Abstract
Background
Patient-reported outcomes (PRO) represent an important measure of cancer therapy effect. For patients with metastatic epidural spinal cord compression (MESCC), hybrid therapy using separation surgery and stereotactic radiosurgery preserves neurologic function and provides tumor control. There is currently a paucity of data reporting PRO after such combined modality therapy for MESCC. Delineation of hybrid surgery–radiosurgery therapy effect on PRO validates the hybrid approach as an effective therapy resulting in meaningful symptom relief.
Patients and Methods
Brief Pain Inventory (BPI) and MD Anderson Symptom Inventory—Spine Tumor (MDASI-SP), PROs validated in the cancer population, were prospectively collected. Patients with MESCC who underwent separation surgery followed by stereotactic radiosurgery were included. Separation surgery included a posterolateral approach without extensive cytoreductive tumor excision. A median postoperative radiosurgery dose of 2700 cGy was delivered. The change in PRO 3 months after the hybrid therapy represented the primary study outcome. Preoperative and postoperative evaluations were analyzed using the Wilcoxon signed-rank test for matched pairs.
Results
One hundred eleven patients were included. Hybrid therapy resulted in a significant reduction in the BPI items “worst” and “right now” pain (P < .0001), and in all BPI constructs (severity, interference with daily activities, and pain experience, P < .001). The MDASI-SP demonstrated reduction in spine-specific pain severity and interference with general activity (P < .001), along with decreased symptom interference (P < .001).
Conclusions
Validated PRO instruments showed that in patients with MESCC, hybrid therapy with separation surgery and radiosurgery results in a significant decrease in pain severity and symptom interference. These prospective data confirm the benefit of hybrid therapy for treatment of MESCC and should facilitate referral of patients with MESCC for surgical evaluation.
Keywords: HRQoL, MESCC, separation surgery, spine tumor, SRS
Malignant epidural spinal cord compression (MESCC) is a common complication of cancer.1 Ten to forty percent of cancer patients will suffer from spinal metastases.2 A population-based study in 2011 revealed that in the United States, 3.4% of all cancer patients required hospitalization due to MESCC.3 As cancer treatments improve and life expectancy increases, this disease burden is likely to continue to grow.
Therapy directed toward improved survival and local tumor control plays a paramount role in the treatment of cancer patients. The effect of oncologic therapy on patient-reported outcomes (PRO), such as the quality of life and symptom burden of cancer patients, requires clear delineation in order to provide a comprehensive understanding of its benefit and to facilitate clinical decision-making. Recommended therapy for patients with solid tumor metastases causing MESCC includes the combination of surgical decompression and stabilization followed by radiotherapy.4 Radiotherapy after surgery has been advocated for regardless of the surgical extent as concerns have been raised with the possible remainders of microscopic tumor cells on the dura.5 The current report provides the first analysis of the effect of combination surgery–radiation therapy, or hybrid MESCC therapy, on the symptoms of patients with MESCC.
The combination therapy implemented in the current study includes separation surgery followed by radiosurgery. Separation surgery provides circumferential decompression of the spinal cord and spinal stabilization via a posterior-only approach.6A major goal of this surgery is to provide a separation between the tumor and spinal cord in order to enable delivery of an ablative dose of radiation. Separation surgery is designed to be accompanied by concomitant radiosurgical treatment, since there is no attempt for complete tumor resection, unlike historical operative strategies involving gross tumor excision. This approach results in short operative time with low morbidity and the combination of such conservative surgery with postoperative radiosurgery has been shown to be a safe and effective strategy for establishing durable local tumor control regardless of tumor histology and prior radiation history.7 The current analysis aims to show that hybrid therapy not only favorably affects clinician-based measures (ie, survival, local control, and gross measures of function), but also improves patient experience through pain relief and improved mobility.
Methods
Design
This is a prospective, single-center, observational cohort study involving a large tertiary cancer center. The local institutional review board approved this study and informed consent was obtained. The change in PRO, measured preoperatively and 3 months postoperatively, was defined as the primary endpoint. Patients with PRO collected 2 to 4.5 months postoperatively were included in the primary endpoint analysis. The change in PRO at long-term follow-up, defined as >4.5 to 12 months, represented the secondary endpoint.
Population
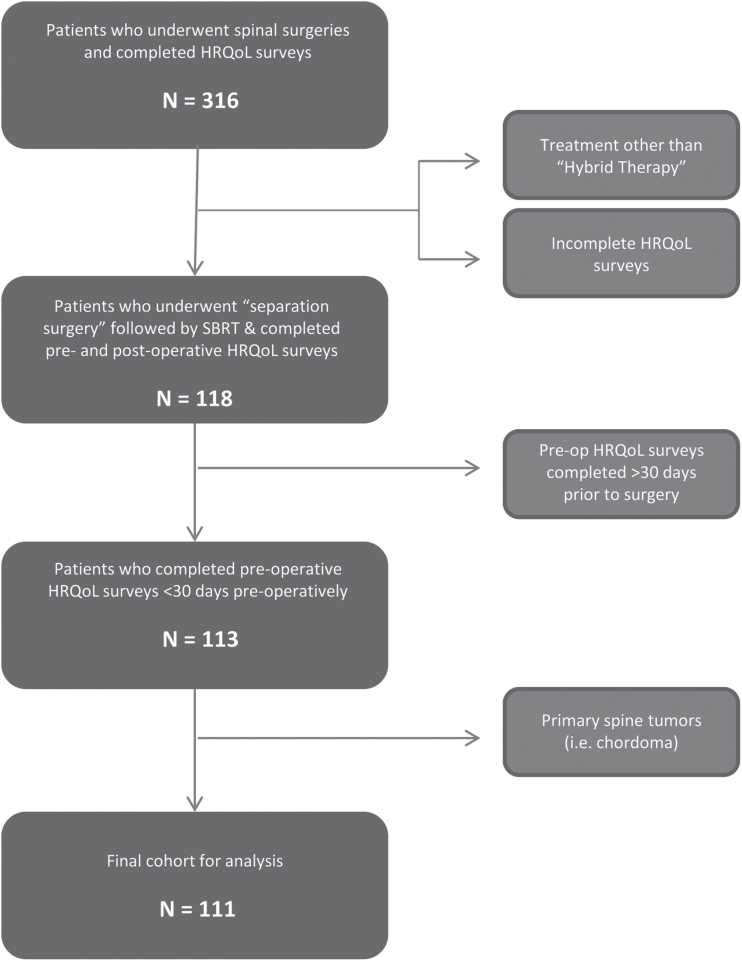
Three hundred thirteen patients who were treated for spinal tumors between October 2013 and July 2016 were screened. Inclusion criteria were a diagnosis of cancer with involvement of the spine, imaging confirming the spinal lesion, surgical treatment for a spinal lesion and ability to use patient reporting tools. Patients who underwent surgery other than separation surgery (ie, percutaneous screw fixation, anterior approach, combined anterior and posterior approaches) were excluded. Patients with intradural tumors as well as those with nonmetastatic lesions were excluded. Patients whose preoperative evaluation was conducted more than 30 days prior to surgery were also excluded. One hundred eleven patients met inclusion criteria and were included in the final analysis (Fig. 1).
Fig. 1.
Flowchart of the study population inclusion criteria implementation. Abbreviations: HRQoL, health-related quality of life; SBRT, stereotactic body radiation therapy.
Patient-Reported Variables
Data were mostly collected electronically. Patients were given either a tablet when in clinic or an electronic link to fill out surveys in the outpatient setting. Patients who preferred handwritten surveys were provided with surveys during admission or in clinic and data were then manually transferred to the electronic database. All data were kept in accordance with HIPAA regulations.
Brief Pain Inventory (BPI) questionnaires included 12 individual items. Of these, 4 were related to pain and 8 were related to disease interference. Combining the 4 pain items generated a BPI pain construct, combining the interference items generated a BPI interference construct, and combining the BPI pain construct items with the BPI interference construct items generated a BPI patient pain experience construct (Supplementary Fig. 1).
MD Anderson Symptom Inventory (MDASI) questionnaires included 13 core symptom items. When combined together they generated a MDASI core symptom construct. MDASI questionnaires also included 6 disease interference items that combined together generate a MDASI disease interference construct. Finally, MDASI questionnaires included 5 spine-tumor-specific items and when these individual items were combined they generated a MDASI spine-tumor-specific construct (Supplementary Fig. 2).
Treatment
One hundred one patients underwent spinal separation surgery with posterior instrumented fusion followed by stereotactic radiosurgery (SRS) and an additional 10 patients had previously placed instrumentation and therefore underwent salvage tumor separation with no added instrumentation followed by SRS. The detailed method for separation surgery has been previously described.6 Briefly, a posterior approach was used for instrumented stabilization and to perform the decompression. Laminectomy, facetectomy, and transpedicular approach to the ventral epidural space were carried out in order to provide circumferential access for circumferential decompression. The posterior longitudinal ligament was resected to provide a margin on the anterior dura and epidural tumor was excised until spinal cord decompression was achieved. Adequate tumor excision provided 2 to 3 mm of separation between the tumor and the spinal cord, generally indicated by complete re-expansion of the thecal sac. Radiosurgery was delivered in a single fraction for 17 patients with a median dose of 2400 cGy (range, 900–2700 cGy), 3 fractions for 70 patients with a median dose of 2700 cGy (range, 3600–2400 cGy), and 5 fractions for 24 patients with a median dose of 3000 cGy (range, 2000–4000 cGy). All patients underwent simulation with CT myelogram during the postoperative recovery period. Radiosurgical contouring and planning was performed in accordance with previously published guidelines.8,9 The dura and the epidural space were included in the treatment volume in order to account for microscopic tumor spread. The gross tumor volume was defined as the entire preoperative tumor volume, and the clinical target volume was defined as the gross tumor volume with the expansion to account for adjacent marrow compartments at risk for microscopic tumor invasion. The presence and location of spinal hardware did not alter the treatment plans that were based entirely on the radiographic tumor volume. Maximum dose constraints for the spinal cord were defined as 14 Gy to any point. All plans were reviewed by radiation oncology and neurosurgery teams. The median time interval from surgery to radiation was 20 days and the mean was 29.4 days.
Statistical Analysis
Descriptive statistics such as frequencies, medians, means, and standard deviations (SD) were used to characterize the cohort. Individual items from the BPI (n = 12) and MDASI (n = 24) were compared preoperatively to postoperatively using the Wilcoxon Signed-Rank test for matched pairs. Three BPI and three MDASI constructs were composed as described above using means for each individual. A majority of each construct’s individual items must have been answered; otherwise, the construct was not calculated for that patient. Constructs were also compared preoperatively to postoperatively using the Wilcoxon signed rank test for matched pairs. All P values were 2-sided with an alpha level of significance of < .05, with the exception of the individual pain items. For the individual pain items (n = 36), Bonferroni correction was used for statistical significance ( < .001 considered statistically significant).
The primary endpoint was the comparison of preoperative to postoperative PROs, where postoperative time point was defined as 2 to 4.5 months following surgery. The secondary endpoint was the comparison of preoperative to postoperative delayed PROs, where the postoperative time point was defined as >4.5 months to 12 months following surgery.
Overall survival (OS) was calculated from the date of surgery until death or last follow-up. Deaths were considered events and all others were censored. Progression-free survival (PFS) was calculated from the date of surgery until progression, death, or last follow-up. Progressions and deaths were considered events and all others were censored. OS and PFS were graphically presented using the method of Kaplan and Meier. All statistical analyses were done in SAS (version 9.4, Cary, NC).
Results
Preoperative Evaluation
Of the 111 patients included, 67 (60%) were males and 44 were females, with a mean age of 61 (SD = 11.9). The most common pathologies were non-small cell lung carcinoma (26 patients, 23%) and renal cell carcinoma (25 patients, 23%). The remaining pathologies varied and are summarized in Table 1. The majority of surgeries included a 2- to 3-level posterolateral bony decompression (75% of cases), allowing ventral separation from the cord, along with instrumented constructs consisting of pedicle screws with connecting rods of 4 to 6 levels (67% of cases). Treatment related variables are summarized in Table 2.
Table 1.
Patient and tumor characteristics
| Variable | Category | N | % |
|---|---|---|---|
| Age | — | 111 | 100 |
| Median 63.9 years | |||
| Mean 61.4 years | |||
| Sex | Female | 44 | 40 |
| Male | 67 | 60 | |
| Surgical treatment level | Cervical | 12 | 11 |
| Thoracic | 75 | 67 | |
| Lumbar | 24 | 22 | |
| Histology |
|
26 | 23 |
| RCC | 25 | 23 | |
| Sarcoma | 13 | 12 | |
| Thyroid | 8 | 7 | |
| Prostate | 7 | 6 | |
| Head and neck | 6 | 5 | |
| Breast | 4 | 3 | |
| Hepatocellular | 3 | 3 | |
| Melanoma | 3 | 3 | |
| Colorectal | 3 | 3 | |
| Other | 13 | 12 | |
| Preoperative SINS | Stable | 10 | 9 |
| Intermediate | 63 | 57 | |
| Unstable | 19 | 17 | |
| n/a | 19 | 17 | |
| Preoperative ECOG | 0 | 10 | 9 |
| 1 | 88 | 79 | |
| 2 | 2 | 2 | |
| 3 | 7 | 6 | |
| 4 | 4 | 4 | |
| Preoperative ASIA | C | 3 | 3 |
| D | 12 | 11 | |
| E | 96 | 86 | |
| Prior spinal procedure | At surgical level | 12 | 11 |
| At other level | 12 | 11 | |
| None | 87 | 78 |
Abbreviations: NSCLC, non-small cell lung carcinoma; RCC, renal cell carcinoma; SINS, Spinal Instability Neoplastic Score; ECOG, Eastern Cooperative Oncology Group performance status; American Spinal Injury Association Impairment Scale score.
Table 2.
Treatment variables
| Variable | Category | N | % | Median | Mean | SD |
|---|---|---|---|---|---|---|
| Number of levels decompressed | — | 111 | 100 | 3 | 2.8 | 1 |
| 1 | 10 | 9 | ||||
| 2 | 26 | 23 | ||||
| 3 | 59 | 53 | ||||
| 4 | 11 | 10 | ||||
| 5 | 3 | 3 | ||||
| 6 | 2 | 2 | ||||
| Number of levels instrumented | — | 111 | 100 | 5 | 5.2 | 2.4 |
| 0 | 10 | 9 | ||||
| 3 | 4 | 4 | ||||
| 4 | 14 | 13 | ||||
| 5 | 43 | 39 | ||||
| 6 | 17 | 15 | ||||
| 7 | 8 | 7 | ||||
| >7 | 15 | 14 | ||||
| SRS dose (cGy) | — | 111 | 100 | 2700 | 2751.4 | 370.7 |
| 2700 | 68 | 61 | ||||
| 2400 | 15 | 14 | ||||
| 3000 | 14 | 13 | ||||
| Other | 14 | 13 | ||||
| SRS treatment fractions | — | 111 | 100 | 3 | 3.1 | 1.2 |
| 1 | 17 | 15 | ||||
| 3 | 70 | 63 | ||||
| 5 | 24 | 22 | ||||
| Concurrent radiation therapy to other level | Yes | 15 | 14 | |||
| No | 96 | 86 |
Abbreviation: SRS, stereotactic radiosurgery.
Twenty-two percent of patients had previous interventions to the spine. Of these, 12 patients (11%) had been previously treated at the current treatment level (9 open surgeries and 3 kyphoplasties) and were re-operated for progression. Twelve others (11%) were previously treated at other spinal levels (7 open surgeries and 5 kyphoplasties). Twenty-one patients (19%) had received, aside from stereotactic body radiation therapy to the postoperative target, concurrent radiation treatment to lesions at other spinal levels.
Preoperatively, the majority (86%) of patients were evaluated as “E” on the American Spinal Injury Association (ASIA) Impairment Scale (normal motor and sensory exam) and had a median muscle strength grade of 5 (range, 2–5) on the Medical Research Council (MRC) Muscle Scale (full strength in all muscle groups). The median Eastern Cooperative Oncology Group (ECOG) Performance Status was 1 (range, 0–4). The median Spinal Instability Neoplastic Score (SINS) was 10 (range, 6–16), which indicates potential instability. SINS score of 0–6 suggests stability, 13–18 suggests instability, and 7–12 are intermediate and suggest potentially unstable spines.10 The SINS score is demonstrated in Supplementary Table 1.
Brief Pain Inventory
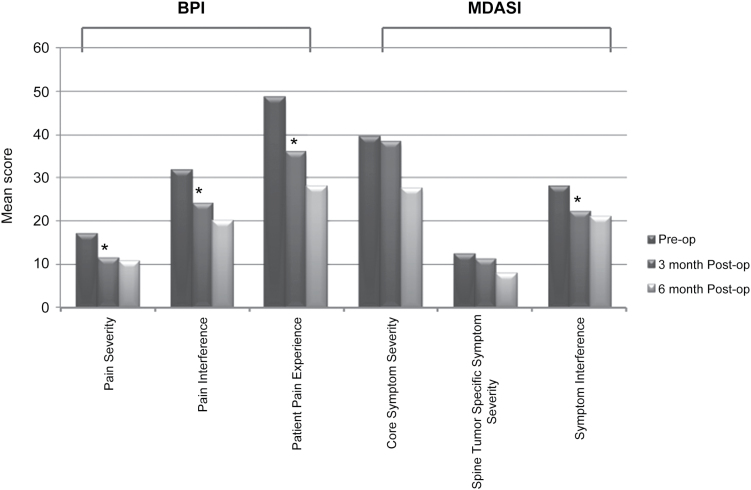
Among BPI individual questionnaire items, “worst pain” and “pain right now” were significantly reduced at 3-month follow-up following hybrid MESCC therapy (P < .0001) (Table 3). Every other single BPI item showed decreased means (ie, a trend of improvement in pain and pain interference) but did not achieve statistical significance at the < .001 alpha level (Table 3). The 3 combined BPI constructs, namely pain severity, pain interference with daily life, and overall patient pain experience, were significantly improved (P < .001) (Fig. 2).
Table 3.
Brief Pain Inventory (BPI) and MD Anderson Symptom Inventory (MDASI) individual item results at baseline and 3-month follow-up (primary end point)
| Preoperative Survey | 3-Month Postoperative Survey | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survey | Individual Item | Mean Score | SD | N | % | Mean Score | SD | N | % | Wilcoxon matched pairs test P value |
| BPI | Worst pain | 6.3 | 3.1 | 111 | 100 | 4.5 | 2.8 | 60 | 54 | < .0001 |
| Least pain | 2.7 | 2.5 | 111 | 100 | 1.6 | 1.8 | 61 | 55 | .0072 | |
| Average pain | 4.3 | 2.6 | 111 | 100 | 3.1 | 2.1 | 61 | 55 | .004 | |
| Right now pain | 3.8 | 2.9 | 111 | 100 | 2.4 | 2.2 | 62 | 56 | < .0001 | |
| General activity | 5.4 | 3.8 | 110 | 99 | 3.7 | 3.2 | 62 | 56 | .01 | |
| Mood | 4.1 | 3.6 | 109 | 98 | 3.2 | 2.8 | 60 | 54 | .02 | |
| Walking ability | 4.7 | 3.6 | 109 | 98 | 3.5 | 2.9 | 61 | 55 | .08 | |
| Normal work | 5.7 | 4 | 109 | 98 | 4.2 | 3.3 | 62 | 56 | .04 | |
| Relations | 2.8 | 3.4 | 109 | 98 | 2.6 | 2.9 | 62 | 56 | .95 | |
| Sleep | 4.1 | 3.6 | 110 | 99 | 3 | 2.8 | 60 | 54 | .2 | |
| Enjoyment of life | 5.2 | 3.7 | 108 | 97 | 4 | 3.2 | 61 | 55 | .01 | |
| Relief | 63.3% | 27.7% | 100 | 90 | 52.7% | 32.7% | 52 | 47 | .06 | |
| MDASI | Pain | 6.6 | 3.2 | 107 | 96 | 5 | 3.1 | 63 | 57 | .06 |
| Fatigue | 5.1 | 3.2 | 108 | 97 | 5.2 | 3.4 | 61 | 55 | .56 | |
| Nausea | 1.2 | 2.4 | 106 | 95 | 2.2 | 2.7 | 62 | 56 | .0001 | |
| Sleep | 4.3 | 3.7 | 108 | 97 | 3.7 | 3.2 | 60 | 54 | .68 | |
| Distress | 4.3 | 3.7 | 105 | 95 | 3.2 | 3.1 | 61 | 55 | .06 | |
| Shortness of breath | 1.7 | 2.6 | 108 | 97 | 2.1 | 2.8 | 62 | 56 | .23 | |
| Memory | 1.5 | 2.3 | 108 | 97 | 2 | 2.6 | 62 | 56 | .62 | |
| Appetite | 2.5 | 3 | 108 | 97 | 2.9 | 3 | 61 | 55 | .02 | |
| Drowsy | 3.1 | 3.1 | 108 | 97 | 3.2 | 3 | 61 | 55 | .13 | |
| Dry mouth | 3.1 | 3.4 | 108 | 97 | 3 | 3 | 61 | 55 | .56 | |
| Sadness | 3.2 | 3.5 | 105 | 95 | 2.8 | 3 | 61 | 55 | .69 | |
| Vomiting | 0.6 | 1.8 | 108 | 97 | 1 | 2 | 60 | 54 | .007 | |
| Numbness | 3 | 3.4 | 107 | 96 | 2.9 | 3.2 | 62 | 56 | .49 | |
| Spine pain | 4.6 | 3.8 | 104 | 94 | 2.6 | 3 | 60 | 54 | .0006 | |
| Limb weakness | 3.2 | 3.6 | 105 | 95 | 3.2 | 3.2 | 60 | 55 | .16 | |
| Bowel/Bladder control | 0.5 | 1.6 | 106 | 95 | 0.6 | 1.6 | 59 | 53 | .84 | |
| Bowel pattern | 2.4 | 3.3 | 105 | 95 | 2.3 | 2.9 | 59 | 53 | .23 | |
| Sexual function | 2.1 | 3.6 | 98 | 88 | 2.8 | 3.9 | 57 | 51 | .81 | |
| General activity | 5.9 | 3.7 | 104 | 94 | 4 | 3.2 | 61 | 55 | .0002 | |
| Mood | 4.2 | 3.4 | 103 | 93 | 3.3 | 2.8 | 61 | 55 | .03 | |
| Work | 5.8 | 4 | 102 | 92 | 4.5 | 3.6 | 59 | 53 | .04 | |
| Relations | 2.8 | 3.3 | 102 | 92 | 2.8 | 3 | 60 | 55 | .97 | |
| Walking | 5.1 | 3.6 | 95 | 86 | 4 | 3.8 | 55 | 50 | .18 | |
| Enjoyment of life | 5.2 | 3.7 | 104 | 94 | 4.1 | 3.5 | 61 | 55 | .04 | |
Fig. 2.
Mean preoperative vs postoperative scores for Brief Pain Inventory (BPI) and MD Anderson Symptom Inventory (MDASI) constructs. * = P < .05.
MD Anderson Symptom Inventory
Analysis of the MDASI questionnaire showed that at the 3-month follow-up, spine pain severity was significantly reduced after treatment (P < .001). General activity also improved significantly at the 3-month follow-up (P < .001). However, patients reported feeling more nauseated (P < .001).
The analysis from the MDASI constructs also yielded symptomatic improvement. Cancer symptom interference in daily life improved significantly following treatment (P = .006) (Fig. 2). Both MDASI and BPI are validated PRO measures to assess patient reported outcome measures in cancer patients.11–15
Long-Term Follow-Up
Thirty-nine patients had fully completed PRO data at long-term follow-up. There was a clinically significant trend toward improvement in all constructs, but due to small patient sample size, this did not reach statistical significance. Ninety-five percent of patients did not require further spinal surgery during the follow-up period.
Overall and Progression-Free Survival
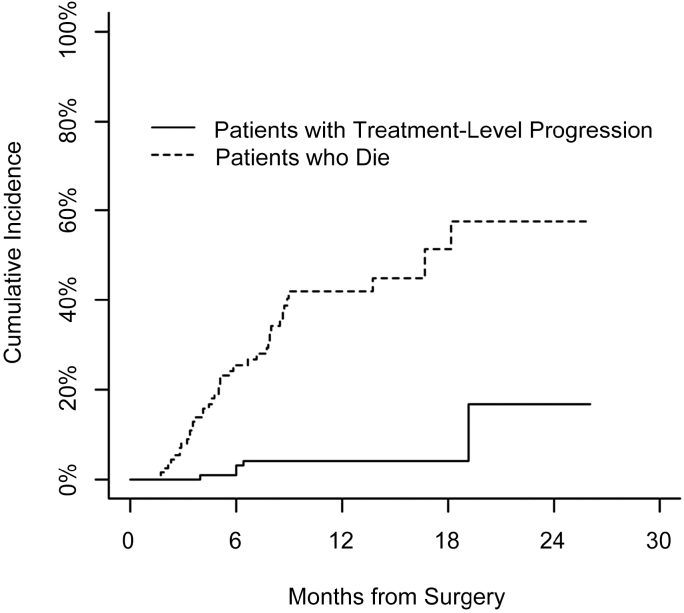
Median survival for the cohort was 16.7 months (95% CI, 8.7 months–no upper confidence limit). Median follow-up of the cohort was 7.2 months (7.8 months for survivors). At the time of data analysis, 41 patients had died (ie, percent dead = 37%) and median time to death was 5 months (Fig. 3). The 6- and 12-month cumulative incidence for treatment-level progression was 2.1% and 4.3%, respectively, and for death was 25.6% and 42.1%, respectively.
Fig. 3.
Cumulative incidence of treatment-level progressions and death as a competing event.
Five cases of progression at the treatment level were found along with 2 at the adjacent level. Twenty patients had documented distal sites of progressive spinal metastases during their follow-up. Median time to progression was 6 months to treatment-level progression, 3.2 months to adjacent-level progression, and 3.6 months to distant-level progression.
Postoperative Complications
Of the 111 patients included, only 2 patients required revision surgery; 1 for wound revision and the other for removal of a postoperative hematoma. Two other patients had symptomatic vertebral compression fractures at the previously operated level for which they underwent kyphoplasty. Two more patients required kyphoplasty at a nonoperated level for a symptomatic vertebral compression fracture and 6 other patients had documented vertebral fractures that did not require interventions.
Discussion
This study evaluated PROs for pain, disease interference, and other cancer-related symptoms in patients undergoing hybrid MESCC therapy consisting of separation surgery and radiosurgery. This was achieved using 2 validated, cancer-specific outcome analysis instruments: the MDASI and the BPI. Retrospective analyses have previously shown that separation surgery complemented with radiosurgery represents a safe and effective strategy for establishing durable local tumor control regardless of tumor histology and previous radiation.7 Herein, we used prospectively collected data to show that this treatment paradigm results in significant reduction in spine-specific cancer symptoms such as “overall pain,” “right now pain,” and “spine-specific pain,” along with combined general pain constructs and disease interference with activity.
With the improvement of cancer therapies, MESCC has become a common occurrence.1–3 Early detection and treatment of spinal metastases prevents development of neurologic deficits and disability.16,17 However, concern about the inability of cancer patients to tolerate extensive surgery, surgical complications, and interruption of systemic therapy may prevent timely referral for surgical evaluation. Hybrid MESCC therapy was developed in order to address these concerns and to capitalize on the strengths of surgery and SRS. Reliance on SRS to provide local tumor control has allowed surgeons to decrease the extent of surgical intervention, thereby minimizing the risk of complications and systemic therapy interruption. Spinal SRS has been shown to be a tumor ablative treatment18 and provide durable and consistent tumor control, with reports of histology-independent 98% local control at 4-year follow-up.19 Separation surgery followed by SRS has been previously shown to be an effective treatment strategy providing a greater than 95% control rate, for previously considered radioresistant tumors in a retrospective review of 186 cases.7 With SRS providing such outstanding tumor control, surgeons no longer need to maximize the extent of tumor excision in order to optimize local control. The role of surgery has shifted to providing optimal SRS conditions through spinal cord decompression and spinal stabilization. This combined, tailored therapy offers short surgery with decreased hemodynamic stress, and the entire surgery–SRS treatment can be completed within 3 to 4 weeks, allowing relatively quick return to systemic therapy. Durable tumor control has been shown with this therapy7 along with low risk of complications such as hardware failure and wound infections.20,21
The NOMS framework,22 which consists of neurologic, oncologic, mechanical stability and systemic considerations, provides evidence-based guidance for treatment decisions in patients with spinal metastases. Patients with high-grade MESCC23 and radioresistant tumors (ie, tumors that are known to be resistant to conventional external beam radiotherapy) require separation of the tumor from the thecal sac, in order to allow adequate SRS dosing to include the entire volume, including the epidural margin, while conforming to safety constraints to the spinal cord. Patients with low-grade cord compression undergo upfront radiation therapy. Patients with tumor-related spinal instability require stabilization, and determination of instability can be facilitated by the Spinal Instability Neoplastic Score10 (Supplementary Table 1).
The role of surgery for patients with MESCC was supported by the clinical trial by Patchell et al,24 in which patients with symptomatic MESCC were randomized to undergo radiation therapy alone or decompressive surgery plus radiation therapy. In this study patients in the surgical arm had significant benefits in survival and ambulation, and decreased steroid and opioid demand. These data support the utilization of surgery followed by radiation therapy in the treatment of MESCC caused by solid tumor metastases. However, since local recurrence rates after conventionally fractionated radiation remain as high, especially in patients with tumor histologies that show resistance to conventional external beam radiotherapy,9,25–27 we advocate integration of stereotactic radiosurgery in the postoperative treatment plan in order to decrease the risk of postoperative tumor recurrence. Recent data suggest that stereotactic body radiation therapy for spinal metastases is associated with high rates of local control and a low risk of marginal failure even in radioresistant histologies.7,19,28,29 These results provide the foundation for hybrid therapy.
We collected PRO data in order to confirm that this therapy confers a positive effect on the life of the patients through providing pain relief, improved mobility, and decreased interference of symptoms with daily life. The previously reported data about clinical outcomes of metastatic spine patients largely focused on clinician-based measures, including survival, local control, and gross measures of function (ambulatory status, Frankel Score),30–32 while PRO measure analyses of spine metastases treatments are scarce. Disease-specific patient self-assessment instruments permit direct measurement of the value of care as perceived by the recipient.23 Examples of spine-cancer-specific tools are the MDASI spine tumor module12 and the BPI,13 which were used in the current study, and the health-related quality of life (HRQoL) Outcome Questionnaire33,34 developed by the Spine Oncology Study Group. MDASI is a validated tool to assess PRO measures in cancer patients.11 Furthermore, MDASI-SP provides a validated, spine-tumor-specific symptom assessment module.12 Similarly, the BPI is a validated13–15 patient-reported pain assessment tool. We elected to use the MDASI-SP along with the BPI because at the time of data collection these represented the only validated instruments for spine tumor patients. Other commonly used quality-of-life tools are the EuroQol 5D (EQ-5D), Oswestry Disability Index, Visual Analog Scale, and Short Form Health Survey (SF-36), though these are not cancer-specific instruments.35
Previously reported spinal metastases treatment PRO data consists of heterogenous surgical series employing a wide range of surgical strategies.36,37 A small number of studies have analyzed the effect of surgery or SRS on patient symptoms. The individual benefit of surgery on HRQoL and SRS on HRQoL has been previously shown. Fehlings et al, in a prospective multicenter study, showed that surgery as an adjunct to radiation and chemotherapy provides improvement in HRQoL measures with acceptable risks.26 In their study the BPI was used to assess pain and HRQoL was measured using the Oswestry Disability Index, SF-36, and EQ-5D forms. The surgical methods used in their analysis were heterogenous, including anterior, posterior, and combined approaches, and radiotherapy information was not provided. Another prospective cohort study,38 from the Global Spine Tumor Study Group database, analyzed 922 consecutive patients with spinal metastases who underwent surgery. Quality of life as measured by the EQ-5D, along with Visual Analog Scale pain score and Karnofsky physical functioning score, improved rapidly after surgery and these improvements were sustained in those patients who survived up to 2 years after surgery. This series also included a large variety of surgical techniques and, of note, almost a quarter of patients experienced postoperative systemic adverse events. The effects of radiotherapy to spinal metastases on HRQoL have been addressed previously.39,40 Wang et al conducted a prospective trial demonstrating that stereotactic body radiation therapy is an effective primary or salvage treatment method for mechanically stable spine metastases.41 They analyzed patient reported pain and other symptoms using the MDASI and BPI and reported a significant and lasting reduction in pain and other symptoms at 6 months after stereotactic body radiation therapy.
The current study demonstrates the favorable impact of hybrid MESCC therapy on the quality of life of cancer patients. The combined use of separation surgery and radiosurgery results in a low risk of complications, superb local tumor control, pain relief, and improved general activity. Our baseline disability as assessed by BPI construct means appear lower than previous reports,26 suggesting that patients with moderate symptom severity from MESCC benefit from hybrid therapy. Early intervention for MESCC prevents development of neurologic deficits and progression of deformity and pain. Furthermore, studies showed an increased risk of complications after emergency surgery for MESCC,42,43 supporting proactive screening for MESCC and early intervention. These data support active surveillance of cancer patients for development of MESCC and referral for hybrid MESCC therapy for the treatment of epidural tumor extension.
There are several limitations to this study. We report the experience of a single tertiary cancer center. The generalizability of this approach has been demonstrated through published work of other medical centers,29 but more such reports are required. Further, while the benefit of hybrid therapy was demonstrated at the primary end point (improvement at 3 months following surgery), patient loss to follow-up precluded demonstration of statistically significant improvement at longer follow-up. As overall survival in this patient population is expected to increase with new treatments, long-term complications and HRQoL will require further investigation. Future studies to determine the minimal clinically significant difference in HRQoL measures in this patient population will be required. This reported treatment strategy requires further testing in prospective studies comparing it to current standards of care.
Conclusion
The data presented demonstrate that patients with MESCC who underwent hybrid therapy with separation surgery and stereotactic body radiation therapy experienced significant improvement in both spine-specific symptoms and overall cancer-related symptoms. When combined into PRO constructs, this treatment significantly and durably reduced the patients’ pain and disease interference. These prospective data confirm that hybrid therapy for the treatment of MESCC significantly and objectively improves pain and HRQoL outcome measures, while providing durable local tumor control. Early referral, multidisciplinary assessment, and a consistent treatment paradigm with hybrid separation surgery–SRS therapy provides durable local control with low risk of complications, but also improves the quality of life in patients with spinal metastases.
Supplementary Material
Supplementary data are available at Neuro-Oncology Practice online.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflict of interest statement. Ori Barzilai—No disclosures, Mary-Kate Amato—No disclosures, Lily McLaughlin—No disclosures, Anne S. Reiner—No disclosures, Eric Lis—Medtronic—consultant, Yoshiya Yamada—Varian medical systems—consultant, Chordoma Foundation—Medical Advisory Board Member, Michael D. Lovelock—No disclosures, Andrew N. Fontanella—No disclosures, Mark H. Bilsky—Globus—consultant, Depuy/Synthes—royalties, BrainLab—consultant, Ilya Laufer—Depuy/Synthes, globus, SpineWave—consultant.
Supplementary Material
References
- 1. Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15–24. [DOI] [PubMed] [Google Scholar]
- 2. Ortiz Gómez JA. The incidence of vertebral body metastases. Int Orthop. 1995;19(5):309–311. [DOI] [PubMed] [Google Scholar]
- 3. Mak KS, Lee LK, Mak RH et al. . Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998–2006. Int J Radiat Oncol Biol Phys. 2011;80:824–831. [DOI] [PubMed] [Google Scholar]
- 4. Bilsky M, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976). 2009;34:S101–S107. [DOI] [PubMed] [Google Scholar]
- 5. Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien). 1998;140(9):957–967. [DOI] [PubMed] [Google Scholar]
- 6. Bilsky MH, Boland P, Lis E et al. . Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine (Phila Pa 1976). 2000;25:2240–2249; discussion 2250. [DOI] [PubMed] [Google Scholar]
- 7. Laufer I, Iorgulescu JB, Chapman T et al. . Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cox BW, Spratt DE, Lovelock M et al. . International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e597–e605. [DOI] [PubMed] [Google Scholar]
- 9. Redmond KJ, Lo SS, Soltys SG et al. . Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg Spine. 2017;26(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher CG, Versteeg AL, Schouten R et al. . Reliability of the spinal instability neoplastic scale among radiologists: an assessment of instability secondary to spinal metastases. AJR Am J Roentgenol. 2014;203(4):869–874. [DOI] [PubMed] [Google Scholar]
- 11. Cleeland CS, Mendoza TR, Wang XS et al. . Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong TS, Gning I, Mendoza TR et al. . Reliability and validity of the M. D. Anderson symptom inventory-spine tumor module. J Neurosurg Spine. 2010;12(4):421–430. [DOI] [PubMed] [Google Scholar]
- 13. Wu JS, Beaton D, Smith PM et al. . Patterns of pain and interference in patients with painful bone metastases: a brief pain inventory validation study. J Pain Symptom Manage. 2010;39(2):230–240. [DOI] [PubMed] [Google Scholar]
- 14. Atkinson TM, Rosenfeld BD, Sit L et al. . Using confirmatory factor analysis to evaluate construct validity of the brief pain inventory (BPI). J Pain Symptom Manage. 2011;41(3):558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dworkin RH, Turk DC, Wyrwich KW et al. . Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 16. George R, Jeba J, Ramkumar G et al. . Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev. 2015;CD006716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dea N, Charest-Morin R, Sciubba DM et al. . Optimizing the adverse event and HRQOL profiles in the management of primary spine tumors. Spine (Phila Pa 1976). 2016;41(Suppl 20):S212–S217. [DOI] [PubMed] [Google Scholar]
- 18. Katsoulakis E, Laufer I, Bilsky M et al. . Pathological characteristics of spine metastases treated with high-dose single-fraction stereotactic radiosurgery. Neurosurg Focus. 2017;42(1):E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamada Y, Katsoulakis E, Laufer I et al. . The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42(1):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amankulor NM, Xu R, Iorgulescu JB et al. . The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14(9):1850–1859. [DOI] [PubMed] [Google Scholar]
- 21. Keam J, Bilsky MH, Laufer I et al. . No association between excessive wound complications and preoperative high-dose, hypofractionated, image-guided radiation therapy for spine metastasis. J Neurosurg Spine. 2014;20(4):411–420. [DOI] [PubMed] [Google Scholar]
- 22. Laufer I, Rubin DG, Lis E et al. . The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bilsky MH, Laufer I, Fourney DR et al. . Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13(3):324–328. [DOI] [PubMed] [Google Scholar]
- 24. Patchell RA, Tibbs PA, Regine WF et al. . Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. [DOI] [PubMed] [Google Scholar]
- 25. Epstein-Peterson ZD, Sullivan A, Krishnan M et al. . Postoperative radiation therapy for osseous metastasis: outcomes and predictors of local failure. Pract Radiat Oncol. 2015;5(5):e531–e536. [DOI] [PubMed] [Google Scholar]
- 26. Fehlings MG, Nater A, Tetreault L et al. . Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol. 2016;34(3):268–276. [DOI] [PubMed] [Google Scholar]
- 27. Howell DD, James JL, Hartsell WF et al. . Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: a subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer. 2013;119(4):888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Omair A, Masucci L, Masson-Cote L et al. . Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15(10):1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bate BG, Khan NR, Kimball BY et al. . Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. 2015;22(4):409–415. [DOI] [PubMed] [Google Scholar]
- 30. Street J, Berven S, Fisher C et al. . Health related quality of life assessment in metastatic disease of the spine: a systematic review. Spine (Phila Pa 1976). 2009;34:S128–S134. [DOI] [PubMed] [Google Scholar]
- 31. Falicov A, Fisher CG, Sparkes J et al. . Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976). 2006;31:2849–2856. [DOI] [PubMed] [Google Scholar]
- 32. Wai EK, Finkelstein JA, Tangente RP et al. . Quality of life in surgical treatment of metastatic spine disease. Spine (Phila Pa 1976). 2003;28:508–512. [DOI] [PubMed] [Google Scholar]
- 33. Street J, Lenehan B, Berven S et al. . Introducing a new health-related quality of life outcome tool for metastatic disease of the spine: content validation using the international classification of functioning, disability, and health; on behalf of the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35:1377–1386. [DOI] [PubMed] [Google Scholar]
- 34. Janssen SJ, Teunis T, van Dijk E et al. . Validation of the Spine Oncology Study Group-Outcomes Questionnaire to assess quality of life in patients with metastatic spine disease. Spine J. 2015;pii: S1529-9430(15)01197-3. [DOI] [PubMed] [Google Scholar]
- 35. DeVine J, Norvell DC, Ecker E et al. . Evaluating the correlation and responsiveness of patient-reported pain with function and quality-of-life outcomes after spine surgery. Spine (Phila Pa 1976). 2011;36:S69–S74. [DOI] [PubMed] [Google Scholar]
- 36. Molina C, Goodwin CR, Abu-Bonsrah N et al. . Posterior approaches for symptomatic metastatic spinal cord compression. Neurosurg Focus. 2016;41(2):E11. [DOI] [PubMed] [Google Scholar]
- 37. Bakar D, Tanenbaum JE, Phan K et al. . Decompression surgery for spinal metastases: a systematic review. Neurosurg Focus. 2016;41(2):E2. [DOI] [PubMed] [Google Scholar]
- 38. Choi D, Fox Z, Albert T et al. . Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. Br J Neurosurg. 2016;30(3):337–344. [DOI] [PubMed] [Google Scholar]
- 39. Zeng L, Chow E, Zhang L et al. . Comparison of pain response and functional interference outcomes between spinal and non-spinal bone metastases treated with palliative radiotherapy. Support Care Cancer. 2012;20:633–639. [DOI] [PubMed] [Google Scholar]
- 40. Wu JS, Monk G, Clark T et al. . Palliative radiotherapy improves pain and reduces functional interference in patients with painful bone metastases: a quality assurance study. Clin Oncol (R Coll Radiol). 2006;18(7):539–544. [DOI] [PubMed] [Google Scholar]
- 41. Wang XS, Rhines LD, Shiu AS et al. . Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chaichana KL, Woodworth GF, Sciubba DM et al. . Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62(3):683–692; discussion 683. [DOI] [PubMed] [Google Scholar]
- 43. Fürstenberg CH, Wiedenhöfer B, Gerner HJ et al. . The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Joint Surg Br. 2009;91(2):240–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.