Abstract
Background and Aims
Although several studies have confirmed the beneficial roles of exogenous melatonin in lateral root (LR) formation, the molecular mechanism is still elusive. Here, the role of hydrogen peroxide (H2O2) in the induction of LR formation triggered by melatonin was investigated.
Methods
Alfalfa (Medicago sativa ‘Biaogan’) and transgenic Arabidopsis seedlings were treated with or without melatonin, diphenyleneiodonium (DPI, NADPH oxidase inhibitor), N,N′-dimethylthiourea (DMTU, H2O2 scavenger), alone or combined. Then, H2O2 content was determined with 2′,7′-dichlorofluorescein diacetate (H2DCFDA)-dependent fluorescence and spectrophotography. Transcript levels of cell cycle regulatory genes were analysed by real-time reverse transcription–PCR.
Key Results
Application of exogenous melatonin not only increased endogenous H2O2 content but also induced LR formation in alfalfa seedlings. Consistently, melatonin-induced LR primordia exhibited an accelerated response. These inducible responses were significantly blocked when DPI or DMTU was applied. Compared with the wild-type, transgenic Arabidopsis plants overexpressing alfalfa MsSNAT (a melatonin synthesis gene) increased H2O2 accumulation and thereafter LR formation, both of which were blocked by DPI or DMTU. Similarly, melatonin-modulated expression of marker genes responsible for LR formation, including MsCDKB1;1, MsCDKB2;1, AtCDKB1;1 and AtCDKB2;1, was obviously impaired by the removal of H2O2 in both alfalfa and transgenic Arabidopsis plants.
Conclusions
Pharmacological and genetic evidence revealed that endogenous melatonin-triggered LR formation was H2O2-dependent.
Keywords: Hydrogen peroxide, lateral root, melatonin, Medicago sativa, transgenic Arabidopsis plants
INTRODUCTION
Since the first discovery of melatonin (N-acetyl-5-methoxytryptamine) in the bovine pineal gland in 1958, its various physiological functions have been investigated in animals (Lerner et al., 1958; Stehle et al., 2011; Rosales-Corral et al., 2012). In higher plants, melatonin was identified in 1995 (Hattori et al., 1995), and it is synthesized from tryptophan via a four-step pathway. Four enzymes – tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT) and N-acetylserotonin methyltransferase (ASMT) – were characterized in this biosynthetic pathway (Byeon and Back, 2014; Arnao and Hernandez-Ruiz, 2015; Lee et al., 2016). Further studies demonstrated that melatonin functions in plant responses against various biotic and abiotic stresses, such as pathogen attacks (Shi et al., 2015), salt (Chen et al., 2017), drought (Zuo et al., 2014), cold (Han et al., 2017) and heavy metal exposure (Hasan et al., 2015; M. Q. Li, 2016; Gu et al., 2017). The promotion of root organogenesis, including lateral root and adventitious root development, by exogenous melatonin was also found in Lupinus albus (Arnao and Hernández-Ruiz, 2007), rice (Liang et al., 2017), cucumber (Zhang et al., 2013, 2014) and Arabidopsis (Pelagio-Flores et al., 2012; Koyama et al., 2013).
Lateral root formation is regarded as a critical avoidance strategy in response to unfavourable conditions and is tightly regulated by intrinsic developmental processes, environmental inputs and hormone signalling in plants (Malamy and Ryan, 2001; Casimiro et al., 2003; Aloni et al., 2006). Among these, auxin positively regulates lateral root development via activating asymmetrical cell division in xylem pole pericycle cells (Ivanchenko et al., 2010). Previous work suggested that melatonin-promoted Arabidopsis lateral root formation might be independent of auxin signalling (Pelagio-Flores et al., 2012). However, genome-wide expression profiling analysis in rice demonstrated that lateral root development controlled by melatonin was promoted by the modulation of auxin signalling (Liang et al., 2017). Further studies found that cyclin-dependent protein kinases (CDKs), cyclins and CDK-inhibitory proteins play key roles in the above development process (Stals and Inzé, 2001; Casimiro et al., 2003; Verkest et al., 2005). It was observed that Kip-related protein (KRP1, the cyclin-dependent kinase inhibitor) interacted with the CDKA;1/CYCD2;1 complex to regulate the G1-to-S phase transition (Verkest et al., 2005). Previous results further showed that gaseous signalling molecules, including nitric oxide (NO) (Correa-Aragunde et al., 2006, 2015), carbon monoxide (CO) (Cao et al., 2007; Guo et al., 2008) and hydrogen sulphide (H2S) (Fang et al., 2014), induced lateral root formation via the modulation of cell cycle regulatory genes (CYCD3;1, KRP2 and auxin-dependent cell cycle gene).
Ample evidence showed that reactive oxygen species (ROS) act as key signalling molecules in regulating stomatal movements and biotic and abiotic stress responses, as well as many aspects of plant development, including lateral root formation (Torres et al., 2002; Foreman et al., 2003; Kwak et al., 2003; Miller et al., 2010; Mittler et al., 2011; Jiang et al., 2013; Ishibashi et al., 2013; Orman-Ligeza et al., 2016). For example, lateral root outgrowth in Arabidopsis was facilitated by RESPIRATORY BURST OXIDASE HOMOLOGS (RBOH)-mediated ROS production by promoting cell wall remodelling of overlying parental tissues (Orman-Ligeza et al., 2016). Similarly, it was found that ROS promoted cell division through accelerating auxin-mediated cell cycle entry (G0-to-G1) in alfalfa (Feher et al., 2008). In guard cells, hydrogen peroxide (H2O2) signalling mediated by AtrbohF was identified as a key mediator of stomatal responses to ethylene (Jiang et al., 2013). Previous results showed that exogenous melatonin enhanced plant tolerance of photo-oxidative stress in an H2O2-dependent manner in cucumber (H. Li, 2016). Recent studies also indicated that NADPH oxidase-dependent regulation of ROS signalling was required for melatonin-induced salinity tolerance (Chen et al., 2017; Gong et al., 2017). Consistently, the production of ROS triggered by melatonin was previously found in animals (Radogna et al., 2009). To date, although exogenous melatonin has been implicated as an inducer responsible for lateral root development, it is not clear whether and how endogenous melatonin could govern lateral root formation.
In this report, pharmacological and molecular evidence reveals that H2O2 was involved in exogenous melatonin-induced lateral root formation in alfalfa seedlings. By using transgenic Arabidopsis overexpressing MsSNAT, a causal link between endogenous melatonin and H2O2 in lateral root formation was further established. Furthermore, a molecular mechanism was preliminarily illustrated. This work may increase our understanding of the mechanisms underlying endogenous melatonin-mediated root organogenesis.
MATERIALS AND METHODS
Plant materials and growth conditions
Commercially available alfalfa (Medicago sativa ‘Biaogan’) seeds were surface-sterilized with 5 % NaClO for 5 min and rinsed comprehensively in distilled water. After soaking overnight in darkness, uniform seedlings were cultured with quarter-strength Hoagland solution in an illuminating incubator with a 14/10-h (25 ± 1/23 ± 1 °C) day/night regime with 200 μmol m−2 s−1 irradiation (Gu et al., 2017).
The SNAT gene of Medicago sativa (MsSNAT), encoding serotonin N-acetyltransferase, is homologous to the Arabidopsis SNAT gene, AtSNAT. Visualized fluorescence indicated that the MsSNAT-GFP signal is localized in the chloroplast. The binary vector pCAMBIA 1302 (AF234298) was used for alfalfa MsSNAT overexpression. Homogenous MsSNAT overexpression Arabidopsis lines driven by the cauliflower mosaic virus were then generated and used, and showed higher levels of endogenous melatonin (Gu et al., 2017). Wild-type (Col-0) and MsSNAT-transgenic lines (MsSNAT-1 and MsSNAT-2) were surface-sterilized and rinsed three times with sterile water, then plated on solid half-strength Murashige–Skoog (MS) medium containing 1 % sucrose and 1 % agar (pH 5.8). Plates were kept at 4 °C for 2 d and then transferred into a growth chamber with a 16/8 h (23/21 °C) day/night regime with 120 μmol m−2 s−1 irradiation (Chen et al., 2017; Gu et al., 2017).
Chemicals and treatments
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless stated otherwise. In the experimental conditions, 3-d-old alfalfa seedlings were treated with 0, 1.0, 10, 50, 100 or 200 μm melatonin (Mel), or treated with or without 1 μm diphenyleneiodonium (DPI) and/or 0.5 mmN,N′-dimethylthiourea (DMTU). The treatment time-points are illustrated in the corresponding figure legends. Control seedlings were grown in quarter-strength Hoagland solution alone. Uniform 4-d-old WT and MsSNAT transgenic seedlings (MsSNAT-1, MsSNAT-2) were chosen, and transferred to 0.1 μm DPI or 0.1 mm DMTU as described in the corresponding figure legends. Control seedlings were grown in half-strength MS medium alone.
After various treatments, photographs were taken and the number of emerged lateral roots (>1 mm) per seedling was recorded. Lateral root length, emerged lateral root density (number of lateral roots per centimetre of primary root) and primary root length were measured using Image J (supplied by NCBI and available at http://rsb.info.nih.gov/ij). Lateral root primordia were also observed in root squash preparations and the number per seedling was quantified with an optical microscope (Stemi 2000-C; Carl Zeiss, Germany). For the subsequent biochemical and molecular analyses, only lateral root-inducible segments (in the regions of root mature zone) were used. The shoots of seedlings were removed by cutting below the root–shoot junction, and the root apical meristems were cut off.
ROS detection
Reactive oxygen species in the maturation zone were detected using a laser scanning confocal microscope (LSCM; Leica Lasertechnik, Heidelberg, Germany; excitation at 488 nm, emission at 500–530 nm). After treatments, seedlings were incubated with 20 μm 2′,7′-dichlorofluorescein diacetate (H2DCFDA; Bright et al., 2006; Chen et al., 2017) in 20 mm HEPES/NaOH buffer (pH 7.5) in darkness (25 °C), followed by washing three times for 15 min each. Six individual samples were randomly selected and measured per treatment. Bright-field (BF) images corresponding to the fluorescent images are shown at the bottom right or left corners of Figs 2, 3 and 5. Fluorescence of ROS accumulation in roots (an area of ~250 000 μm2 in alfalfa and 50 000 μm2 in Arabidopsis) was quantified based on 20 overlapping confocal planes using the Leica software package. Fluorescence was expressed as relative fluorescence units (Xie et al., 2011).
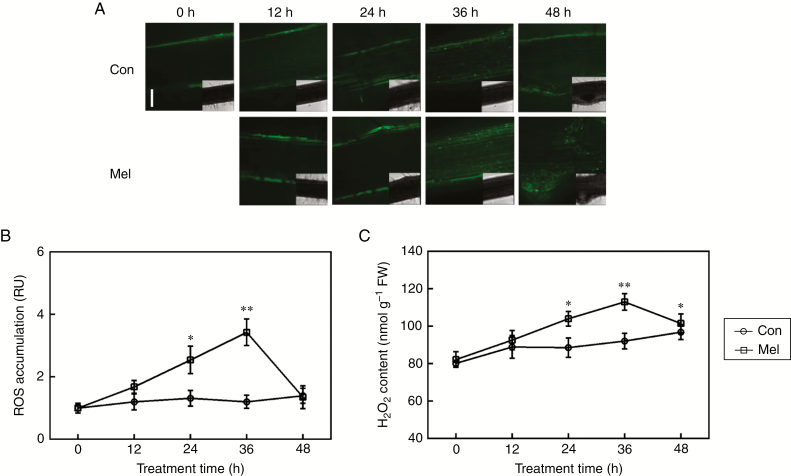
Fig. 2.
ROS generation is induced by melatonin. Three-day-old alfalfa seedlings were treated with or without 10 μm melatonin (Mel) for the indicated times. (A, B) Then, root tissues were loaded with 20 μm 2′,7′-dichlorofluorescein diacetate (H2DCFDA; a fluorescent dye for ROS) and regions of root mature zone were detected by LSCM. Scale bar (A) = 100 μm. Six individual samples were randomly selected and measured per treatment. Fluorescence is shown relative to control (Con) samples without added melatonin at 0 h. RU, relative units. (C) H2O2 content was determined by spectrophotography. FW, fresh weight. Means and standard errors were calculated from at least three independent experiments with at least three replicates for each. Asterisks denote significant differences in comparison with control (one-way ANOVA followed by Tukey’s multiple range test, *P < 0 .05, **P < 0 .01).
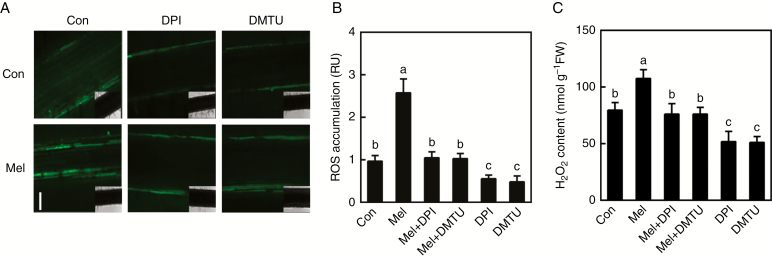
Fig. 3.
Melatonin-induced H2O2 generation is impaired by DPI or DMTU. Three-day-old alfalfa seedlings were treated with or without 10 μm melatonin (Mel), 1 μm DPI or 0.5 mm DMTU, or a combination of Mel with DPI or DMTU. (A, B) After treatment for 36 h, root tissues were loaded with 20 μm H2DCFDA and regions of root mature zone were detected by LSCM. Scale bar (A) = 100 μm. Fluorescence is shown relative to control samples (Con). RU, relative units. Six individual samples were randomly selected and measured per treatment. (C) H2O2 content was determined by spectrophotography. Means and standard errors were calculated from at least three independent experiments with at least three replicates for each. In (B) and (C) bars with different letters denote significant differences (one-way ANOVA followed by Tukey’s multiple range test, P < 0 .05).
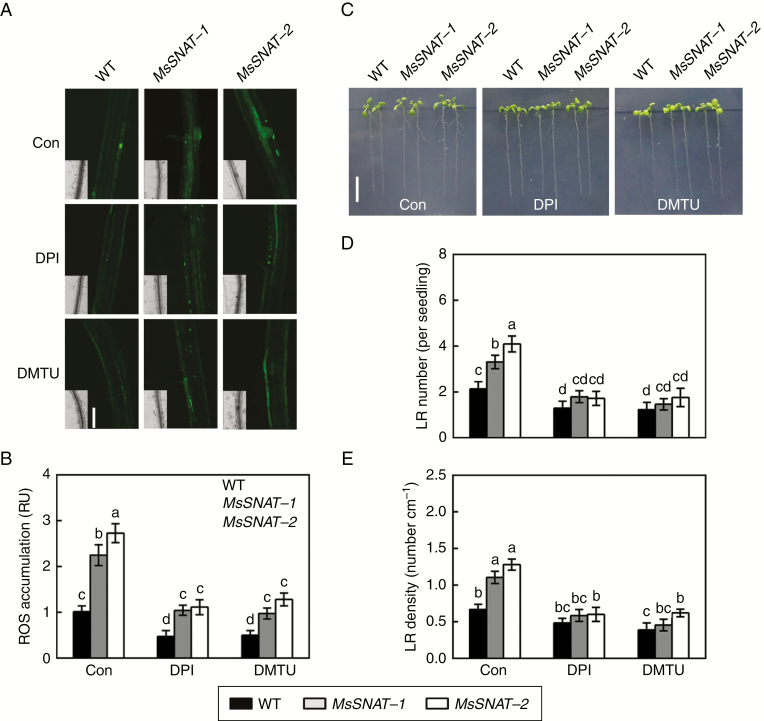
Fig. 5.
Genetic evidence showed that endogenous melatonin-induced ROS generation and thereafter lateral root formation are sensitive to DPI and DMTU. The SNAT gene of Medicago sativa (MsSNAT), encoding serotonin N-acetyltransferase, is homologous to the AtSNAT gene. Transgenic Arabidopsis lines overexpressing MsSNAT driven by the cauliflower mosaic virus were generated, and showed higher levels of endogenous melatonin (Gu et al., 2017). Four-day-old wild-type (WT) and transgenic seedlings (MsSNAT-1 and MsSNAT-2) were treated with or without 0.1 μm DPI or 0.1 mm DMTU. (A, B) After treatment for 36 h, root tissues were loaded with 20 μm H2DCFDA and regions of root mature zone were detected by LSCM. Fluorescence is shown as relative units (RU) of pixel intensity with reference to wild-type (Con). Scale bar (A) = 100 μm. Six individual plants were randomly selected and measured for each genotype per treatment. (C) Lateral root (LR) phenotypes after treatment for 5 d. Scale bar (C) = 1 cm. (D) Number of emerged LRs (>1 mm) per seedling. (E) Density of emerged LRs. Means and standard errors were calculated from at least three independent experiments with at least three replicates for each. Bars with different letters (B, D, E) denote significant differences (one-way ANOVA followed by Tukey’s multiple range test, P < 0 .05).
Quantitative analysis of H2O2 was performed according to a previous method (Ma et al., 2014). Regions of root mature zone (0.2 g) were ground with a mortar and pestle and extracted into 2 mL of 0.2 m HClO4 on ice. The combined extracts were then centrifuged (about 10 000 rpm, 4 °C) for 15 min. Briefly, an aliquot of supernatant (500 μL) was added to 500 μL of assay reagent (0.5 mm ammonium ferrous sulphate, 50 mm H2SO4, 0.2 mm xylenol orange and 200 mm sorbitol). The absorbance at 560 nm was determined after 1 h of incubation in darkness (25 °C). Standard curves were obtained by adding different amounts of H2O2.
Gene expression analysis
Total RNA was extracted from the maturation zone of roots using a TransZol Up Kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. RNA concentration and quality were checked using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). cDNAs were synthesized from 1 μg of total RNA using an EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix System (TransGen Biotech, Beijing, China).
By using the gene-specific primers (Supplementary Data Table S1), real-time quantitative reverse transcription (RT) PCR was conducted using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with TransStart® Green qPCR SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. The expression levels of corresponding genes were normalized against an internal control gene in alfalfa and Arabidopsis seedlings (MSC27 and Atactin7, respectively). The data were based on three independent biological replicates, and each sample was prepared in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software. Means and standard errors were calculated from at least three independent experiments with at least three biological replicates for each. For statistical analysis, data were analysed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test, and P values <0.05 or <0.01 were considered statistically significant.
RESULTS
Exogenous melatonin-induced lateral root formation in alfalfa seedlings
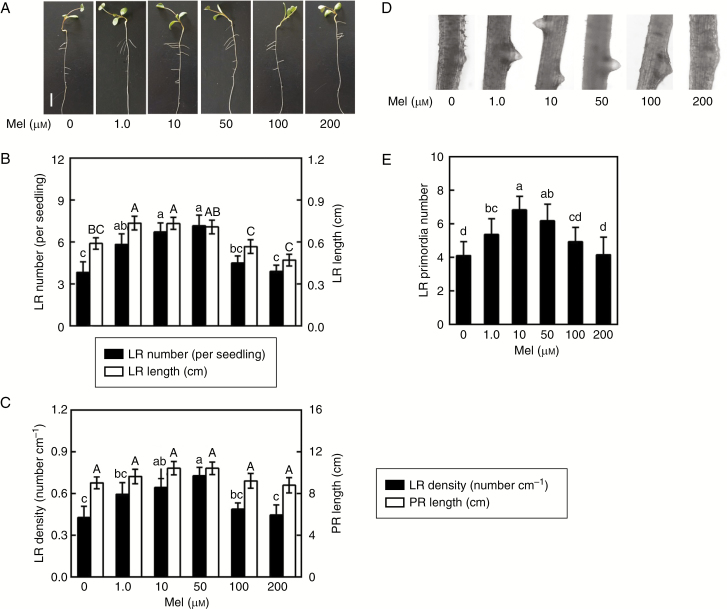
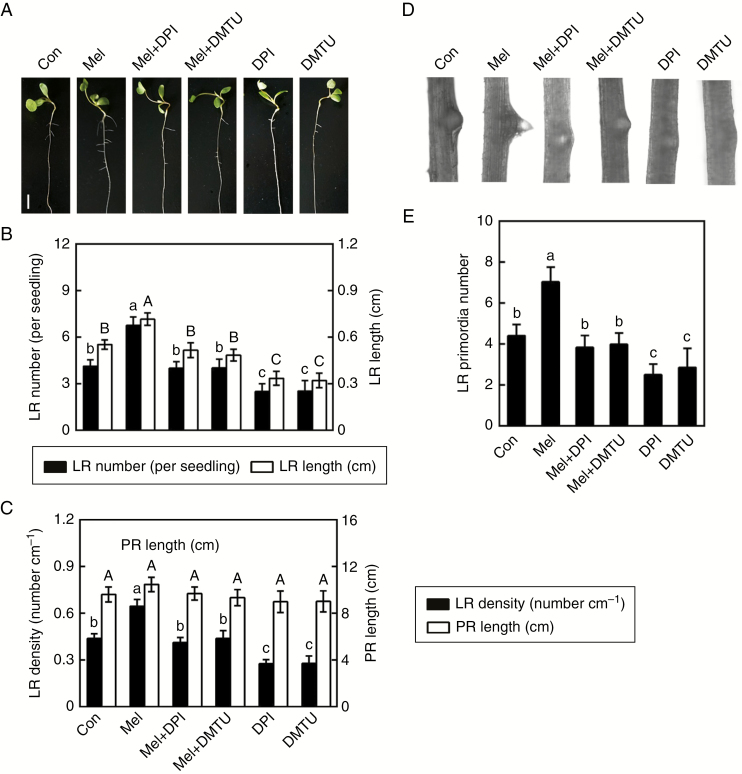
To assess the role of exogenous melatonin in the regulation of lateral root formation in alfalfa seedlings, a dose–response study of melatonin in vitro was performed. As expected, the results shown in Fig. 1A–C indicate that the addition of exogenous melatonin (1.0, 10, and 50 μm) could bring about significant increases in lateral root number and length (Fig. 1B) and lateral root density (Fig. 1C), while the changes in primary root length were not so obvious. Responses to 10 and 50 μm melatonin were maximal. A similar accelerated response in lateral root primordia was also observed (Fig. 1D, E). High concentrations (100 and 200 μm) of melatonin failed to induce lateral root formation. Considering the above results and the cost of chemicals, 10 μm melatonin was used in the following experiments.
Fig. 1.
Melatonin induces lateral root (LR) formation. Three-day-old alfalfa seedlings were treated with 0, 1.0, 10, 50, 100 or 200 μm melatonin (Mel). (A) Lateral root phenotypes after treatment for 5 d. Scale bar = 1 cm. (B) Number of emerged LRs (>1 mm) per seedling and LR length. (C) Density of emerged LRs and primary root (PR) length. (D, E) After treatment for 60 h, photographs of LR primordia formation were taken and the number of LR primordia was recorded. The sample without added melatonin was the control. Means and standard errors were calculated from at least three independent experiments with at least three replicates for each. Within each set of experiments (B, C, E), bars with different letters denote significant differences (one-way ANOVA followed by Tukey’s multiple range test, P < 0.05).
H2O2 accumulation in response to melatonin
To unravel the molecular mechanism underlying melatonin-mediated lateral root formation, the levels of endogenous H2O2, a well-known signalling molecule responsible for lateral root development, were tested. Seedlings were loaded with the ROS-specific fluorescent dye H2DCFDA, and an LSCM was used to investigate changes in ROS-related fluorescence. Time-course analysis revealed that the accumulation of ROS in the maturation zone was induced in melatonin-treated roots, with a strong and substantial peak at 36 h over a 48-h period (Fig. 2A, B). Further analysis using spectrophotography indicated that exogenous melatonin elicited an increase in H2O2 content as well (Fig. 2C). These results suggest that the melatonin-induced dichlorofluorescein-dependent fluorescence was, at least partially, caused by endogenous H2O2.
Melatonin-induced lateral root formation is impaired by removal of endogenous H2O2
Subsequent work investigated the causal link between melatonin and H2O2 in lateral root development, using the NADPH oxidase inhibitor DPI and the H2O2 scavenger DMTU. In the experimental conditions, both 1 μm DPI and 0.5 mm DMTU dramatically blocked the induction of endogenous H2O2 content triggered by melatonin, determined by fluorescence analysis and spectrophotography (Fig. 3). For example, the presence of DPI or DMTU caused significant decreases in H2O2 accumulation by ~59.5 % and ~60.5 % (determined by LSCM) in melatonin-treated seedlings, compared with melatonin alone (Fig. 3A, B). H2O2 content, checked by using spectrophotography, showed a similar tendency (Fig. 3C).
Consistently, exogenous melatonin-triggered lateral root formation was apparently impaired by the presence of DPI or DMTU in alfalfa seedlings (Fig. 4A–C). Microscopic analysis showed that the inducing effects of melatonin on lateral root primordia could be prevented by DMTU or DPI (Fig. 4D, E). Combined with endogenous H2O2 accumulation (Fig. 3), these pharmacological tests indicated that H2O2 might be required for melatonin-induced lateral root formation in alfalfa seedlings. It was noticed that DPI or DMTU alone not only decreased the corresponding fluorescence (Fig. 3), but also inhibited lateral root formation (Fig. 4).
Fig. 4.
Melatonin-induced lateral root (LR) formation is inhibited by DPI and DMTU. Three-day-old alfalfa seedlings were treated with or without 10 μm melatonin (Mel), 1 μm DPI or 0.5 mm DMTU, or a combination of Mel with DPI or DMTU. (A) Lateral root phenotypes after treatment for 5 d. Scale bar = 1 cm. (B) Number of emerged LRs (>1 mm) per seedling and LR length. (C) Density of emerged LRs and primary root (PR) length. (D, E) After treatment for 60 h, photographs of LR primordia formation were taken and the number of LR primordia was recorded. The sample without added chemicals was the control (Con). Means and standard errors were calculated from at least three independent experiments with at least three replicates for each. Within each set of experiments (B, C, E), bars with different letters denote significant differences (one-way ANOVA followed by Tukey’s multiple range test, P < 0 .05).
Genetic evidence confirmed that endogenous melatonin-induced lateral root formation was H2O2-dependent
To further explore the function of endogenous melatonin in plants, MsSNAT was overexpressed in transgenic Arabidopsis under the control of a CaMV 35S promoter (Gu et al., 2017). Two transgenic lines (MsSNAT-1 and MsSNAT-2) were used to investigate the effect of MsSNAT on lateral root formation. The dichlorofluorescein-dependent fluorescence analysis revealed the accumulation of ROS in MsSNAT-1 and MsSNAT-2 plants, compared with the wild-type (Fig. 5A, B). Pharmacological tests further showed that the ROS level was less impaired in two transgenic lines compared with wild-type when DPI or DMTU was added. These results further confirmed that the fluorescence was mainly caused by endogenous H2O2. Further results revealed that transgenic lines had more lateral roots than the wild-type seedlings, indicating that endogenous melatonin stimulated lateral root formation (Fig. 5C–E). By contrast, lateral root number and density were seriously inhibited when transgenic lines were exposed to DPI or DMTU. These results clearly suggest that endogenous melatonin-induced lateral root formation was, at least partially, H2O2-dependent.
Expression of cell cycle regulatory genes
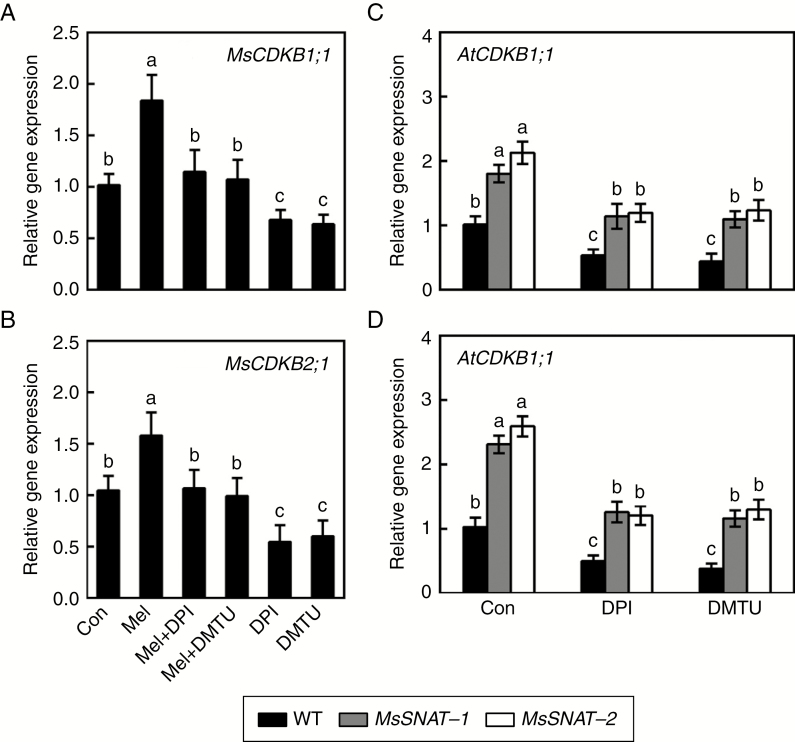
To further investigate the corresponding molecular mechanism, the transcript levels of two cell cycle regulatory genes related to lateral root formation, namely CDKB1;1 and CDKB2;1, were examined in alfalfa and Arabidopsis seedlings. Results shown in Fig. 6A, B reveal that expression of MsCDKB1;1 and MsCDKB2;1 was induced significantly in alfalfa seedlings treated with exogenous melatonin, and that both effects were obviously reversed when DPI or DMTU was applied. DPI and DMTU alone differentially inhibited MsCDKB1;1 and MsCDKB2;1 expression.
Fig. 6.
Melatonin affects expression profiles of cell cycle regulatory genes in alfalfa and transgenic Arabidopsis seedlings. (A, B) Three-day-old alfalfa seedlings were treated with or without 10 μm melatonin (Mel), 1 μm DPI or 0.5 mm DMTU, or combinations of Mel with DPI or DMTU. After treatment for 36 h, transcript levels of MsCDKB1;1 (X97315; A) and MsCDKB2;1 (X97317; B) in the mature zone of the root were analysed by real-time RT–PCR. (C, D) Four-day-old wild-type (WT) and transgenic seedlings were treated with or without 0.1 μm DPI or 0.1 mm DMTU. After treatment for 36 h, AtCDKB1;1 (At3g54180; C) and AtCDKB2;1 (At1g76540; D) transcript levels in the mature root zone were analysed by real-time RT–PCR. Expression levels are shown relative to control samples after normalization to alfalfa MSC27 (X63872) and Arabidopsis Atactin7 (At5g09810). Means and standard errors were calculated from three independent experiments with three replicates for each. Bars with different letters denote significant differences (one-way ANOVA followed by Tukey’s multiple range test, P < 0 .05).
Further genetic evidence showed that AtCDKB1;1 and AtCDKB2;1 were upregulated in the transgenic lines compared with wild-type (Fig. 6C, D). We also noticed that the expression of these two genes decreased to a greater degree when DPI or DMTU was added, in both the transgenic lines and in the wild-type. Combined with the changes in ROS levels (Figs 3 and 5) and corresponding phenotypes (Figs 4 and 5), it can be deduced that H2O2 might be the downstream messenger of melatonin signalling responsible for lateral root formation by modulating the expression of cell cycle regulatory genes.
DISCUSSION
It is well established that melatonin fulfils many important roles in plants, and it is proposed to be an important regulator in controlling root development, including the induction of lateral root formation in Lupinus albus (Arnao and Hernández-Ruiz, 2007), rice (Liang et al., 2017), cucumber (Zhang et al., 2013, 2014) and Arabidopsis (Pelagio-Flores et al., 2012). In this study, by using pharmacological, genetic and molecular approaches we extended the previous results and further discovered that (1) endogenous melatonin might modulate lateral root formation, and (2) H2O2 might be involved in melatonin-induced lateral root formation via modulating the expression of cell cycle regulatory genes.
Lateral root formation might be induced by endogenous melatonin
This report provides evidence that the application of exogenous melatonin was able to induce alfalfa lateral root formation, confirmed by changes in lateral root number, lateral root length and lateral root density (Fig. 1). Importantly, 10 and 50 μm melatonin exhibited maximal responses. Microscopic analyses of lateral root primordia shown in Fig. 1D, E supported the above results. This finding was consistent with previous findings in cucumber, rice and Arabidopsis (Pelagio-Flores et al., 2012; Zhang et al., 2013, 2014; Liang et al., 2017). Comparatively, the most effective concentration(s) of melatonin in plants were different, which might be explained by different plant species or treatment time-points. In addition, although melatonin can act as a potential modulator of plant growth and development, high concentrations of melatonin (100 and 200 μm) had no obvious effect (Fig. 1) and even suppressed cell proliferation and endoreduplication in Arabidopsis (Wang et al., 2017). These results suggest that the beneficial role of exogenous melatonin in plants might act within a narrow dose range.
Since the application of exogenous melatonin may not completely mimic the function of endogenous melatonin, two transgenic Arabidopsis lines, MsSNAT-1 and MsSNAT-2, showing high levels of melatonin (Gu et al., 2017), were used. Consistently, MsSNAT-1 and MsSNAT-2 transgenic lines had more lateral roots than wild-type (Fig. 5C–E). The above pharmacological and genetic evidence indicates that lateral root formation might be regulated by endogenous melatonin.
Involvement of H2O2 in melatonin-induced lateral root formation
Previous results showed that ROS signalling was specifically required during lateral root emergence in Arabidopsis, tomato and rice (Chen et al., 2013; Cao et al., 2014; Ma et al., 2014; Manzano et al., 2014). It is well established that melatonin and H2O2 have similar physiological roles in root organogenesis. Therefore, it is most likely that there is interaction in the process of lateral root formation.
In this subsequent study, time-course analyses by using LSCM and spectrophotography revealed that exogenous melatonin could simultaneously induce H2O2 generation in alfalfa seedlings (Fig. 2; reaching a maximum at 36 h of treatment). Additionally, MsSNAT-1 and MsSNAT-2 transgenic lines had higher endogenous H2O2 contents compared with the wild-type (Fig. 5A, B). Combined with the corresponding phenotypes shown in Figs 1 and 5C–E, we speculate that there is a potential interrelationship between melatonin and H2O2 during lateral root formation.
Further pharmacological and microscopical evidence revealed the requirement for endogenous H2O2 in the induction of lateral root formation triggered by melatonin. This conclusion is based on several pieces of evidence. (1) Exogenously applied DPI (NADPH oxidase inhibitor) or DMTU (H2O2 scavenger) inhibited exogenous melatonin-induced ROS accumulation determined by fluorescence analysis and spectrophotography (Fig. 3). (2) Melatonin-triggered lateral root formation was obviously impaired by DPI or DMTU in alfalfa (Fig. 4). (3) Similar to the beneficial responses to exogenous melatonin, the removal of endogenous ROS by using DPI or DMTU obviously blocked melatonin-triggered lateral root formation in wild-type and, in particular, transgenic plants (Fig. 5), confirming the possible role of H2O2 in root organogenesis elicited by melatonin. This deduction was consistent with the recent discovery that lateral root emergence was modulated by ROS accumulation in Arabidopsis (Orman-Ligeza et al., 2016). Related signalling receptor molecules should be elucidated in future work.
Cell cycle reactivation might be mediated by H2O2 through regulation of the expression of multiple cell cycle genes in early lateral root initiation (Himanen et al., 2004). Alfalfa MsCDKB1;1 and MsCDKB2;1 belong to the plant-specific CDK class, and MsCDKB2;1 was also activated as a consequence of wounding and treatment with ethephon in a non-cell cycle-dependent fashion (Zhiponova et al., 2006). The subsequent experiment found that the expression of above two cell cycle genes was upregulated by exogenous melatonin (Fig. 6A, B). These inducing effects were impaired by treatment with DPI, or DMTU, which was consistent with the reduced H2O2 levels (Fig. 3) and subsequently decreased lateral root formation in alfalfa seedlings (Fig. 4). Genetic evidence further revealed that, compared with the wild-type, the expression of AtCDKB1;1 and AtCDKB2;1 transcripts was upregulated in transgenic seedlings (Fig. 6C, D), while these increased transcript levels were significantly reversed by DPI or DMTU treatment. These results are consistent with the changes in lateral root formation (Fig. 5C–E). Therefore, this study clearly demonstrates that cell cycle regulatory genes might be the target genes of the action of H2O2 triggered by melatonin, thus leading to lateral root development. In agreement with the above results, an RNA-seq approach revealed that root development was modulated by melatonin via the ROS system in cucumber (Zhang et al., 2014). A recent study has indicated that Arabidopsis root development processes elicited by melatonin are likely independent of auxin responses (Pelagio-Flores et al., 2012). By contrast, Liang et al (2017) suggested that melatonin shaped root architecture by activating the auxin signalling pathway in rice. Previous findings also showed a close interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis (Ma et al., 2014). Thus, whether the auxin signalling is involved in the above process should be elucidated in the future.
In summary, the ability of endogenous melatonin to induce lateral root formation is a new finding. Further pharmacological and genetic evidence demonstrated that H2O2 signalling might be required for melatonin-induced lateral root development, and that the regulation of cell cycle regulatory gene expression might be an indispensable and crucial strategy in this process (Fig. 7). Thus, the above results will open a new window to the understanding of molecular mechanisms related to lateral root formation induced by melatonin.
Fig. 7.
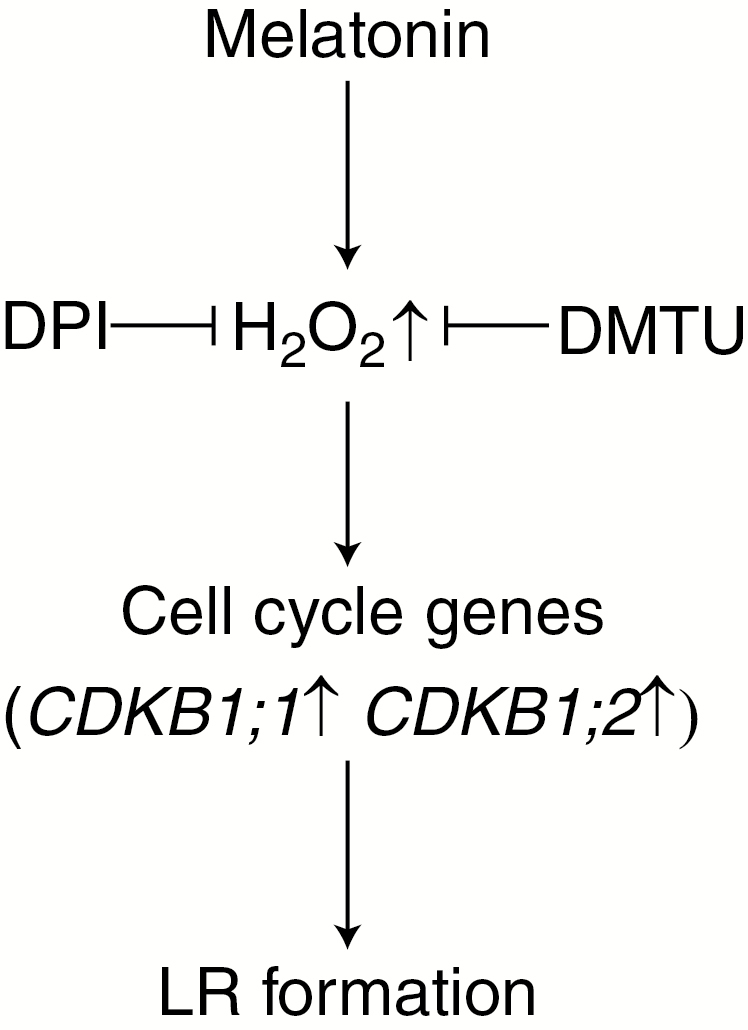
Schemolving endogenous H2O2 during lateral root (LR) formation triggered by melatonin. Cell cycle genes (CDKB1;1, CDKB1;2) are involved in this response. T bars indicate inhibition.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: sequences of primers for real-time RT–PCR.
ACKNOWLEDGEMENTS
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20141361), Scientific Innovation Research of College Graduate in Jiangsu Province (KYZZ15_0180), the National Natural Science Foundation of China (31201617), the Fundamental Research Funds for the Central Universities (KYTZ201402) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
LITERATURE CITED
- Aloni R, Aloni E, Langhans M, Ullrich CI. 2006. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany 97: 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J. 2007. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. Journal of Pineal Research 42: 147–152. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernandez-Ruiz J. 2015. Function of melatonin in plants: a review. Journal of Pineal Research 59: 133–150. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. 2006. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant Journal 45: 113–122. [DOI] [PubMed] [Google Scholar]
- Byeon Y, Back K. 2014. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. Journal of Pineal Research 56: 189–195. [DOI] [PubMed] [Google Scholar]
- Cao Z, Fang T, Chen M, Li J, Shen W, Huang L. 2014. Involvement of haem oxygenase-1 in hydrogen peroxide-induced lateral root formation in tomato. Acta Physiologiae Plantarum 36: 931–943. [Google Scholar]
- Cao ZY, Xuan W, Liu ZY et al. 2007. Carbon monoxide promotes lateral root formation in rapeseed. Journal of Integrative Plant Biology 49: 1070–1079. [Google Scholar]
- Casimiro I, Beeckman T, Graham N et al. 2003. Dissecting Arabidopsis lateral root development. Trends in Plant Science 8: 165–171. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chao YY, Hsu YY, Kao CH. 2013. Heme oxygenase is involved in H2O2-induced lateral root formation in apocynin-treated rice. Plant Cell Reports 32: 219–226. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xie Y, Gu Q et al. 2017. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radical Biology and Medicine 108: 465–477. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L. 2006. Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. Journal of Experimental Botany 57: 581–588. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Cejudo FJ, Lamattina L. 2015. Nitric oxide is required for the auxin-induced activation of NADPH-dependent thioredoxin reductase and protein denitrosylation during root growth responses in arabidopsis. Annals of Botany 116: 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T, Cao Z, Li J, Shen W, Huang L. 2014. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiology and Biochemistry 76: 44–51. [DOI] [PubMed] [Google Scholar]
- Feher A, Ötvös K, Pasternak TP, Pettkó-Szandtner A. 2008. The involvement of reactive oxygen species (ROS) in the cell cycle activation (G0-to-G1 transition) of plant cells. Plant Signaling & Behavior 3: 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF et al. 2003. Reactive oxygen species produced by NADPH oxidases regulate plant cell growth. Nature 422: 442–446. [DOI] [PubMed] [Google Scholar]
- Gong B, Yan Y, Wen D, Shi Q. 2017. Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiologia Plantarum 160: 396–409. [DOI] [PubMed] [Google Scholar]
- Gu Q, Chen Z, Yu X et al. 2017. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Science 261: 28–37. [DOI] [PubMed] [Google Scholar]
- Guo K, Xia K, Yang ZM. 2008. Regulation of tomato lateral root development by carbon monoxide and involvement in auxin and nitric oxide. Journal of Experimental Botany 59: 3443–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QH, Huang B, Ding CB et al. 2017. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Frontiers in Plant Science 8: 785. doi: 10.3389/fps.2017.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MK, Ahammed GJ, Yin L et al. 2015. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Frontiers in Plant Science 6: 601. doi: 10.3389/fps.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Migitaka H, Iigo M et al. 1995. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochemistry and Molecular Biology International 35: 627–634. [PubMed] [Google Scholar]
- Himanen K, Vuylsteke M, Vanneste S et al. 2004. Transcript profiling of early lateral root initiation. Proceedings of the National Academy of Sciences of the USA 101: 5146–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y, Koda Y, Zheng SH, Yuasa T, Iwaya-Inoue M. 2013. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Annals of Botany 111: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Napsucialy-Mendivil S, Dubrovsky JG. 2010. Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. Plant Journal 64: 740–752. [DOI] [PubMed] [Google Scholar]
- Jiang C, Belfield EJ, Cao Y, Smith JAC, Harberd NP. 2013. An Arabidopsis soil-salinity-tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis. Plant Cell 25: 3535–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama FC, Carvalho TLG, Alves E et al. 2013. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. Journal of Eukaryotic Microbiology 60: 646–651. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM et al. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO Journal 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Zawadzka A, Czarnocki Z, Reiter RJ, Back K. 2016. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). Journal of Pineal Research 61: 470–478. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. 1958. Isolation of melatonin, a pineal gland factor that lightens melanocytes. Journal of the American Chemical Society 80: 2587–2587. [Google Scholar]
- Liang C, Li A, Yu H et al. 2017. Melatonin regulates root architecture by modulating auxin response in rice. Frontiers in Plant Science 8: 134. doi: 10.3389/fpls.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, He J, Yang X et al. 2016. Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L.). Journal of Pineal Research 60: 206–216. [DOI] [PubMed] [Google Scholar]
- Li MQ, Hasan M, Li CX et al. 2016. Melatonin mediates selenium‐induced tolerance to cadmium stress in tomato plants. Journal of Pineal Research 61: 291–302. [DOI] [PubMed] [Google Scholar]
- Ma F, Wang L, Li J et al. 2014. Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Molecular Biology 85: 49–61. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. 2001. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiology 127: 899–909. [PMC free article] [PubMed] [Google Scholar]
- Manzano C, Pallero M, Casimiro I et al. 2014. The emerging role of ROS signaling during lateral root development. Plant Physiology 165: 1105–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2010. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell and Environment 33: 453–467. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N et al. 2011. ROS signaling: the new wave. Trends in Plant Science 16: 300–309. [DOI] [PubMed] [Google Scholar]
- Orman-Ligeza B, Parizot B, De Rycke R et al. 2016. RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143: 3328–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelagio-Flores R, Muñoz-Parra E, Ortiz-Castro R, López-Bucio J. 2012. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. Journal of Pineal Research 53: 279–288. [DOI] [PubMed] [Google Scholar]
- Radogna F, Paternoster L, Nicola MD et al. 2009. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicology and Applied Pharmacology 239: 37–45. [DOI] [PubMed] [Google Scholar]
- Rosales-Corral SA, Acuña-Castroviejo D, Coto-Montes A et al. 2012. Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. Journal of Pineal Research 52: 167–202. [DOI] [PubMed] [Google Scholar]
- Shi H, Chen Y, Tan DX, Reiter RJ, Chan Z, He C. 2015. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. Journal of Pineal Research 59: 102–108. [DOI] [PubMed] [Google Scholar]
- Stals H, Inzé D. 2001. When plant cells decide to divide. Trends in Plant Science 6: 359–364. [DOI] [PubMed] [Google Scholar]
- Stehle JH, Saade A, Rawashdeh O et al. 2011. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. Journal of Pineal Research 51: 17–43. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences of the USA 99: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest A, Weinl C, Inzé D, De Veylder L, Schnittger A. 2005. Switching the cell cycle. Kip-related proteins in plant cell cycle control. Plant Physiology 139: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, An B, Shi H, Luo H, He C. 2017. High concentration of melatonin regulates leaf development by suppressing cell proliferation and endoreduplication in Arabidopsis. International Journal of Molecular Science 18: 991. doi: 10.3390/ijms18050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Xu S, Han B et al. 2011. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant Journal 66: 280–292. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhao B, Zhang HJ et al. 2013. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). Journal of Pineal Research 54: 15–23. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang HJ, Zhao B et al. 2014. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. Journal of Pineal Research 56: 39–50. [DOI] [PubMed] [Google Scholar]
- Zhiponova MK, Pettkó-Szandtner A, Stelkovics É et al. 2006. Mitosis-specific promoter of the alfalfa cyclin-dependent kinase gene (Medsa;CDKB2;1) is activated by wounding and ethylene in a non-cell division-dependent manner. Plant Physiology 140: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo B, Zheng X, He P et al. 2014. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. Journal of Pineal Research 57: 408–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.