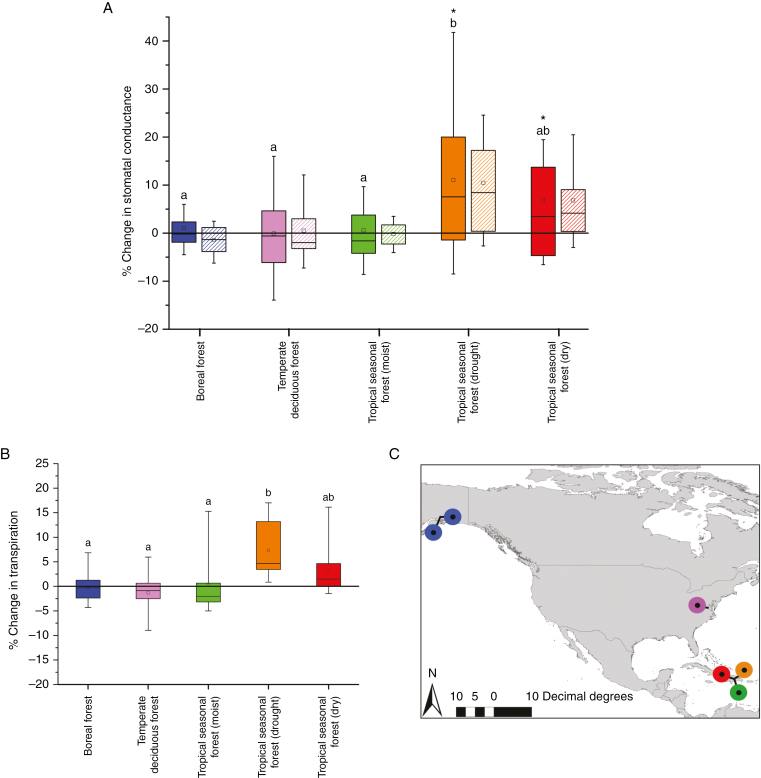
Measurements were carried out (see Fig. 3) at two boreal forest sites [16 species, Bird Creek (60°58′N, 149°28′W) and Kenai (60°33.3′N, 151°12.8′W), Alaska, USA], one temperate deciduous forest site [11 species, Smithsonian Environmental Research Centre (38°53′N, 76°32′W), Maryland, USA], two tropical seasonal forest (wet) sites [15 species, Cambalache (18°27′N, 66°35′W) and Guajataca (18°24′N, 66°58′W), Puerto Rico] one of which had undergone a long drought period (Cambalache), and one tropical seasonal forest (dry) site [nine species, Guanica (17°93′N, 66°92′W), Puerto Rico]. See Supplementary Data Table S1 for a complete species list.
Fig. 3.
Dynamic gs responses to a subtle CO2 increase across four biomes observed in field conditions compared with modelled responses. (A) Percentage change in gs during the transition from 354 (sub-ambient) to 400 ppm (modern ambient) atmospheric CO2, which is representative of the atmospheric changes that have occurred over the past ~25 years. The boxes signify the distribution of the 25–75 % quartiles, with median and average values represented by a vertical line and an open square within the box, respectively. Whiskers indicate the distribution of the 5–95 % quartiles. Solid boxes represent the field measurements. Striped boxes represent the modelled percentage responses of gs using the Farquhar–Ball–Berry model and the A, Tv and ea/ei values measured in the field. Different lower-case letters denote statistically significant differences between biomes (P ≤ 0.05). Asterisks indicate within-biome statistically significant differences between the conductance values at 354 and 400 ppm CO2. n = 24–66 independent measurements depending on biome (see Table S1 for species list). (B) Percentage change in transpiration between 354 and 400 ppm atmospheric CO2. (C) Locations of expedition sites visited during this study. See Table S1 for geographical coordinates and site information.
Stomatal responses were assessed on an average of four individuals per species between 0900 and 1300 h. A sun-exposed branch was sampled following standard protocols (Dang et al., 1997; Koch et al., 2004; Berveiller et al., 2007; Domingues et al., 2010; Rowland et al., 2015) from each individual using either a pruner (shrubs) or a pole with a scythe fitted on its top (trees) and was immediately recut under water. Following this, a fully expanded leaf from each branch was enclosed in the cuvette of the gas analyser, which was running at a sub-ambient ~year 1990 reference CO2 concentration of 354 ppm (Betts et al., 2016). Stomatal conductance at sub-ambient CO2 concentration was recorded upon stabilization of its value, which typically took less than 15 min. Subsequently, reference CO2 was established at 400 ppm (year 2016 values) (Betts et al., 2016) and the leaf was left to equilibrate for at least 15 min before gs at modern ambient CO2 was recorded. Randomization of the sequence of the two treatments was ensured; overall about 65 % of the measurements started at 400 ppm (386.6 ± 0.5) and were reduced to 354 ppm (342.4 ± 0.5), while the remaining measurements (35 %) started at 354 ppm and were increased to 400 ppm. On several occasions the reversibility of the CO2 effects on gs was tested. This was done by measuring gs at a starting CO2 concentration of 400 ppm, after which CO2 was reduced to 354 ppm for several minutes, before it was returned to the initial concentration of 400 ppm. The final gs values at 400 ppm were the same as those initially recorded (data not shown).
Stomatal responses to a subtle increase in CO2 were estimated as the percentage change in the gs values between sub-ambient CO2 and modern ambient CO2. Air flow, light intensity and incoming mole fraction of water during the measurements were maintained at 200 cm3 min−1, 1000 μmol m−2 s−1 and 80–90 % of ambient, respectively. Since ambient and leaf temperatures varied significantly between the beginning and the end of the daily measurement time window in all biomes, the measurements were taken at the calculated mean and biome-specific leaf temperature at 0900 h. Calculation was performed early on the first measurement day at each site by running the gas analyser at the set points mentioned above (i.e. 1000 µmol m−2 s−1 of light, 80–90 % of ambient water vapour, 400 µmol mol−1 CO2, no temperature control) and by recording the leaf temperatures of at least ten leaves belonging to ten different species growing at the site. Differences in gs responses between biomes were tested on the normal data using analysis of variance (ANOVA). Moreover, a linear model was used to test for the correlation of gs to vapour pressure deficit (VPD) and leaf temperature and the modelled and observed gs data. Mixed effects models were used to test which variables best explain the observed changes in gs and the best model was selected following Akaike’s Information Criterion (AIC).
Farquhar–Ball–Berry model (combined photosynthesis and gs)
The model relates gs to net leaf photosynthesis, scaled by the relative humidity at the leaf surface and the CO2 concentration at the leaf surface (Collatz et al., 1991; Sellers et al., 1996). It solves the following three equations:
| (1) |
| (2) |
| (3) |
where gs is the stomatal conductance to water vapour, A is the photosynthetic uptake flux of CO2, ca and ci are partial pressures of CO2 just outside and inside the stomata, respectively, pa = 105 Pa is atmospheric pressure, ea and ei are the water vapour pressures just outside and inside the stomata, respectively (the latter computed as the saturation vapour pressure at leaf temperature Tv), and m and b are empirical constants taken as m = 6 and b = 3 × 104 µmol m–2 s–1. The uptake flux is taken to be the minimum of three rate-limiting processes for C3 plants: Rubisco limitation, wc = Vcmax (ci– Γ*)/(ci+ Kc+ oiKc/Ko); light limitation, wj = α PAR (ci– Γ*)/(ci+ 2Γ*); and export limitation we = 0.5 Vcmax. In these expressions Kc and Ko are Michaelis–Menten constants for CO2 and O2, respectively, which vary with leaf temperature Tv (expressed in °C) as and where Kc25 = 30 and Ko25 = 30 000 are reference values while akc = 2.1 and ako = 1.2. The CO2 compensation point is taken as Γ* = 0.105 oiKc/Ko with oi the partial pressure of oxygen. PAR = 1000 µmol m–2 s–1 is the photosynthetically active radiation flux falling on the leaf, and α = 0.06 is the quantum efficiency of photosynthesis. Finally, Vcmax is the temperature-dependent maximum carboxylation rate modelled following Katul et al. (2010) as Vcmax = Vcmax25e0.88(Tv–25)/(1 + e0.29(Tv–41)) where Vcmax25 = 60 µmol m–2 s–1 is the maximum carboxylation rate at 25 °C. Given values of ca, ea, Tv, PAR and Vcmax25, the equations are solved numerically using an iterative method to yield ci, A and gs.
Optimization model
For the comparison of our field data with the optimum gs model of Medlyn et al. (2011) we used measured values of A, ca and VPD and Biome-specific gl values and the version of the model equation from Lin et al. (2015).
| (4) |
where D is VPD (kPa), and gl is the model coefficient.
The Community Land Model version 4 (CLM4)
The Community Land Model version 4 (CLM4), released in 2010 (Oleson et al., 2010; Lawrence et al., 2011) was used in this study. Land cover and atmospheric weather conditions serve as boundary conditions for CLM4. Grid cells in CLM4 may include vegetation, wetlands, lakes, glacier and urban regions. CLM4 can be used in conjunction with the other models in the Community Earth System Model (CESM), or independently (stand-alone), as is the case here. This is referred to as an I-compset. Specifically, we have used the I-compset with an f19g16 resolution and CLM4 satellite phenology. This simulation has the carbon and nitrogen cycling (biogeophysics ‘CN’) turned off. CLM4 parameterizes stomatal responses via an FBB scheme as described above.
CLM4 uses atmospheric boundary conditions for integration. We use the QIAN atmospheric input data set, for 1972–2004 (Qian et al., 2006). This is a global forcing dataset for the period 1948–2004 with 3-hourly temporal and T62 spatial resolution (1.875°). The dataset was developed by combining analyses of monthly precipitation and surface air temperature with intra-monthly variations from the National Centers for Environmental Prediction – National Center for Atmospheric Research (NCEP–NCAR) re-analysis (Qian et al., 2006). Using the I-compset we performed experiments at 350, 400 and 700 ppm. Results are provided as climatological mean values over the forcing period (1974–2004). Atmospheric forcing, as per Qian et al. (2006), is identical between each of the 350, 400 and 700 ppm runs.
RESULTS
Free air CO2 enrichment studies (FACE)
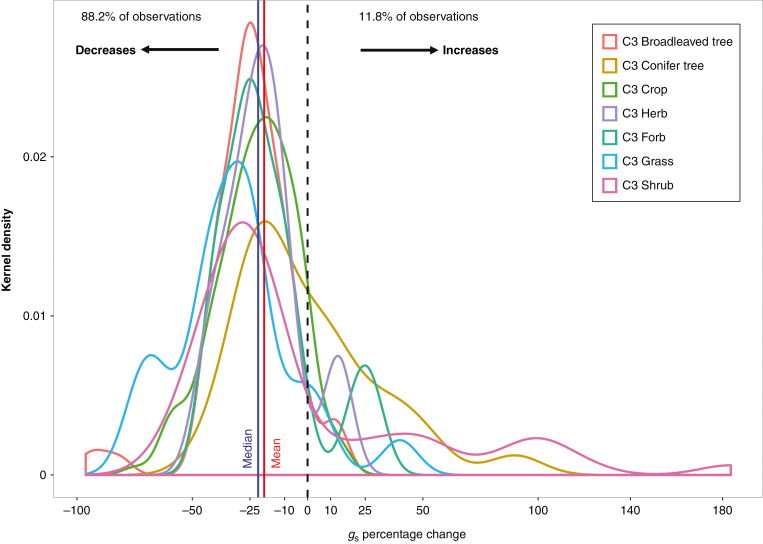
To investigate the range of responses of gs across global sites (Fig. 1) we performed a synthesis of data from 51 FACE studies. Of the 1313 independent measurements across 52 species, 88.2 % of the measurements showed a decrease in gs in response to elevated CO2 (Fig. 2). However, 11.8 % of the measurements showed an increase in gs (Fig. 2). Such increases have gone largely unreported in the past, with most meta-analyses focusing on the overall mean negative response (decrease) of gs to increasing CO2 concentration (e.g. Ainsworth and Rogers, 2007). Overall, gs decreased by ~19 % on average across all FACE studies (Fig. 2).
Fig. 2.
FACE synthesis of gs responses to increasing CO2 concentration. Kernel density probability distribution of the percentage change of gs to increasing CO2 concentration. Each colour represents a different plant functional type (PFT). The percentage gs change is expressed as the delta change of gs between ambient and high CO2 treatments. Solid lines are median (blue) and mean (red) change in gs across all PFTs. The dashed line is the zero percentage change mark. See Materials and Methods for details of the synthesis and cited FACE studies used.
Field survey of gs responses to a 50 ppm CO2 rise
A total of 51 C3 tree and shrub species (n = 209) were sampled during the in situ CO2 gas exchange measurements across four biomes (Fig. 3). Measurements reveal significant variation in the dynamic gs responses to an ~50 ppm CO2 increase, which was selected to represent anthropogenic climate change over the past 25 years (from 354 to 400 ppm) across the different biomes (Fig. 3). The species of the boreal, temperate deciduous forest and tropical seasonal forest (moist) biomes displayed an overall negligible response to increasing CO2 (Fig. 3). In contrast, the species of the tropical seasonal forest (dry) and, to an even greater extent, the species of the tropical seasonal forest (drought), which had been subjected to a 1-month-long drought period prior to the measurements, displayed statistically significant mean increases in gs in response to a 50 ppm rise in CO2 (6.8 and 11.1 %, respectively) (Fig. 3). The grouping of stomatal responses between wet [i.e. boreal forest, temperate deciduous forest and tropical seasonal forest (moist)] and dry regions [i.e. tropical seasonal forest (dry) and tropical moist seasonal forest (drought)] is also clearly reflected in the corresponding changes in plant transpiration; decreasing and increasing mean transpiration are observed, respectively (Fig. 3).
Field gs data – model comparison
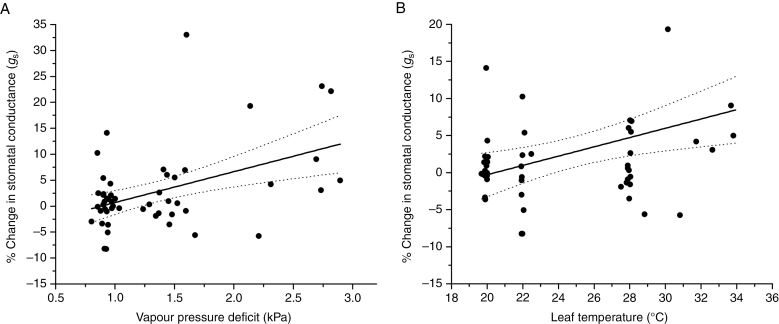
Our finding that gs can respond positively to increasing CO2 is supported by the theoretical predictions of the combined FBB photosynthesis and gs model. The model simulations, under an ~50 ppm CO2 rise scenario, demonstrate that increases in atmospheric CO2 drive increases in gs (Fig. 4) under conditions of high VPD (expressed as ea/ei in the model) and medium to high leaf temperature (Tv). The dependence of gs responses to increasing CO2 on air moisture and leaf temperature is also observed in the field gas analysis data by positive correlations between gs responses and VPD and leaf temperature (Fig. 5). This was also confirmed using mixed effects models, which showed that the measured relative changes in gs are best explained when the relative changes in A and ea/ei are used as fixed factors (AIC = 1633.8, χ2 = 4.0348, P = 0.044). The FBB simulations provide a theoretical underpinning for the field observations by demonstrating that plants can increase gs as a response to increasing CO2, while simultaneously optimizing water use efficiency (WUE) (Fig 4). In the model, increases in WUE are observed across all values of Tv and humidity. However, increases in WUE are highest in the parameter space where leaf humidity is low (dry regions) and Tv is high (warm–hot regions). A second simulation shows that the model produces an even higher gs increase in response to a doubling of CO2 (to 700 ppm) in dry and warm–hot regions of the parameter space (not shown).
Fig. 4.
Results from the Farquhar–Ball–Berry combined photosynthesis and gs model. (A) A–ci response curves at two different leaf temperatures, as indicated in the key. (B) A–ca response curves at two different temperatures and humidities (see key in C). (C) Sensitivity of A to ca, normalized by A/ca, as a function of ca at two different temperatures and humidities, as indicated in the key. (D) Predicted gs at ca = 350 ppm as a function of leaf temperature and humidity. (E) Predicted percentage change in gs when ca changes from 350 to 400 ppm, with zero contour highlighted by solid black line. (F) Predicted percentage change in water use efficiency (WUE) when ca changes from 350 to 400 ppm. Symbols in all panels indicate three selected cases: high temperature, low humidity (circles); high temperature, high humidity (squares); and low temperature, low humidity (triangles).
Fig. 5.
Gas analysis relationship between gs and vapour pressure deficit and leaf temperature. Linear relationship and 95 % confidence bands (dotted lines) between the percentage change in gs during the transition from 354 (sub-ambient) to 400 ppm (modern ambient) atmospheric CO2 and (A) VPD (kPa) (y = 5.94x − 5.24, r2 = 0.21, P < 0.01) and (B) leaf temperature (°C) (y = 0.63x − 12.82, r2 = 0.14, P < 0.01). Data represent species averages with an average number of four individuals measured per species.
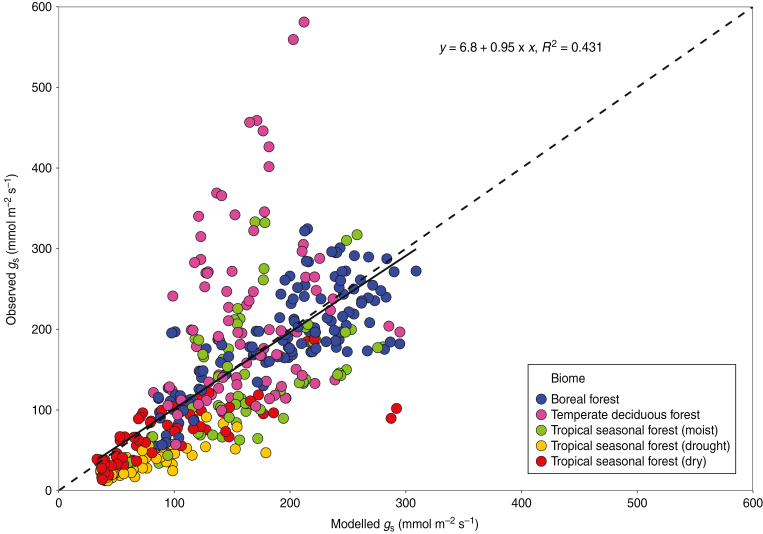
To test how well the field infrared-gas-analyser measured gs is described by the FBB model, as well as the optimal gs model of Medlyn et al. (2011), we used the recorded values of photosynthesis (A), Tv and water vapour concentration to calculate the model-implemented gs of all 51 taxa analysed. For the Medlyn et al. (2011) model we used published gl values by Lin et al. (2015) for evergreen and deciduous trees and shrubs. Here g0 values of 20 mmol m−2 s−1 are used. The comparison of modelled and recorded data revealed that the FBB model can accurately predict the observed gs, with the regression between estimated and observed gs falling very close to the 1: 1 line (Fig. 6). Furthermore, the model-implemented gs responses are strikingly similar to those observed in the field (Fig. 3). A similarly good fit was found when observed gs values were plotted against the optimal gs model of Medlyn et al. (2011) (Supplementary Data Fig. S1).
Fig. 6.
Comparison of measured and modelled gs values under 354 and 400 ppm of atmospheric CO2. Relationship (0.95x+6.8, r2 = 0.431, solid line) between measured and modelled gs values. Stomatal conductance was modelled using the Farquhar–Ball–Berry model and the A, Tv and ea/ei values measured in the field. The dashed line represents the 1: 1 relationship. Mixed effects model results showed that the relative changes in gs are best explained when the relative changes in A and ea/ei are used as fixed factors (AIC = 1633.8, χ2 = 4.0348, P = 0.044).
The Community Land Model – a spatial investigation of global gs
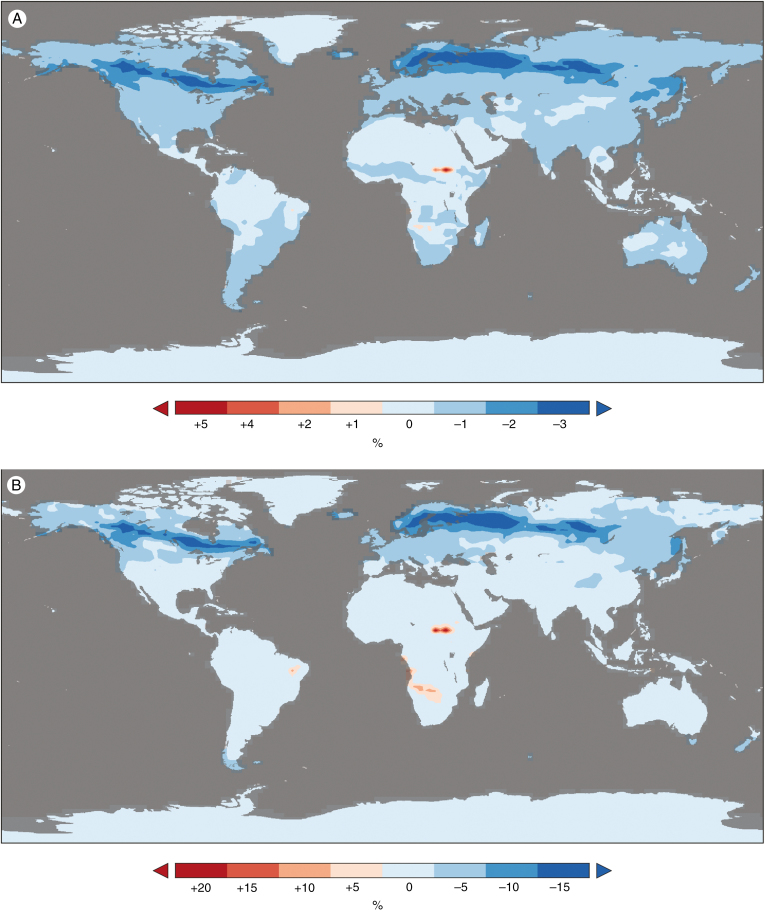
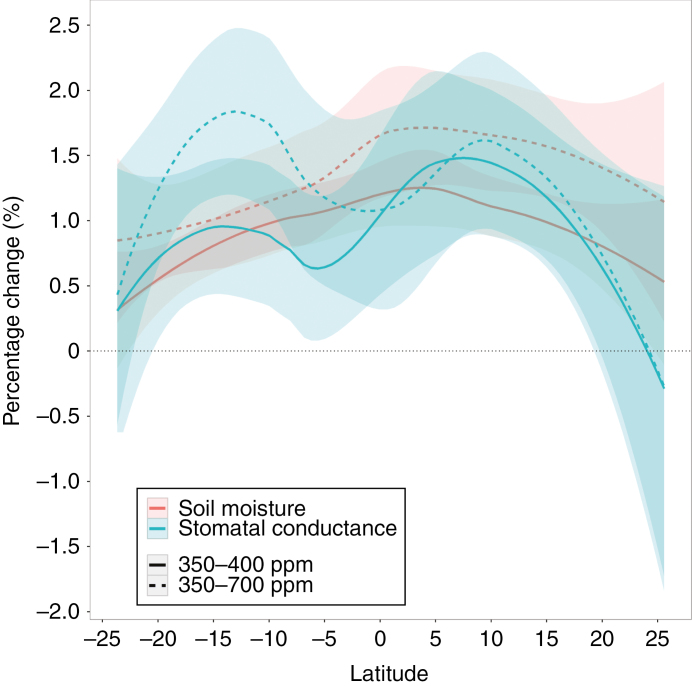
To gain a deeper understanding of the land–vegetation system response to increases in CO2 at a spatial global scale, we performed simulations using the CLM4 land–vegetation model. The FBB model is also used for the parameterization of CLM4. Simulations of the same CO2 increases in CLM4 resulted in a similar pattern of gs responses (Fig. 7). In response to a 50 ppm CO2 increase the CLM4 simulation produces predominantly negative changes (decreases) in gs (Fig. 7). An ~3.2 % annual global climatological maximum decrease in gs is simulated (Table 1). However, positive gs responses are also simulated, with a maximum increase of ~4.9 % (Fig. 7, Table 1). A second annual global simulation, forcing the system with a doubling of CO2 (to 700 ppm), resulted in a larger ~16.8 % global climatological maximum decrease in gs (Fig. 7). As in the 50 ppm scenario, positive gs responses were also simulated across the low latitudes, this time with higher maximum positive changes of ~18.9 % (Fig. 7, Table 1). There was a clear seasonal latitudinal and regional trend in the magnitude of gs change between months in the simulation (Fig. S2). For example, positive gs increases (to 50 ppm) were mostly observed in the months between December and May in Central Africa and between June and October in South Africa. In contrast, positive gs increases in Central America were observed in the months between January and June and in South America between June and November. Interestingly, the gs increases were accompanied by increases in soil moisture (Fig. 8, Table 1). Annual modelled regions experiencing an increasing gs response to CO2 include Mexico, the Galapagos Islands, Dominican Republic, Columbia, Venezuela, Brazil, Bolivia, Sudan, South Sudan, Somalia, Tanzania, Democratic Republic of Congo (D.R.C.), Angola, Namibia, Botswana and Indonesia (Fig. 7, Table 2). Similar to our field observations, areas that showed positive gs increases were situated in hot and dry biomes (Table 2).
Fig. 7.
Annual gs response to increasing CO2 in the CLM4 land–vegetation model. Negative and positive gs responses to increasing CO2 in CLM4, for (A) a 400 ppm and (B) a 700 ppm scenario, relative to 350 ppm. Modelled regions experiencing positive gs responses for both A and B include parts of Central America, South America, Africa and Asia (see Table 2 for more detail). Note that the majority of the land surface experiences decreases in gs in response to increasing CO2.
Table 1.
Community Land Model maximum annual increases/decreases and percentage of grid cells showing increases/decreases or no change in gs and soil moisture worldwide
| CO2 (ppm) | Variable | Max. decreases | Max. increases | Percentage no. of grid cells | ||
|---|---|---|---|---|---|---|
| Increase | Decrease | No change | ||||
| 400–350 | Stomatal conductance (mmol m–2 s–1) | 0.00075 (3.15 %) | 0.00004 (4.92 %) | 1.94 | 64.22 | 33.83 |
| Soil moisture (kg m−2) | 0.1 (0.21 %) | 1.1 (2.3 %) | 48.55 | 0.15 | 51.33 | |
| 700–350 | Stomatal conductance (mmol m–2 s–1) | 0.00004 (16.82 %) | 0.00001 (18.94 %) | 1.45 | 65.81 | 32.74 |
| Soil moisture (kg m−2) | 2.6 (5.6 %) | 0.01 (0.02 %) | 80.87 | 0.03 | 19.11 | |
Fig. 8.
Detailed analysis of Community Land Model grid cells showing positive gs responses under a 400 and 700 ppm CO2 scenario. Percentage change of soil moisture and gs for a 400 ppm (solid lines) and a 700 ppm (dashed lines) scenario, relative to 350 ppm. Only grid cells that showed positive increases in gs are used for this analysis (geographical areas coloured in red and orange in Fig. 7).
Table 2.
Countries and associated biomes that showed annual positive increases in gs under a 50 ppm increase in CO2
| Continent | Country | Biome |
|---|---|---|
| Central America | Mexico | Tropical & Subtropical Dry Broadleaved Forest |
| South America | Galapagos Islands | Mediterranean Forests, Woodland & Shrub |
| South America | Dominican Republic | Tropical & Subtropical Dry Broadleaved Forest |
| South America | Columbia | Tropical & Subtropical Dry Broadleaved Forest & Deserts & Xeric Shrublands |
| South America | Venezuela | Deserts & Xeric Shrublands |
| South America | Brazil | Deserts & Xeric Shrublands |
| South America | Bolivia | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | Sudan | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | South Sudan | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | Somalia | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | Tanzania | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | D.R.C. | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | Angola | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | Namibia | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Africa | Botswana | Tropical & Subtropical Grasslands, Savannas & Shrublands |
| Asia | Indonesia | Tropical & Subtropical Dry Broadleaved Forest |
DISCUSSION
Overall, our results clearly demonstrate that in dry, warm environments, or during drought periods, plants can respond to increases in CO2 by increasing their gs, while, crucially, maximizing the increase in their WUE (Figs 3, 4 and 7) compared to plants growing in the cooler moist conditions of the temperate latitudes. Implementation of the FBB model clearly shows a region of parameter space where CO2, gs and WUE increases can coincide (Fig. 4). The FBB model, when supplied with independently measured values of Vcmax, was able to accurately predict field observations, including the unexpected increases in gs at high Tv and high VPD (Figs 3 and 6), a region of parameter space not often explored in standard gas analysis protocols, which typically run under standardized temperatures and VPD of 22 °C and 1 kPa, respectively. Although the measured gs responses are small and difficult to capture under field conditions, Figs 3 and 6 show excellent agreement between modelled and observed values and strongly support our claims.
For a more mechanistic understanding of the gs responses documented above, we turn to a more detailed analysis of the FBB model. Firstly, we note that in the light-saturated conditions we are exploring here, A is Rubisco-limited and is thus expected to increase with temperature. In the particular formulation used here (see Materials and Methods), Vcmax increases roughly exponentially with temperature at temperatures below ~35 °C, leading to a strong steepening of the A–ci response curve as temperature increases (Fig. 4). This steepening carries over to the A–ca response, as shown in Fig. 4; this figure also shows that higher humidity yields greater A at a given temperature and ca, because greater humidity promotes stomatal opening (Fig. 4) and thus greater ci, enhancing photosynthesis. Furthermore, we note that eqn (1) in the model (see Materials and Methods) implies that the sensitivity of gs to ambient CO2, dgs/dca, at fixed temperature and humidity is given by:
| (5) |
Thus, increasing gs in response to increasing ca is possible when the first term on the right-hand side is greater than one, i.e. when the relative change in A is greater than the relative change in ca. This condition can be met when temperature is high and humidity is low (as exemplified by the solid circles in Fig. 4): in that case, dA/dca is high while A is low, bringing dgs/dca above zero (Fig. 4). When both temperature and humidity are high (squares in Fig. 4), A is large enough to make the first term on the right less than one; conversely, when both temperature and humidity are low (triangles in Fig. 4), A is low but dA/dca is also low, and the first term on the right is still less than one.
In summary, the FBB model predicts dgs/dca> 0 at high temperature and low humidity under light-saturated conditions because high temperature promotes a strong gain in A per unit increase in ci (or ca), while low humidity keeps the base value of A low. Naturally, different model formulations would give quantitatively different results; in particular, the threshold values of temperature and humidity required for dgs/dca> 0 are likely to be strongly model-dependent. However, the qualitative nature of the result appears robust, because increasing Vcmax with increasing temperatures and stomatal opening with increasing humidity are both well-known features of plant physiology. Note in particular that the optimization models of Medlyn et al. (2011) also predict increasing gs as humidity increases (or VPD decreases), and would thus give qualitatively similar behaviour to the empirical Ball–Berry closure reported here (Fig. S1).
It is surprising that the possibility of gs increasing as a response to rising CO2 under these particular climatic conditions has not been highlighted before. As implied above, optimization models also predict similar increases within the CO2 envelope tested in the present study (i.e. 354–400 ppm CO2) (Arneth et al., 2002; Konrad et al., 2008; Medlyn et al., 2011, 2013). For example, the optimization model of Konrad et al. (2008) demonstrates that the inflection point between rising and falling gs response to CO2 is dependent on the ‘cost of water’ (Fig. 4 in their article). In particular, high cost of water shifts the inflection point to higher values, which are similar to those used in the present study. These predictions fit well with both our measured and modelled gs responses.
It is intriguing that a substantial number of the FACE studies (see Materials and Methods) also report increases in gs under super-ambient CO2. These increases in gs are generally not discussed, or are disregarded as methodological artefacts (Gunderson et al., 2002). Due to a lack of standardized FACE protocols, the exact reasons why positive gs responses are observed across these studies remain largely unclear. Possible reasons for the observed increases might include: (1) differences in the climatic and/or cuvette measurement conditions; (2) differences in soil nutrient and water status; (3) differences in the signal to noise ratio with regard to gs (i.e. species with low gs show a greater propensity for erroneous measurements); and (4) studies do not consistently record the time when measurements are taken, despite literature which shows that gs responses to CO2 are highly dependent on the time of day (Konrad et al., 2008). Unfortunately, FACE studies inherently include a range of weather regimes/cuvette conditions and measurement times, which are inconsistent amongst studies and typically unreported. It is therefore not possible to assess the role of these conditions with regard to the reported gs increases. Secondly, nutrient concentrations and soil water content naturally vary between sites, but are inconsistently documented across studies (e.g. Naumburg et al., 2003) making direct comparison unfeasible at this time. Regarding the potential low signal to noise ratio of the species that display increases in gs as a response to increased CO2, our meta-analysis of FACE studies showed that there is no significant difference in the gs values between species that show either positive or negative responses to CO2 (F = 1.663, P = 0.198). The same was found for the gs responses of different PFTs, with the exception of shrubs (F = 4.122, P < 0.001). Thus, the observed positive gs responses in FACE studies may arise for several reasons. It is likely that at least some of them are due to warm, dry conditions, as demonstrated by our field data (Figs 3 and 5) and model comparisons (Fig. 6 and Fig. S1).
Positive gs responses have the potential to alter regional or even global hydrological and carbon cycles, and other ecological processes. We acknowledge that there are limitations in assessing long-term gs trends through field measurements, as they cannot account for long-term water availability changes resulting from the CO2 effects on gs. Several studies have shown that decreasing soil moisture can elicit greater stomatal closure under elevated CO2 than ambient CO2 (Leakey et al., 2006; Piao et al., 2007; Gray et al., 2016). Similarly, increases in LAI have been shown to reduce soil moisture, thus indirectly affecting gs (Field et al., 1995; Wenfang et al., 2013). Our global simulations using the CLM can only partially test for this, as LAI was not simulated here. It also needs to be noted that current CLM parameterizations do not account for many morphological plant responses to elevated CO2 (e.g. changes in stomatal density). Keeping these reservations in mind and although predictions of future gs are somewhat beyond the scope of the present study, Fig. 8 shows that in regions where gs is predicted to increase in response to a 50 and 350 ppm CO2 rise, soil moisture also increases (in this instance the increased soil moisture may be caused by water savings due to suppressed gs in prior months, and may in fact cause the annual mean increase of gs. at these locations). Coupled with potential increases in LAI in response to elevated CO2 (Piao et al., 2007; Wu et al., 2012; Niu et al., 2013; Frank et al., 2015; Schymanski et al., 2015), regionally increasing gs may act to offset the much studied effects of decreasing gs, such as increasing river runoff (Gedney et al., 2006; Betts et al., 2007; de Boer et al., 2011; Gopalakrishnan et al., 2011; Lammertsma et al., 2011), or even drive enhanced drought and desertification in certain regions (Dai, 2013). Areas that were predicted by the CLM to show increases in gs with elevated CO2 (~50 and 350 ppm) are located in hot and dry biomes (Fig. 7 and Table 2). A monthly analysis of gs for the CLM also suggests that the relative timing of temperature and relative humidity is important in driving the gs increases, which leads us to expect increases in gs in monsoonal regions (Fig. S2). However, due to other confounding factors (e.g. vegetation types and/or soil moisture) this expectation is not always met (e.g. India) and requires further investigation, which is beyond the scope of the current study. Continued land–vegetation model development based on field data at the biome (and community–species) level, as well as further Earth System Model inter-comparison studies, will be required to assess the implications of this shift in our understanding of vegetation responses to elevated CO2, and for improved prediction of the global hydrological cycle, particularly in dry and warm–hot regions.
We have demonstrated that increases in gs can occur under elevated CO2 in environments that are hot and dry (high VPD). Our field observations across several global biomes are in excellent agreement with predictions from optimization models and fall within a previously unrecognized parameter space within the FBB model. The implications of our findings are of global significance for future modelling of soil–vegetation–climate feedbacks, as the FBB model is also implemented in the CLM. Although most of the global vegetation responds by decreasing gs under elevated CO2, biomes that already experience drought conditions are likely to show increases in gs. It remains to be seen how these increases will affect soil–canopy–atmosphere climate feedbacks in the future, particularly in areas that are already expected to be more threatened as a result of predicted changes in climate.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: Species list and site descriptions. Fig. S1: Comparison of measured and modelled gs values under 354 and 400 ppm of atmospheric CO2 using the optimal gs model of Medlyn et al. (2011). Fig. S2. Stomatal conductance response to increasing CO2 in the CLM4 land–vegetation model for each month of the year. Negative and positive gs responses to increasing CO2 in CLM4 (400 ppm relative to 350 ppm).
ACKNOWLEDGEMENTS
We are grateful for the highly constructive discussions we had with Joseph White (Baylor University), Andrew Leakey (University of Illinois), Aidan Holohan (University College Dublin) and Christiana Evans-FitzGerald (University College Dublin). We also thank staff at the National Center for Atmospheric Research (NCAR), Boulder, Colorado, particularly Keith Oleson, for helpful discussion, and Lisa Ainsworth, University of Illinois, for access to her stomatal conductance database from FACE sites. This work was supported by an SFI (Science Foundation Ireland) PI grant [11/PI/1103] and two Irish Research Council (IRC) Fellowship grants [GOIPD/2016/261, GOIPD/2016/320]. CLM simulations were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) at NSC.
LITERATURE CITED
- Adachi M, Hasegawa T, Fukayama H et al. 2014. Soil and water warming accelerates phenology and down-regulation of leaf photosynthesis of rice plants grown under free-air CO2 enrichment (FACE). Plant and Cell Physiology 55: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant, Cell & Environment 30: 258–270. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Blum H, Nösberger J, Long SP. 2003. Variation in acclimation of photosynthesis in Trifolium repens after eight years of exposure to Free Air CO2 Enrichment (FACE). Journal of Experimental Botany 54: 2769–2774. [DOI] [PubMed] [Google Scholar]
- Arneth A, Lloyd J, Šantrůčková H et al. 2002. Response of central Siberian Scots pine to soil water deficit and long-term trends in atmospheric CO2 concentration. Global Biogeochemical Cycles 16: 5–13. [Google Scholar]
- Bader MK-F, Siegwolf R, Körner C. 2010. Sustained enhancement of photosynthesis in mature deciduous forest trees after 8 years of free air CO2 enrichment. Planta 232: 1115–1125. [DOI] [PubMed] [Google Scholar]
- Berveiller D, Kierzkowski D, Damesin C. 2007. Interspecific variability of stem photosynthesis among tree species. Tree Physiology 27: 53. [DOI] [PubMed] [Google Scholar]
- Betts RA, Boucher O, Collins M et al. 2007. Projected increase in continental runoff due to plant responses to increasing carbon dioxide. Nature 448: 1037–1041. [DOI] [PubMed] [Google Scholar]
- Betts RA, Jones CD, Knight JR, Keeling RF, Kennedy JJ. 2016. El Nino and a record CO2 rise. Nature Climate Change 6: 806–810. [Google Scholar]
- Bhattacharya NC, Radin JW, Kimball BA et al. 1994. Leaf water relations of cotton in a free-air CO2-enriched environment. Agricultural and Forest Meteorology 70: 171–182. [Google Scholar]
- Borjigidai A, Hikosaka K, Hirose T, Hasegawa T, Okada M, Kobayashi K. 2006. Seasonal changes in temperature dependence of photosynthetic rate in rice under a free-air CO2 enrichment. Annals of Botany 97: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J, Taylor G, Frehner M. 1998. Photosynthetic acclimation to elevated CO2 is modified by source:sink balance in three component species of chalk grassland swards grown in a free air carbon dioxide enrichment (FACE) experiment. Plant, Cell & Environment 21: 159–168. [Google Scholar]
- Calfapietra C, Tulva I, Eensalu E et al. 2005. Canopy profiles of photosynthetic parameters under elevated CO2 and N fertilization in a Poplar plantation. Environmental Pollution 137: 525–535. [DOI] [PubMed] [Google Scholar]
- Chen CP, Sakai H, Tokida T, Usui Y, Nakamura H, Hasegawa T. 2014. Do the rich always become richer? Characterizing the leaf physiological response of the high-yielding rice cultivar takanari to free-air CO2 enrichment. Plant and Cell Physiology 55: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491: 752–755. [DOI] [PubMed] [Google Scholar]
- Collatz GJ, Ball JT, Grivet C, Berry JA. 1991. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary layer. Agricultural and Forest Meteorology 54: 107–136. [Google Scholar]
- Dai A. 2013. Increasing drought under global warming in observations and models. Nature Climate Change 3: 52–58. [Google Scholar]
- Dang Q-L, Margolis HA, Coyea MR, Sy M, Collatz GJ. 1997. Regulation of branch-level gas exchange of boreal trees: roles of shoot water potential and vapor pressure difference. Tree Physiology 17: 521–535. [DOI] [PubMed] [Google Scholar]
- de Boer HJ, Lammertsma EI, Wagner-Cremer F, Dilcher DL, Wassen MJ, Dekker SC. 2011. Climate forcing due to optimization of maximal leaf conductance in subtropical vegetation under rising CO2. Proceedings of the National Academy of Sciences USA 108: 4041–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kauwe MG, Zhou SX, Medlyn BE et al. 2015. Do land surface models need to include differential plant species responses to drought? Examining model predictions across a mesic-xeric gradient in Europe. Biogeosciences 12: 7503–7518. [Google Scholar]
- Domingues TF, Meir P, Feldpausch TR et al. 2010. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant, Cell & Environment 33: 959–980. [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzàlez-Meler MA, Long SP. 1997. More efficient plants: a consequence of rising atmospheric CO2?Annual Review of Plant Physiology and Plant Molecular Biology 48: 609–639. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS. 1999. CO2 enrichment in a maturing pine forest: are CO2 exchange and water status in the canopy affected?Plant, Cell & Environment 22: 461–472. [Google Scholar]
- Ellsworth DS, Oren R, Huang C, Phillips N, Hendrey GR. 1995. Leaf and canopy responses to elevated CO2 in a pine forest under free-air CO2 enrichment. Oecologia 104: 139–146. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Thomas R, Crous KY et al. 2012. Elevated CO2 affects photosynthetic responses in canopy pine and subcanopy deciduous trees over 10 years: a synthesis from Duke FACE. Global Change Biology 18: 223–242. [Google Scholar]
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33: 317–345. [Google Scholar]
- Field CB, Jackson RB, Mooney HA. 1995. Stomatal responses to increased CO2: implications from the plant to the global scale. Plant, Cell & Environment 18: 1214–1225. [Google Scholar]
- Frank DC, Poulter B, Saurer M et al. 2015. Water-use efficiency and transpiration across European forests during the Anthropocene. Nature Climate Change 5: 579–583. [Google Scholar]
- Garcia RL, Long SP, Wall GW et al. 1998. Photosynthesis and conductance of spring-wheat leaves: field response to continuous free-air atmospheric CO2 enrichment. Plant, Cell & Environment 21: 659–669. [Google Scholar]
- Gedney N, Cox PM, Betts RA, Boucher O, Huntingford C, Stott PA. 2006. Detection of a direct carbon dioxide effect in continental river runoff records. Nature 439: 835–838. [DOI] [PubMed] [Google Scholar]
- Ghini R, Torre-Neto A, Dentzien AFM et al. 2015. Coffee growth, pest and yield responses to free-air CO2 enrichment. Climatic Change 132: 307–320. [Google Scholar]
- Gopalakrishnan R, Bala G, Jayaraman M, Cao L, Nemani R, Ravindranath NH. 2011. Sensitivity of terrestrial water and energy budgets to CO2 - physiological forcing: An investigation using an offline land model. Environmental Research Letters 6: 044013. [Google Scholar]
- Grant RF, Wall GW, Kimball BA et al. 1999. Crop water relations under different CO2 and irrigation: Testing of ecosys with the free air CO2 enrichment (FACE) experiment. Agricultural and Forest Meteorology 95: 27–51. [Google Scholar]
- Gray SB, Dermody O, Klein SP et al. 2016. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nature Plants. https://www.nature.com/articles/nplants2016132?WT.feed_name=subjects_photosynthesis [DOI] [PubMed] [Google Scholar]
- Gunderson CA, Sholtis JD, Wullschleger SD, Tissue DT, Hanson PJ, Norby RJ. 2002. Environmental and stomatal control of photosynthetic enhancement in the canopy of a sweetgum (Liquidambar styraciflua L.) plantation during 3 years of CO2 enrichment. Plant, Cell & Environment 25: 379–393. [Google Scholar]
- Hamerlynck EP, Huxman TE, Nowak RS et al. 2000. Photosynthetic responses of Larrea tridentata to a step-increase in atmospheric CO2 at the Nevada Desert FACE Facility. Journal of Arid Environments 44: 425–436. [Google Scholar]
- Hamerlynck EP, Huxman TE, Charlet TN, Smith SD. 2002. Effects of elevated CO2 (FACE) on the functional ecology of the drought-deciduous Mojave Desert shrub, Lycium andersonii. Environmental and Experimental Botany 48: 93–106. [Google Scholar]
- Hao X, Li P, Feng Y et al. 2013. Effects of fully open-air CO2 elevation on leaf photosynthesis and ultrastructure of Isatis indigotica Fort. PLoS ONE 8: e74600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hättenschwiler S, Handa IT, Egli L, Asshoff R, Ammann W, Körner C. 2002. Atmospheric CO2 enrichment of alpine treeline conifers. New Phytologist 156: 363–375. [DOI] [PubMed] [Google Scholar]
- Herrick JD, Thomas RB. 1999. Effects of CO2 enrichment on the photosynthetic light response of sun and shade leaves of canopy sweetgum trees (Liquidambar styraciflua) in a forest ecosystem. Tree Physiology 19: 779–786. [DOI] [PubMed] [Google Scholar]
- Herrick JD, Thomas RB. 2003. Leaf senescence and late-season net photosynthesis of sun and shade leaves of overstory sweetgum (Liquidambar styraciflua) grown in elevated and ambient carbon dioxide concentrations. Tree Physiology 23: 109–118. [DOI] [PubMed] [Google Scholar]
- Herrick JD, Maherali H, Thomas RB. 2004. Reduced stomatal conductance in sweetgum (Liquidambar styraciflua) sustained over long-term CO2 enrichment. New Phytologist 162: 387–396. [Google Scholar]
- Hileman DR, Bhattacharya NC, Ghosh PP, Biswas PK, Lewin KF, Hendrey GR. 1992. Responses of photosynthesis and stomatal conductance to elevated carbon dioxide in field-grown cotton. Critical Reviews in Plant Sciences 11: 227–231. [Google Scholar]
- Hileman D, Huluka G, Kenjige P et al. 1994. Canopy photosynthesis and transpiration of field-grown cotton exposed to free-air CO2 enrichment (FACE) and differential irrigation. Agricultural and Forest Meteorology 70: 189–207. [Google Scholar]
- Huxman TE, Smith SD. 2001. Photosynthesis in an invasive grass and native forb at elevated CO2 during an El Niño year in the Mojave Desert. Oecologia 128: 193–201. [DOI] [PubMed] [Google Scholar]
- Ji G, Xue H, Seneweera S et al. 2015. Leaf photosynthesis and yield components of mung bean under fully open-air elevated CO2. Journal of Integrative Agriculture 14: 977–983. [Google Scholar]
- Katul G, Manzoni S, Palmroth S, Oren R. 2010. A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Annals of Botany 105: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel SG, Pepin S, Leuzinger S, Körner C. 2006. Stomatal conductance in mature deciduous forest trees exposed to elevated CO2. Trees 21: 151–159. [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD. 2004. The limits to tree height. Nature 428: 851–854. [DOI] [PubMed] [Google Scholar]
- Konrad W, Roth-Nebelsick A, Grein M. 2008. Modelling of stomatal density response to atmospheric CO2. Journal of Theoretical Biology 253: 638–658. [DOI] [PubMed] [Google Scholar]
- Lammertsma EI, Boer HJd, Dekker SC, Dilcher DL, Lotter AF, Wagner-Cremer F. 2011. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proceedings of the National Academy of Sciences USA 108: 4035–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Oleson KW, Flanner MG et al. 2011. Parameterization improvements and functional and structural advances in Version 4 of the Community Land Model. Journal of Advances in Modeling Earth Systems 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Bernacchi CJ, Ort DR, Long SP. 2006. Long-term growth of soybean at elevated CO2 does not cause acclimation of stomatal conductance under fully open-air conditions. Plant, Cell & Environment 29: 1794–1800. [DOI] [PubMed] [Google Scholar]
- Lee TD, Tjoelker MG, Ellsworth DS, Reich PB. 2001. Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytologist 150: 405–418. [Google Scholar]
- Leuzinger S, Körner C. 2007. Water savings in mature deciduous forest trees under elevated CO2. Global Change Biology 13: 2498–2508. [Google Scholar]
- Limousin J-M, Bickford CP, Dickman LT et al. 2013. Regulation and acclimation of leaf gas exchange in a piñon–juniper woodland exposed to three different precipitation regimes. Plant, Cell & Environment 36: 1812–1825. [DOI] [PubMed] [Google Scholar]
- Lin Y-S, Medlyn BE, Duursma RA et al. 2015. Optimal stomatal behaviour around the world. Nature Climate Change 5: 459–464. [Google Scholar]
- Marchi S, Tognetti R, Vaccari FP et al. 2004. Physiological and morphological responses of grassland species to elevated atmospheric CO2 concentrations in FACE-systems and natural CO2 springs. Functional Plant Biology 31: 181–194. [DOI] [PubMed] [Google Scholar]
- McElrone AJ, Reid CD, Hoye KA, Hart E, Jackson RB. 2005. Elevated CO2 reduces disease incidence and severity of a red maple fungal pathogen via changes in host physiology and leaf chemistry. Global Change Biology 11: 1828–1836. [Google Scholar]
- Medlyn BE, Duursma RA, Eamus D et al. 2011. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Global Change Biology 17: 2134–2144. [Google Scholar]
- Medlyn BE, Duursma RA, De Kauwe MG, Prentice IC. 2013. The optimal stomatal response to atmospheric CO2 concentration: Alternative solutions, alternative interpretations. Agricultural and Forest Meteorology 182–183: 200–203. [Google Scholar]
- Mencuccini M, Minunno F, Salmon Y, Martínez-Vilalta J, Hölttä T. 2015. Coordination of physiological traits involved in drought-induced mortality of woody plants. New Phytologist 208: 396–409. [DOI] [PubMed] [Google Scholar]
- Naumburg E, Ellsworth SD. 2000. Photosynthetic sunfleck utilization potential of understory saplings growing under elevated CO2 in FACE. Oecologia 122: 163–174. [DOI] [PubMed] [Google Scholar]
- Naumburg E, Housman DC, Huxman TE, Charlet TN, Loik ME, Smith SD. 2003. Photosynthetic responses of Mojave Desert shrubs to free air CO2 enrichment are greatest during wet years. Global Change Biology 9: 276–285. [Google Scholar]
- Naumburg E, Loik ME, Smith SD. 2004. Photosynthetic responses of Larrea tridentata to seasonal temperature extremes under elevated CO2. New Phytologist 162: 323–330. [Google Scholar]
- Neal AR, Wall GW, Kimball BA et al. 2000. Acclimation response of spring wheat in a free-air CO2 enrichment (FACE) atmosphere with variable soil nitrogen regimes. 1. Leaf position and phenology determine acclimation response. Photosynthesis Research 66: 65–77. [DOI] [PubMed] [Google Scholar]
- Nijs I, Ferris R, Blum H, Hendrey G, Impens I. 1997. Stomatal regulation in a changing climate: a field study using Free Air Temperature Increase (FATI) and Free Air CO2 Enrichment (FACE). Plant, Cell & Environment 20: 1041–1050. [Google Scholar]
- Niu J, Sivakumar B, Chen J. 2013. Impacts of increased CO2 on the hydrologic response over the Xijiang (West River) basin, South China. Journal of Hydrology 505: 218–227. [Google Scholar]
- Noormets A, Sôber A, Pell EJ et al. 2001. Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (Populus tremuloides Michx.) clones exposed to elevated CO2 and/or O3. Plant, Cell & Environment 24: 327–336. [Google Scholar]
- Nowak RS, DeFalco LA, Wilcox CS et al. 2001. Leaf conductance decreased under free-air CO2 enrichment (FACE) for three perennials in the Nevada desert. New Phytologist 150: 449–458. [Google Scholar]
- Oleson KW, Lawrence DM, Gordon B et al. 2010. Technical description of version 4.0 of the Community Land Model (CLM). https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=4&cad=rja&uact=8&ved=0ahUKEwiHtuDZz77YAhUIElAKHcaoA00QFgg5MAM&url=http%3A%2F%2Fwww.cesm.ucar.edu%2Fmodels%2Fccsm4.0%2Fclm%2FCLM4_Tech_Note.pdf&usg=AOvVaw1X8SWcwo87itYngvTzLsG2 [Google Scholar]
- Pataki DE, Huxman TE, Jordan DN et al. 2000. Water use of two Mojave Desert shrubs under elevated CO2. Global Change Biology 6: 889–897. [Google Scholar]
- Pearson M, Davies WJ, Mansfield TA. 1995. Asymmetric responses of adaxial and abaxial stomata to elevated CO2: impacts on the control of gas exchange by leaves. Plant, Cell & Environment 18: 837–843. [Google Scholar]
- Piao S, Friedlingstein P, Ciais P, de Noblet-Ducoudré N, Labat D, Zaehle S. 2007. Changes in climate and land use have a larger direct impact than rising CO2 on global river runoff trends. Proceedings of the National Academy of Sciences USA 104: 15242–15247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian T, Dai A, Trenberth KE, Oleson KW. 2006. Simulation of global land surface conditions from 1948 to 2004. Part I: Forcing data and evaluations. Journal of Hydrometeorology 7: 953–975. [Google Scholar]
- Rogers A, Allen DJ, Davey PA et al. 2004. Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant, Cell & Environment 27: 449–458. [Google Scholar]
- Rowland L, Lobo-do-Vale RL, Christoffersen BO et al. 2015. After more than a decade of soil moisture deficit, tropical rainforest trees maintain photosynthetic capacity, despite increased leaf respiration. Global Change Biology 21: 4662–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhil K, Sheeba , Ahmad A, Iqbal M, Tripathy BC. 2015. Photosynthesis and growth responses of mustard (Brassica juncea L. cv Pusa Bold) plants to free air carbon dioxide enrichment (FACE). Protoplasma 252: 935–946. [DOI] [PubMed] [Google Scholar]
- Schymanski SJ, Roderick ML, Sivapalan M. 2015. Using an optimality model to understand medium and long-term responses of vegetation water use to elevated atmospheric CO2 concentrations. AoB Plants 7: 20150527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers PJ, Randall DA, Collatz GJ et al. 1996. A revised land surface parameterization (SiB2) for atmospheric GCMS. Part I: Model formulation. Journal of Climate 9: 676–705. [Google Scholar]
- Shimono H, Okada M, Inoue M, Nakamura H, Kobayashi K, Hasegawa T. 2010. Diurnal and seasonal variations in stomatal conductance of rice at elevated atmospheric CO2 under fully open-air conditions. Plant, Cell & Environment 33: 322–331. [DOI] [PubMed] [Google Scholar]
- Singsaas EL, Ort DR, DeLucia EH. 2000. Diurnal regulation of photosynthesis in understory saplings. New Phytologist 145: 39–49. [Google Scholar]
- Tricker PJ, Trewin H, Kull O et al. 2005. Stomatal conductance and not stomatal density determines the long-term reduction in leaf transpiration of poplar in elevated CO2. Oecologia 143: 652–660. [DOI] [PubMed] [Google Scholar]
- Wall GW, Adam NR, Brooks TJ et al. 2000. Acclimation response of spring wheat in a free-air CO2 enrichment (FACE) atmosphere with variable soil nitrogen regimes. 2. Net assimilation and stomatal conductance of leaves. Photosynthesis Research 66: 79–95. [DOI] [PubMed] [Google Scholar]
- Wall GW, Brooks TJ, Adam NR et al. 2001. Elevated atmospheric CO2 improved Sorghum plant water status by ameliorating the adverse effects of drought. New Phytologist 152: 231–248. [Google Scholar]
- Wechsung F, Garcia RL, Wall GW et al. 2000. Photosynthesis and conductance of spring wheat ears: Field response to free-air CO2 enrichment and limitations in water and nitrogen supply. Plant, Cell & Environment 23: 917–929. [Google Scholar]
- Wenfang X, Wenping Y, Wenjie D, Jiangzhou X, Dan L, Yang C. 2013. A meta-analysis of the response of soil moisture to experimental warming. Environmental Research Letters 8: 044027. [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327: 617–618. [Google Scholar]
- Wu Y, Liu S, Abdul-Aziz OI. 2012. Hydrological effects of the increased CO2 and climate change in the Upper Mississippi River Basin using a modified SWAT. Climatic Change 110: 977–1003. [Google Scholar]
- Wullschleger SD, Gunderson CA, Hanson PJ, Wilson KB, Norby RJ. 2002. Sensitivity of stomatal and canopy conductance to elevated CO2 concentration – interacting variables and perspectives of scale. New Phytologist 153: 485–496. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Oue H, Kobayashi K. 2005. Energy balance and water use efficiency of rice canopies under free-air CO2 enrichment. Agricultural and Forest Meteorology 133: 226–246. [Google Scholar]
- Zhou S, Duursma RA, Medlyn BE, Kelly JWG, Prentice IC. 2013. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agricultural and Forest Meteorology 182–183: 204–214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.