Abstract
Study Objectives
Sleep quality is poor among patients with chronic obstructive pulmonary disease (COPD), and studies show that sleep disturbance is associated with low overall quality of life in this population. We evaluated the impact of patient-reported sleep quality and sleep apnea risk on disease-specific and overall quality of life within patients with COPD enrolled in the SPIROMICS study, after accounting for demographics and COPD disease severity.
Methods
Baseline data from 1341 participants [892 mild/moderate COPD (FEV1 ≥ 50% predicted); 449 severe COPD (FEV1 < 50%)] were used to perform three nested (blocks) regression models to predict quality of life (Short Form-12 mental and physical components and St. George’s Respiratory Questionnaire). Dependent measures used for the nested regressions included the following: Block1: demographics and smoking history; Block 2: disease severity (forced expiratory volume 1 s; 6 min walk test); Block 3: risk for obstructive sleep apnea (OSA; Berlin questionnaire); and Block 4: sleep quality (Pittsburgh Sleep Quality Index [PSQI]).
Results
Over half of participants with COPD reported poor sleep quality (Mean PSQI 6.4 ± 3.9; 50% with high risk score on the Berlin questionnaire). In all three nested regression models, sleep quality (Block 4) was a significant predictor of poor quality of life, over and above variables included in blocks 1–3.
Conclusions
Poor sleep quality represents a potentially modifiable risk factor for poor quality of life in patients with COPD, over and above demographics and smoking history, disease severity, and risk for OSA. Improving sleep quality may be an important target for clinical interventions.
Clinical Trial
SPIROMICS
Clinical Trial URL
Clinical Trial Registration
ClinicalTrials.gov NCT01969344
Keywords: sleep, COPD, QOL
Statement of Significance.
Patients with COPD have a high incidence of sleep disruption and small studies have shown that sleep disorders in this population reduce quality of life. Large cohort studies evaluating sleep in COPD are lacking. In the SPIROMICS study, a large COPD cohort study, we found that poor sleep quality was a risk factor for poor overall and disease specific quality of life over and above variables associated with poor quality of life, such as demographics, COPD disease severity, and risk for sleep apnea. Disease severity, depression, and anxiety were all associated with poor sleep quality in COPD patients, and improving quality of life may require a multifaceted approach, including treatment of sleep disorders. These results suggest that clinically addressing poor sleep quality has the potential to improve overall and disease-specific quality of life in COPD.
Introduction
Patients with chronic obstructive pulmonary disease (COPD) have high rates of sleep disturbances, including insomnia and sleep fragmentation. Potential sources of disturbed sleep in patients with COPD include impaired lung function and hyperinflation, which are exacerbated during sleep [1], specific sleep disorders such as obstructive sleep apnea (OSA), insomnia disorder, restless leg syndrome, and periodic limb movements of sleep [2–5]. Maladaptive sleep-related behaviors associated with worsening disease, including decreased physical activity and extended time in bed, also play a role [6]. Irrespective of the etiology, research suggests that poor sleep quality, as measured by questionnaires or wrist actigraphy, is associated with negative outcomes such as increased COPD exacerbations, increased emergency healthcare utilization, poor quality of life, and increased mortality in patients with COPD [7–9].
Multiple small studies have shown that sleep difficulties are associated with poorer disease specific-quality of life in COPD suggesting that sleep difficulties may affect aspects of life quality that are specific to the disease-state itself [8, 10–13]. In addition, sleep-disordered breathing (SDB, including OSA) is common among patients with COPD, and some studies suggest that high rates of SDB may account for the impact of poor sleep on quality of life in COPD [14]. Data from the Tucson Epidemiologic Study of Chronic Lung Disease demonstrate a strong association between respiratory complaints and poor sleep quality [15]; individuals with respiratory complaints, such as cough and/or wheeze, reported higher rates of insomnia and daytime sleepiness compared with individuals without these symptoms. In COPD, dyspnea related to hyperinflation, accumulation of secretions with associated mucous plugging, and worsening ventilation and oxygenation during rapid eye movement (REM) sleep may also contribute to poor sleep quality [1]. A limitation of the existing literature, however, is that studies have not concurrently assessed risk for OSA and impaired sleep quality, making it difficult to evaluate whether both might affect overall quality of life. Understanding whether OSA and poor sleep quality affect quality of life is important because sleep quality does not always improve with treatment of OSA, and poor sleep may represent another modifiable risk factor for poor quality of life in patients with COPD. One study showed that cognitive-behavioral therapy could improve insomnia in patients with COPD, suggesting behavioral sleep interventions may be beneficial to patients with COPD [16].
The current study was designed to examine the role of sleep quality in predicting overall quality of life, over and above demographic factors, COPD disease severity, and OSA risk in a large, well-characterized cohort of patients with COPD participating in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) (http://www2.cscc.unc.edu/spiromics/home). We hypothesized that poorer patient-reported sleep quality would be associated with lower overall and COPD disease-specific quality of life, even after accounting for the effects of demographic variables, measures of COPD disease severity, and risk for OSA (using the Berlin Questionnaire).
Given the complex relationship between COPD-related factors and sleep, we tested a separate multivariable regression model to further our understanding of factors associated with poor sleep quality in patients with COPD. We hypothesized that additional clinical variables including COPD symptoms, medication use, and anxiety and depression among others would contribute to poor sleep quality and may suggest COPD-related targets for interventions to improve sleep.
Methods
SPIROMICS is a prospective, multicenter cohort study conducted at sites covering major geographic regions across the country, including Los Angeles, San Francisco bay area, Salt Lake City, Denver, Alabama, Chicago, Ann Arbor and Detroit, Winston-Salem, Philadelphia, Baltimore, and New York City. Individuals with COPD of varying severity as well as comparison groups of age-matched smokers without COPD and nonsmokers were enrolled at all sites. Participants were enrolled between 2010 and 2015. Results presented here are based on data gathered during the baseline study visits for the first 1341 patients with COPD enrolled into the study. Details of the study design have been published previously [17].
Participants
All participants provided written informed consent to participate, and Human Subjects Committees at each site approved the study. Inclusion criteria for the larger study included age of 40–80 years, and for patients with COPD, a smoking history of ≥20 pack years. The main exclusion criteria were as follows: non-COPD obstructive lung disease, a history of diseases likely to interfere with the planned SPIROMICS study tests [17], BMI > 40 at baseline, intolerance of bronchodilators used in study assessments, or unstable cardiovascular disease. Participants were categorized into four strata: Stratum 1 included nonsmoker comparison participants; Stratum 2 included smokers without COPD; Stratum 3 included individuals with mild/moderate COPD, and Stratum 4 included individuals with severe–very severe COPD.
For this analysis, data from the SPIROMICS baseline visit for individuals in Strata 3 and 4 (n = 1341; i.e. those with COPD) were analyzed. This included 892 individuals with mild/moderate COPD (>20 pack years of tobacco; FEV1/FVC < 0.7; FEV1 ≥ 50% predicted) and 449 with severe COPD (>20 pack years of tobacco; FEV1/FVC <0.7; FEV1 < 50%). Demographic information for all participants and for participants in each stratum is shown in Table 1.
Table 1.
Descriptive statistics (Mean [SD] or n%) for overall sample (N = 1341) and for individuals with mild/moderate (N = 892) and severe (N = 449) COPD
| Variable | Overall (n = 1341) | Mild/moderate COPD (n = 892) | Severe COPD (n = 449) | N | % missing |
|---|---|---|---|---|---|
| Block 1 variables: Demographics | |||||
| Age (years)** | 65.7 (7.9) | 66.2 (7.9) | 64.7 (7.8) | 1341 | 0.0% |
| Gender (female) | 42.8% | 41.4% | 45.7% | 1341 | 0.0% |
| Race | 1341 | 0.0% | |||
| White | 81.1% | 83.1% | 77.3% | ||
| Black | 15.0% | 13.1% | 18.7% | ||
| Asian | 1.4% | 1.5% | 1.3% | ||
| American Indian | 0.5% | 0.6% | 0.4% | ||
| More than 1 race | 1.4% | 1.2% | 1.8% | ||
| Missing | 0.5% | 0.6% | 0.4% | ||
| Ethnicity; % Hispanic | 3.9% | 4.1% | 3.3% | 1341 | 0.0% |
| Smoking (pack years) | 53.4 (24.8) | 53.8 (25.6) | 52.7 (23.2) | 1341 | 0.0% |
| Currently Smoking (1 month ago)*** | 33.3% | 37.1% | 25.7% | 1310 | 2.3% |
| Block 2 variables: Disease severity | |||||
| Six minute walk test (distance in m)*** | 396.9 (125.0) | 418.9 (109.3) | 348.3 (142.8) | 1249 | 6.9% |
| FEV1 observed/predicted (%)*** | 61.5 (23.1) | 74.7 (15.6) | 35.3 (9.1) | 1341 | 0.0% |
| Currently using Theophylline*** | 2.6% | 0.9% | 6.0% | 1341 | 0.0% |
| Currently using Oral Corticosteroids*** | 4.0% | 1.2% | 9.4% | 1341 | 0.0% |
| Using supplemental oxygen*** | 22.8% | 9.4% | 49.3% | 1336 | 0.4% |
| Used inhaled steroids in last three months*** | 45.2% | 33.4% | 68.6% | 1341 | 0.0% |
| Used inhaled bronchodilators in last three months*** | 62.6% | 51.1% | 85.5% | 1341 | 0.0% |
| Used nebulized bronchodilators in the last three months*** | 15.1% | 7.3% | 30.5% | 1341 | 0.0% |
| Frequency of cough last night*** | 1341 | 0.0% | |||
| None/Rare | 78.8% | 80.6% | 75.3% | ||
| Occasional | 13.6% | 13.8% | 13.1% | ||
| Frequent/Constant | 3.7% | 3.4% | 4.2% | ||
| Missing | 4.0% | 2.2% | 7.3% | ||
| Sleep Apnea Risk | 1341 | 0.0% | |||
| High Sleep Apnea Risk | 40.6% | 42.2% | 37.6% | ||
| Low sleep apnea Risk | 32.5% | 32.5% | 32.5% | ||
| Missing/Don’t Know | 26.8% | 25.3% | 29.8% | ||
| PSQI total score | 6.4 (3.9) | 6.5 (4.0) | 6.4 (3.7) | 1198 | 10.7% |
| PSQI total score > 5 | 51.1% | 51.0% | 51.2% | 1198 | 10.7% |
| Subjective sleep quality | 1.0 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 1314 | 2.0% |
| Sleep latency | 1.1 (1.0) | 1.1 (1.0) | 1.1 (1.0) | 1307 | 2.5% |
| Sleep Duration | 0.7 (0.9) | 0.7 (0.9) | 0.7 (0.9) | 1308 | 2.5% |
| Habitual sleep efficiency | 0.7 (1.0) | 0.7 (1.0) | 0.7 (1.0) | 1232 | 8.1% |
| Sleep disturbances | 1.5 (0.6) | 1.5 (0.7) | 1.5 (0.6) | 1297 | 3.3% |
| Sleep medication use | 0.8 (1.2) | 0.8 (1.2) | 0.7 (1.2) | 1312 | 2.2% |
| Daytime dysfunction* | 0.8 (0.7) | 0.8 (0.7)* | 0.9 (0.7)* | 1310 | 2.3% |
| Quality of life outcome variables | |||||
| SF-12 Mental Health Score | 46.2 (8.8) | 46.3 (8.6) | 45.9 (9.1) | 1312 | 2.2% |
| SF-12 Physical health Score*** | 27.0 (10.6) | 29.5 (10.7) *** | 22.1 (8.6) *** | 1312 | 2.2% |
| St. George’s Respiratory Questionnaire*** | 36.9 (19.7) | 31.2 (18.8) *** | 47.9 (16.4) *** | 1248 | 6.9% |
Significance of comparison of mild/moderate versus severe: *p < .05; **p < .01; ***p < .001.
Procedures
All measures used in this study were collected at the baseline SPIROMICS study visit. At the baseline visit, demographic information as well as morphometric measures (including BMI), spirometry, 6 min walk test, and medical history (including mediations) were collected, and a series of questionnaires was administered. The questionnaire battery included sleep and quality of life measures (described below).
Demographics (Block 1 variables)
At the baseline visit, demographic information was gathered from participants, including age, gender, race/ethnicity, smoking status (smoked within the past month yes/no), and smoking history (total pack years).
Disease severity (Block 2 variables)
Spirometry was performed according to the American Thoracic Society guidelines [18]. Metrics assessed included forced vital capacity (FVC), Forced Expiratory Volume in 1 second (FEV1), ratio of FEV1/FVC, and forced expiratory flow rate 25%–75% (FEF25%–75%). Six minute walk distance in meters was tested and recorded as per SPIROMICS protocol [17]. Measures of COPD disease severity included in Block 2 were baseline FEV1 percent predicted and distance (in meters) walked during a 6 min walk study. Additional variables representing disease severity were also included in Block 2. This included as follows: (1) current use of theophylline, (2) current use of oral corticosteroids, (3) current use of supplemental oxygen, (4) use of inhaled steroids in last 3 months, (5) use of inhaled bronchodilators in last 3 months, (6) use of nebulized bronchodilators in the last 3 months, and (7) frequency of cough last night (none/rare, occasional, and frequent/constant).
Sleep measures (Block 3 and 4 variables)
The main measure of self-reported OSA risk in SPIROMICS was the patient-reported Berlin Questionnaire, which is a well-accepted OSA screening tool [19, 20]. The Berlin Questionnaire includes body mass index, snoring and other respiratory symptoms, tiredness/sleepiness, and self-reported history of high blood pressure. The overall risk score (high/low risk) was used in the analysis. For the predictive regression models (described below), Berlin Questionnaire score was coded as high risk, low risk, or missing. Low risk was used as the reference group.
The main measure of self-reported sleep quality was the Pittsburgh Sleep Quality Index (PSQI) [21]. The PSQI is an 18-item questionnaire (score range 0–21) that assesses overall sleep quality. Participants were asked about their sleep for the previous 1 month period. Higher scores indicate more disturbed sleep. The PSQI is comprised of seven subscales including (1) Subjective Sleep Quality; (2) Sleep Latency, (3) Sleep Duration; (4) Habitual Sleep Efficiency; (5) Sleep Disturbances; (6) Use of Sleeping Medication; and (7) Daytime Dysfunction. These seven variables were entered into the predictive regression model in Block 4. For descriptive purposes, we also computed the PSQI total score and present the proportion of patients who fell above and below the clinical cutoff score of 5.
Quality of life measures (outcome variables)
The Short Form-12 Health Survey (SF-12) [22] was completed at the baseline visit and was used to asses overall quality of life. The SF-12 is a 12-item measure evaluating mental and physical functioning as well as overall health-related-quality of life (score 0–100 with lower scores indicating higher impairment). We tested separate models predicting mental health and physical health functioning (Models 1 and 2, respectively).
The main measure of disease-specific quality of life was the St. George Respiratory Questionnaire (SGRQ). The SGRQ measures disease impact on overall health, daily life, and perceived well-being in patients with COPD (score range 0–100 with higher scores indicating higher level of impairment). The SGRQ is well validated in the COPD population [23].
Additional variables examined as predictors of poor sleep quality
Depression and anxiety were also assessed as potential predictors of poor sleep quality. These constructs were assessed with the 14-item Hospital Anxiety and Depression Scale (HADS) [24], which focuses on nonsomatic symptoms making it appropriate for use in patient populations with physical symptoms due to underlying diseases.
Data analysis
To test the effects of demographics, disease severity, OSA risk factors, and sleep quality on quality of life, we used three nested regression models (also known as hierarchical regression models), one for each outcome variable (SF12-MH, SF12-PH, and SGRQ). This method enters variables in “blocks,” permitting assessment of the variance uniquely explained by each block of variables, after accounting for variance explained by variables in prior blocks.
In each model, Block 1 was composed of demographic variables including age, gender, race, ethnicity, smoking history, and current smoking status. Block 2 represented disease severity and was composed of the variables specified previously (see Block 2, disease severity, above). Block 3 was composed of OSA risk on the Berlin Questionnaire. Block 4 was composed of the seven subscales of the PSQI. An F-test was used to assess the significance of each block of variables, after accounting for variables entered in previous blocks. The R2 was computed to assess variance explained by variables in the current block plus prior block(s), and an R2-change (ΔR2) was computed expressing additional variance explained by Blocks 2, 3, and 4, above and beyond variables in prior block(s). A sensitivity analysis was performed using the composite PSQI total score Block 4.
To examine possible predictors of poor sleep quality, a linear multiple regression model was fit using sleep quality (PSQI total score) as the outcome variable. The predictors included demographics (as described above, see block 1), disease severity (as described above, see block 2), sleep apnea risk (as described above, see block 3), and measures of anxiety and depression (HADS).
For all statistical tests, p < .05 was considered statistically significant. Analyses were conducted using Stata Version 13.1, StataCorp [25]. The nested regression models (with list-wise deletion) were estimated using the regress command prefixed with nestreg. (Maximum likelihood nested regression models were fit using the sem command with the method(mlmv) option to request maximum likelihood estimation with missing values. These results were compared with the list-wise results as a sensitivity analysis.)
Results
Demographics and descriptives
Table 1 presents descriptive information on demographics, disease characteristics, sleep quality, and quality of life metrics for the overall sample (N = 1341), and for those with mild/moderate COPD (N = 892) and severe COPD (N = 449). Individuals with mild/moderate COPD were significantly older and were more likely to be current smokers than those in the severe COPD group. As expected, there were significant differences in the COPD disease severity measures between the groups as well. Individuals with mild/moderate COPD were also more likely to be at high risk for sleep apnea on the Berlin Questionnaire. Poor sleep quality was common, with 51.1% of participants scoring above the clinical cutoff for sleep disturbances on the PSQI (>5). The only difference in sleep quality between groups was that participants with severe COPD had higher (worse) scores on the daytime dysfunction subscale of the PSQI compared with mild/moderate COPD; however, total PSQI score did not differ between groups. Individuals with severe COPD had a worse physical and disease specific quality of life as well.
Impact of missing values on results
To evaluate the impact of missing values on the results, we examined the R2 and R2-change values for all models, comparing the results obtained with list-wise deletion to the results obtained with maximum likelihood estimation with missing values. Differences between these two approaches were trivial (R2 differences of <1% to 2%). For simplicity, only the traditional nested regression results are presented for each outcome variable below.
Predictors of overall quality of life
The results of the nested regression models showed that sleep quality was a strong predictor of all three metrics of quality of life (Table 2) after accounting for the variables entered in blocks 1, 2, and 3. In Model 1, demographics explained a significant proportion of variance in the mental health component of the SF-12. In total, the first two blocks (demographics and disease severity) explained a total of 12.5 per cent of the variance in the Mental Health component of the SF-12. The third block (Berlin Questionnaire) explained an additional 0.5 per cent, and the fourth Block of predictors (sleep quality) explained an additional 14.0 per cent of the variance in this outcome (p < .001).
Table 2.
Nested regression models predicting overall and disease-specific quality of life (Model 1: SF-12: Mental health score; Model 2: SF-12 Physical Health Score; and Model 3: St. George’s Respiratory Questionnaire)
| Variable block | Model 1: SF-12 Mental Health (n = 1086) | Model 2: SF-12 Physical Health (n = 1088) | Model 3: St. George’s Respiratory Questionnaire (n = 1043) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F df and p-value | R 2 | ΔR2 | F df and p-value | R 2 | ΔR2 | F df and p-value | R 2 | ΔR2 | |
| 1: Demographics | F 10,1075=12.08; p < .001 | .101 | — | F 10,1077=5.99; p < .001 | .052 | — | F 10,1032=18.35; p < .001 | .151 | — |
| 2: Disease severity | F 11,1064=2.60; p = .003 | .125 | .024 | F 11,1066=41.10; p < .001 | .334 | .282 | F 11,1021=69.30; p < .001 | .514 | .363 |
| 3: SDB risk factors | F 2,1062=2.99; p = .005 | .130 | .005 | F 2,1064=20.92; p < .001 | .359 | .025 | F 2,1019=31.71; p < .001 | .542 | .029 |
| 4: Sleep Quality | F 7,1055=28.99; p < .001 | .270 | .140 | F 7,1057=23.41; p < .001 | .445 | .086 | F 7,1012=39.83; p < .001 | .641 | .099 |
Block 1: Demographics and smoking: Age, gender, race, ethnicity, smoking history, and current smoking status.
Block 2: Disease severity: Six minute walk distance, FEV1 % predicted, currently using theophylline, currently using oral corticosteroids, supplemental oxygen usage, inhaled steroids usage in the last 3 months, inhaled bronchodilators in the last 3 months, nebulized bronchodilators usage in the last 3 months, and frequency of cough last night.
Block 3: Sleep apnea risk: Berlin questionnaire.
Block 4: PSQI total score 7 components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction.
Results of Model 2 predicting the Physical Health component of SF-12 (Table 2) showed that each of the first two blocks (demographics and disease severity) explained a significant proportion of variance, with disease severity predominating. These two blocks of variables overall explained 33.4 per cent of the variance in the Physical Health component of the SF-12. The third block (Berlin Questionnaire) explained an additional 2.5 per cent, and the fourth block (sleep quality) explained an additional 8.6 per cent of the variance in this outcome (p < .001).
Predictors of disease-specific quality of life
In Model 3, the predictors in each of the first two blocks explained a significant proportion of the variance in disease-specific quality of life (SGRQ). As expected, disease severity was the most powerful predictor, explaining 36.3 per cent of the variance. In total, the first two blocks of variables explained 51.4 per cent of the variance, and block three (Berlin Questionnaire) explained an additional 2.9 per cent, yet sleep quality still explained an additional 9.9 per cent of the variance in disease specific quality of life (p < .001).
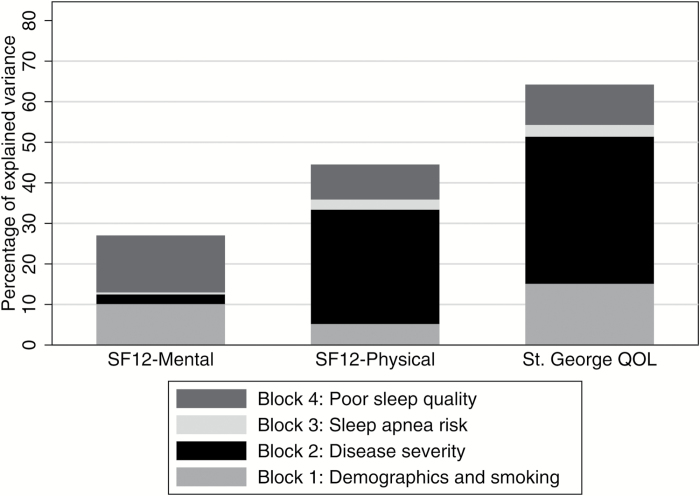
The relative percentage of variance explained for each model of the three models is depicted in Figure 1.
Figure 1.
Percentage of variance explained in SF12-Mental Health, SF-12 Physical Health, and St. George QOL as a function of Block 1, Block 2, Block 3, and Block 4 of the nested regression model.
Components of sleep quality and quality of life
A regression was used to evaluate how individual components of the PSQI were associated with quality of life (Table 3). For Model 1, four of the seven PSQI subscales were significantly associated with the SF-12 Mental Health component: Subjective Sleep Quality, Sleep Latency, Use of Sleeping Medications, and Daytime Dysfunction. For Model 2, two of the seven PSQI subscales were significantly associated with the SF-12 Physical Health Component: Sleep Disturbances and Daytime Dysfunction. In predicting the St. George’s Respiratory Questionnaire (Model 3), three of the seven predictors were significant: Sleep Latency, Sleep Disturbances, and Daytime Dysfunction. Across all three models, daytime dysfunction was consistently associated with lower quality of life. Additionally, sleep disturbances was also significant in models 2 and 3.
Table 3.
Regression coefficients for PSQI subscale score variables (Block 4 variables) from nested regression models shown in Table 2 (Model 1: SF-12: Mental health score; Model 2: SF-12 Physical Health Score; Model 3: St. George’s Respiratory Questionnaire). Shading indicates subscale scores that were significant predictors of the quality of life outcome for each model.
| Block 4: Sleep Quality Variables | Model 1: SF-12 Mental Health | Model 2: SF-12 Physical Health | Model 3: St. George’s Respiratory Questionnaire | |||
|---|---|---|---|---|---|---|
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | |
| Subjective sleep quality | −0.96* | [−1.75,−0.16] | 0.36 | [−0.49,1.21] | 0.22 | [−1.05,1.49] |
| Sleep latency | −0.65* | [−1.20,−0.10] | −0.01 | [−0.61,0.58] | 0.89* | [0.01,1.78] |
| Sleep duration | −0.04 | [−0.77,0.70] | −0.77 | [−1.56,0.03] | 0.69 | [−0.48,1.87] |
| Habitual sleep efficiency | −0.10 | [−0.72,0.51] | −0.24 | [−0.90,0.42] | 0.34 | [−0.65,1.33] |
| Sleep disturbances | −0.76 | [−1.62,0.11] | −3.57*** | [−4.50,−2.64] | 5.43*** | [4.02,6.83] |
| Sleep medication use | −0.72*** | [−1.11,−0.33] | −0.18 | [−0.60,0.24] | 0.22 | [−0.41,0.84] |
| Daytime dysfunction | −3.63*** | [−4.38,−2.87] | −2.71*** | [−3.52,−1.89] | 6.11*** | [4.89,7.32] |
| N observations | 1086 | 1088 | 1043 | |||
*p < .05; **p < .01; ***p < .001.
Multivariable model to explore predictors of poor sleep quality
Given the unique contribution of sleep quality to quality of life in patients with COPD described above, we conducted a second analysis examining predictors of poor sleep quality (PSQI total score). We included all variables in Blocks 1–3 in the regression models described above, plus depression and anxiety as measured with the HADS. The results from the multiple regression predicting poor sleep quality are shown in Table 4. In addition to the expected effects of older age and female gender, several metrics of disease severity over and above predicted poor sleep quality, including lower FEV1, more nighttime cough, and use of bronchodilators were all associated with poorer sleep quality. In addition, those at high risk for sleep apnea and those with missing scores on the Berlin Questionnaire had worse sleep than those at low risk for sleep apnea on the Berlin Questionnaire. Depression and anxiety were also significant predictors of poor sleep in this population of patients with COPD.
Table 4.
Multiple regression predicting poor sleep quality (PSQI Total Score)
| Coefficient | [95% CI] | |
|---|---|---|
| Age at Baseline (years) | −0.03* | [−0.06,−0.00] |
| Gender (female = 1) | 0.53** | [0.13,0.92] |
| Race (reference = white) | ||
| Black | −0.04 | [−0.64,0.56] |
| Asian | 1.17 | [−0.41,2.75] |
| American Indian | 2.03 | [−0.37,4.43] |
| More than one | −0.97 | [−2.73,0.79] |
| Missing | 1.52 | [−0.98,4.03] |
| Ethnicity (1 = Hispanic) | 0.48 | [−0.63,1.58] |
| Smoking, pack years | −0.00 | [−0.01,0.01] |
| Currently smoking, as of 1 month ago | 0.35 | [−0.10,0.81] |
| Six minute walk distance (m) | 0.00 | [−0.00,0.00] |
| FEV1 as % predicted (%) | 0.01* | [0.00,0.02] |
| Currently using Theophylline | −0.74 | [−1.99,0.52] |
| Currently using Oral Corticosteroids | −1.05 | [−2.12,0.03] |
| Supplemental oxygen usage | −0.13 | [−0.69,0.44] |
| Inhaled steroids usage in the last 3 months | 0.06 | [−0.39,0.51] |
| Inhaled bronchodilators in the last 3 months | 0.59* | [0.12,1.06] |
| Nebulized bronchodilators usage in the last 3 months | 1.03** | [0.42,1.65] |
| Coughing last night (Reference = None/Rare) | ||
| Occasional | 0.60* | [0.03,1.18] |
| Frequent/Constant | 2.52*** | [1.38,3.66] |
| Missing | −0.83 | [−1.98,0.32] |
| Berlin Questionnaire Sleep Apnea Risk (Reference = Low Risk) | ||
| High Risk | 1.04*** | [0.58,1.49] |
| Missing | 0.65* | [0.14,1.15] |
| HADS Anxiety Score | 0.25*** | [0.18,0.32] |
| HADS Depression Score | 0.29*** | [0.21,0.37] |
| Observations | 1087 | |
95% confidence intervals in brackets; overall model F25,1061 = 23.11, p < .001, RMSE = 3.16, R2 = 0.35, Adjusted R2 = 0.34.
*p < .05; **p < .01; ***p < .001.
Conclusion/discussion
This study represents a comprehensive assessment of patient-reported sleep quality and overall quality of life among patients with COPD. We aimed to investigate the association between sleep quality and quality of life. Using an approach that statistically accounts for demographic and clinical variables, including COPD severity and risk for OSA, we found that poor sleep quality per se accounted for 9%–14% of the variance in quality of life measures. Prior research has suggested that poor sleep affects overall quality of life among patients with COPD, as it does in the population at large. Our study supports these prior findings. This is particularly notable as poor sleep is a potentially modifiable risk factor for poor overall and disease-specific quality of life.
In this study, poor sleep quality was common, with 51.1 per cent of participants scoring above the clinical cut off for sleep disturbances on the PSQI although there were no differences in sleep quality between the disease severity groups. This finding corroborates previous studies demonstrating poor sleep quality in patients with COPD [8, 10, 26]. An unanswered question, however, has been whether poor sleep quality was simply related to other factors such as COPD disease severity or OSA. Our analytic approach allowed us to test whether poor sleep quality may represent distinct clinical issues faced by patients with COPD. In fact, a key difference between this study and prior research is the inclusion of multiple potential “confounders” including demographics, disease severity, and OSA risk. To examine this relationship, we used the seven subscales of the PSQI and found that Subjective Sleep Disturbance and Daytime Dysfunction were the two subscales within the PSQI that most strongly affected both overall and disease-specific quality of life. Importantly, this highlights that it is the impact of poor sleep on daytime functioning that likely affects overall quality of life. Using the PSQI total score in lieu of the seven subscales showed the same pattern of significance, although the total explained variance was lower.
Although our method of analysis did allow us to account for disease severity’s impact on quality of life, the question of how disease severity might affect sleep quality directly remained. To understand how disease severity metrics might affect sleep quality (PSQI total score), we tested a model in which all disease severity metrics were entered separately in addition to demographics and sleep apnea risk (Berlin Questionnaire). As expected, COPD severity was associated with worse sleep as was more frequent nighttime cough. The use of bronchodilators (inhaled or nebulized) may be an additional marker of disease severity (i.e. patients using bronchodilators are experiencing more symptoms and therefore using more medications) or the medications themselves may have sleep-disruptive properties. OSA risk was also a significant predictor of poor sleep quality suggesting treatment of OSA may benefit overall sleep quality in patients with COPD.
The implications of our findings are that to improve quality of life it may be necessary to optimally manage symptoms, including nighttime cough, and to address factors that affect sleep quality directly such as OSA and other sleep disorders.
The study design had multiple strengths, including a large well-characterized group of patients with COPD from across the United States. We also have validated questionnaires to assess sleep quality and risk for OSA. Despite these strengths, our study also had weaknesses. An important limitation of this study is the lack of a diagnostic study to assess for OSA in this population. Self-reported OSA risk factors represented only a small contribution to quality of life, explaining only 0.5%–2% of the variance in quality of life outcomes. Objective measurement of OSA severity might have altered the pattern of results. Previous studies have shown that patients with COPD with overlap syndrome (i.e. COPD + OSA) are more likely to experience COPD exacerbations that lead to hospitalization and to have a higher mortality than patients with COPD alone. When OSA is treated in these patients with positive airway pressure, such treatment is associated with improved survival and decreased hospitalizations [27, 28]. Additional research using objective monitoring of OSA in patients with COPD is needed to further our understanding of OSA and QOL in COPD. Additionally other breathing disorders during sleep associated with COPD (e.g. sleep-related hypoventilation and hypoxemia) may have been present, particularly in the patients within the severe COPD subset, which may have affected these analyses.
Objectively measured sleep architecture and sleep efficiency (e.g. with polysomnography) and measures of insomnia were also not included in the SPIROMICS study. Nonetheless, sleep complaints from patients often trigger evaluation and treatment of sleep disorders and therefore represent an important construct on their own. The study also did not include measures of daytime and nighttime lung hyperinflation, which may adversely affect REM and non-REM sleep in patients with COPD [1, 29]. Although we were able to account for disease severity, this additional information would have furthered our understanding about the mechanisms underlying the relationships among disease severity, sleep quality, and quality of life.
There also are limitations to the analytic approach used in this study, which we attempted to mitigate. For example, one drawback of a traditional nested regression is that observations with missing data on any predictor are removed in a list-wise fashion, and as more predictor variables are added, the number of observations omitted due to missing items increases. In this study, the percentage of observations omitted due to missing data on the predictors ranged from 18.4% to 21.3%. To address concerns regarding an analysis based on the omission of observations, a second nested regression model was fit using maximum likelihood estimation. This showed that missing data on select variables did not alter the pattern of results, suggesting that missing data did not affect our findings in a meaningful way [30].
In summary, patient-reported sleep quality was a significant and clinically meaningful predictor of multiple metrics of quality of life among well-characterized COPD patients in the SPIROMICS cohort study. Sleep quality itself was related to disease severity measures, OSA risk, and depression and anxiety among patients with COPD. Based on these findings, patients with COPD with subjective sleep disturbance should be encouraged to seek treatment for OSA and other sleep disorders such as insomnia when present. Studies that combine optimal management of COPD symptoms with treatment of sleep disorders would inform management of COPD patients and potentially improve their quality of life.
Funding
Provided by the National Heart, Lung, and Blood Institute (NHLBI) of the NIH, contract and grant numbers: NHLBI contracts HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN2682009000019C, and HHSN268200900020C; NHLBI grant R01HL110906.
Acknowledgments
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, PhD; Wayne H. Anderson, PhD; R. Graham Barr, MD, DrPH; Eugene R. Bleecker, MD; Richard C. Boucher, MD; Russell P. Bowler, MD, PhD; Elizabeth E. Carretta, MPH; Stephanie A. Christenson, MD; Alejandro P. Comellas, MD; Christopher B. Cooper, MD, PhD; David J. Couper, PhD; Ronald G. Crystal, MD; Jeffrey L. Curtis, MD; Claire M. Doerschuk, MD; Mark T. Dransfield, MD; Christine M. Freeman, PhD; MeiLan K. Han, MD, MS; Nadia N. Hansel, MD, MPH; Annette T. Hastie, PhD; Eric A. Hoffman, PhD; Robert J. Kaner, MD; Richard E. Kanner, MD; Eric C. Kleerup, MD; Lisa M. LaVange, PhD; Stephen C. Lazarus, MD; Fernando J. Martinez, MD, MS; Deborah A. Meyers, PhD; John D. Newell Jr, MD; Elizabeth C. Oelsner, MD, MPH; Wanda K. O’Neal, PhD; Robert Paine, III, MD; Nirupama Putcha, MD, MHS; Stephen I. Rennard, MD; Donald P. Tashkin, MD; Mary Beth Scholand, MD; J. Michael Wells, MD; Robert A. Wise, MD; and Prescott G. Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Thomas Croxton, PhD, MD.
Notes
Conflict of interest statement. None declared.
References
- 1. Kwon JS, et al. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD. 2009;6(6):441–445. [DOI] [PubMed] [Google Scholar]
- 2. Shiina K, et al. Overlap syndrome: additive effects of COPD on the cardiovascular damages in patients with OSA. Respir Med. 2012;106(9):1335–1341. [DOI] [PubMed] [Google Scholar]
- 3. Weitzenblum E, et al. Chronic obstructive pulmonary disease and sleep apnea syndrome. Sleep. 1992;15(6Suppl):S33–S35. [PubMed] [Google Scholar]
- 4. Budhiraja R, et al. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavalcante AG, et al. Restless legs syndrome, sleep impairment, and fatigue in chronic obstructive pulmonary disease. Sleep Med. 2012;13(7):842–847. [DOI] [PubMed] [Google Scholar]
- 6. Jonsdottir H, et al. Effectiveness of a partnership-based self-management programme for patients with mild and moderate chronic obstructive pulmonary disease: a pragmatic randomized controlled trial. J Adv Nurs. 2015;71(11):2634–2649. [DOI] [PubMed] [Google Scholar]
- 7. Omachi TA, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13(5):476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dignani L, et al. Sleep and quality of life in people with COPD: a descriptive-correlational study. Clin Nurs Res. 2016; 25(4):432–447. [DOI] [PubMed] [Google Scholar]
- 9. Nunes DM, et al. Actigraphic assessment of sleep in chronic obstructive pulmonary disease. Sleep Breath. 2013;17(1):125–132. [DOI] [PubMed] [Google Scholar]
- 10. Arikan H, et al. The relationship between cough-specific quality of life and abdominal muscle endurance, fatigue, and depression in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kohli P, et al. Functional capacity, health status, and inflammatory biomarker profile in a cohort of patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2015;35(5):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akinci AC, et al. Factors affecting health status in patients with chronic obstructive pulmonary disease. Int J Nurs Pract. 2013;19(1):31–38. [DOI] [PubMed] [Google Scholar]
- 13. Scharf SM, et al. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zohal MA, et al. Sleep quality and quality of life in COPD patients with and without suspected obstructive sleep apnea. Sleep Disord. 2014;2014:508372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klink ME, et al. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105(1):151–154. [DOI] [PubMed] [Google Scholar]
- 16. Kapella MC, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis. 2011;6:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Couper D, et al. ; SPIROMICS Research Group Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax. 2014;69(5):491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller MR, et al. ; ATS/ERS Task Force General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. [DOI] [PubMed] [Google Scholar]
- 19. Netzer NC, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 20. Abrishami A, et al. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423–438. [DOI] [PubMed] [Google Scholar]
- 21. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 22. Ware J, Jr, et al. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 23. Beretta L, et al. Validity of the Saint George’s Respiratory Questionnaire in the evaluation of the health-related quality of life in patients with interstitial lung disease secondary to systemic sclerosis. Rheumatology (Oxford). 2007;46(2):296–301. [DOI] [PubMed] [Google Scholar]
- 24. Zigmond AS, et al. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 25. Stata Statistical Software: Release 13.1 Version 13.1. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 26. Agusti A, et al. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marin JM, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. [DOI] [PubMed] [Google Scholar]
- 28. Machado MC, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35(1):132–137. [DOI] [PubMed] [Google Scholar]
- 29. Krachman SL, et al. ; National Emphysema Treatment Trial Research Group Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest. 2005;128(5):3221–3228. [DOI] [PubMed] [Google Scholar]
- 30. Allison P. Handling Missing Data by Maximum Likelihood: Paper 312–2012. 2012. SAS Global Forum; 2012. [Google Scholar]