Abstract
Epidemiologic studies have demonstrated that women account for two-thirds of Alzheimer’s disease (AD) cases, for which the decline in circulating gonadal hormone is considered to be one of the major risk factors. In addition, ovarian hormone deficiency may affect β-amyloid (Aβ) deposition, which has a close relationship with autophagic flux. In this study, we investigated the impact of short-term or long-term ovarian hormone deprivation on two mouse models, the non-transgenic (wild-type) and the APP/PS1 double-transgenic AD (2×TgAD) model. Autophagy-related proteins (Beclin1, LC3, and p62) and lysosome-related proteins were detected to evaluate Aβ deposition and autophagy. Our results showed that in the group with short-term depletion of ovarian hormones by ovariectomy (ovx), Beclin1, Cathepsin B (Cath-B), and LAMP1 levels were significantly decreased, while the levels of LC3-II and p62 were increased. In the long-term group, however, there was a sharp decline in Beclin1, LC3-II, Cath-B, and LAMP1 expression but not in p62 expression which is increased. It is worthwhile to note that the occurrence of neuritic plaque-induced ovarian hormone loss increased both the Aβ level and neuritic plaque deposition in 2×TgAD mice. Therefore, autophagy may play an important role in the pathogenesis of female AD, which is also expected to help post-menopausal patients with AD.
Keywords: Alzheimer’s disease, transgenic mice, neuritic plaques, ovariectomy, autophagy
Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder characterized by intracellular accumulation of β-amyloid (Aβ) and extracellular senile plaque (SP) deposition. With a rapidly aging population in the world, the incidence of AD is rising quickly. The Alzheimer’s Association estimates that the number of persons with dementia is increasing annually, and every 33 s a new AD case appears [1]. More than 46 million people currently live with dementia worldwide, and this number is predicted to triple by 2050 [2]. Among AD patients, two-thirds are women, and this phenomenon is ever-present in both prevalence and severity [3,4]. Previous studies have shown that female patients suffer a faster deterioration than males [5], especially post-menopausal women, whose circulating ovarian hormone concentrations gradually decrease as age increases [6,7]. Epidemiological analyses have revealed that women who received hormone therapy in their perimenopausal period are at a lower risk for AD [8], while those untreated are more likely to suffer from AD [9,10]. A growing number of reports suggest that ovarian hormones [11,12], including E2 and progesterone, play a neuroprotective role. However, the effect of sex hormone treatment for AD still remains to be explored [13].
Aβ, such as intraneuronal Aβ1–42, is commonly found in mild cognitive impairment or Alzheimer’s patients and is regarded as a pathological hallmark of AD [14]. A high Aβ level is often accompanied by an increase in production as well as defective clearance [14,15]. Autophagic vacuoles are considered to be the source of Aβ production [19,20], and evidence has shown that autophagy dysregulation occurs in both AD patients and animal models of AD [16–18]. Recent studies have also shown that the formation of Aβ plaques may result in autophagy−lysosome pathway disorder, which plays an important role in the intracellular clearance of damaged organelles and misfolded proteins [21,22]. Ovarian hormone loss may contribute to the development of AD pathogenesis in menopausal females through regulation of autophagic activity.
Macroautophagy (‘self-eating’ or ‘autophagy’) is an ongoing catabolic process that occurs in physiologic conditions in various cells to maintain protein metabolism. Autophagy is a kind of intracellular degradation involving several steps, such as sequestration of cytosolic proteins into double-membrane vesicles, transportation of autophagosomes (which fuse with lysosomes either directly or after a prior fusion with endosomes and subsequently cause the formation of amphisomes), and degradation and reutilization in lysosomes, which is perturbed in neurodegenerative diseases such as AD [23,24]. Beclin1, also known as Atg6, plays an important role in membrane isolation and nucleation, contributing to the formation of early autophagosomes [25]. An appearance of autophagosomes proves the induction of autophagy [26]. The upregulated level and cytosol-to-vesicle translocation of LC3-II are widely used as a specific marker of autophagosome formation and play a key role in phagophore elongation [27]. LC3-I and phosphatidylethanolamine are conjugated to produce lipidated LC3-II, which can induce elongation and closure of autophagosomal membranes and can bind to the autophagosome and fuse with lysosomes [28,29]. SQSTM1/p62, with multiple protein–protein interaction motifs, binds with both LC3 and polyubiquitinated proteins to recruit protein cargo into autophagosomes which are then degraded as aggregates [30–32]. SQSTM1/p62, as a marker of lysosomal protein degradation, is usually found in ubiquitinated inclusion bodies and can be quickly accumulated when autophagy flux is disturbed [33]. Cathepsin B (Cath-B) is a cysteine lysosomal hydrolase, which has been found to cleave Aβ1–42 into less amyloidogenic species [34]. Neurons in AD-vulnerable subfields can take up extracellular Aβ1–42 and be sequestered into lysosomes [35]. Cath-B is a major lysosomal protease that can control AD-like amyloidogenic processing and is involved in the degradation of Aβ [36]. Thus, lysosomal cathepsin activity may be important for the clearance of Aβ.
In the present study, we sought to determine the impact of short-term or long-term deprivation of ovarian hormones on autophagy in 2×TgAD female mouse brains and investigated the effect of ovarian hormone loss on Aβ. This study may provide essential information for the prevention and treatment of AD.
Materials and Methods
Transgenic mice
The animal care and experimental protocol used in this study were performed according to the guidelines of Laboratory Animals from Ethics Committee of Chongqing Medical University. Mice were kept in the individual ventilated cage room of Chongqing Medical University Animal Center at constant temperature, with food and water ad libitum. Heterozygous B6C3-Tg (APP swe/PS1ENdE9) mice that overexpress mouse/human amyloid precursor protein (Mo/Hu APP swe) and mutant human presenilin-1 (PS1-dE9) were used (Certificate No. 11401300010893; HFK Bio-Technology Co., Ltd, Beijing, China). Mice weaned on postnatal Day 28 were genotyped with tail biopsies by polymerase chain reaction using B40013 Mouse Direct PCR Kit for Rapid Genotyping (Bimake, Houston, USA). The wild-type (WT) littermates were used as normal controls. All efforts were made to minimize animal suffering and the number of animals used.
Experimental design and surgery
To investigate the change in autophagy and Aβ after removal of ovaries, 3-month-old female 2×TgAD and WT mice were randomly assigned to two groups (n = 6–8 per group): sham ovariectomized (sham) and ovariectomized (ovx). Mice were anesthetized with 3.5% chloral hydrate (0.1 ml/g, intraperitoneally) and fixed in the prone position to remove the ovaries. The ovaries of the sham operation animals were exposed but not removed. Animals were examined 1 week, 1 month, or 3 months after treatment.
Tissue preparation
Mice were sacrificed, and the cortex and hippocampus were isolated from each half of the brain. One half was stored at −80°C and homogenized for protein analysis as soon as possible. The other half was fixed in freshly prepared 4% paraformaldehyde overnight at 4°C, dehydrated in a gradient of 10%, 20%, and 30% sucrose in 0.1 M phosphate-buffered saline (PBS, pH 7.4) until it progressed through the sucrose gradient, and then embedded in tissue embedding medium (4583, OCT Compound; Sakura Finetek Tissue-Tek, Tokyo, Japan). A freezing microtome (CM1860; Leica, Wetzlar, Germany) was used to cut the brain into 10-μm thick coronal serial sections. All sections encompassing the hippocampus were collected serially (ten sections in 1 well) in 24-well plates filled with 0.01 M PBS. Two slices in every 100 μm were mounted onto one slide for immunohistochemical staining. All slides were stored at −20°C. The uteri were collected from all groups and weighed.
Immunohistochemistry analysis
The brain tissue sections were immunohistochemically stained according to a special kit (SA1020; BOSTER, Wuhan, China). The sections were immunostained with anti-pan Aβ antibody 4G8 (800704; BioLegend, San Diago, USA) at a 1:250 dilution overnight at 4°C. The next day, the primary antibody was tagged with a Cy3-conjugated goat anti-mouse IgG secondary antibody (11G05A, 1:200; BOSTER). Plaques were visualized using diaminobenzidine with a DAB Kit (ZLI-9018, solution 1:solution 2 = 1:20; ZSGB-BIO, Beijing, China) and images were captured with a digital microscope camera (DFC425 C; Leica). In addition, thioflavin S staining of plaques was performed with 1% thioflavin S, and the green fluorescence-stained plaques were visualized by a fluorescence microscope (Leica DM 4000; Leica).
ELISA
The protease inhibitor phenylmethanesulfonyl fluoride (PMSF) (ST506; Beyotime, Shanghai, China) was added to tissue samples to prevent Aβ degradation. The concentration of Aβ40 or Aβ42 was detected by an Amyloid β (1–40) or Amyloid β (1–42) Colorimetric ELISA Kit (27713 or 27711; Immuno-Biological Laboratories Co., Ltd, Fujioka, Japan) according to the manufacturer’s instructions. Briefly, 100 μl of the Aβ standard or the sample was added into each well and incubated the precoated plate overnight at 4°C. Wash the plate with the prepared wash buffer and remove all liquid. And then, pipette 100 μl of labeled antibody solution into the wells of test samples, diluted standard and test sample blank. After 1 h of incubation at 4°C, the plates were washed and then incubated with 100 μl of the stabilized chromogen for 30 min at room temperature. The reaction was terminated by addition of 100 μl of the stop solution. Finally, absorbance at 450 nm was measured with a microplate reader (Multiskan 1510; Thermo Fisher Scientific, Vantaa, Finland).
Western blot analysis
Brain tissue (cortex and hippocampus) was lysed in a mixture of radio immunoprecipitation assay (RIPA) lysis buffer (P0013B; Beyotime) and PMSF (RIPA:PMSF = 100:1, 100 mg tissue/1 ml mixture), and homogenized with an MT-30K homogenizer (MIU Instruments Co., Hangzhou, China) on ice. Homogenates were centrifuged twice at 12,000 g for 10 min, and the supernatant protein concentration was determined with a BCA Kit (P0010S; Beyotime). After addition of 5× sample loading buffer (P0015; Beyotime) and heating at 100°C for 5 min, the protein samples (40 μg per lane) were separated by 10% SDS-PAGE (P0012A; Beyotime), and transferred to polyvinylidene fluoride membranes (IJ-58; Millipore, Darmstadt, Germany). Membranes were blocked with blocking buffer (P0023B; Beyotime) and then incubated with the primary antibodies, including anti-Beclin1 (ab55878, 1:500; Abcam, Cambridge, USA), anti-LC3 (12741 T, 1:1000; Cell Signaling Technology, Boston, USA), anti-p62 (5114 S, 1:1000; Cell Signaling Technology), and anti-β-actin (AF0003, 1:1000; Beyotime) overnight at 4°C. After washing, membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated anti-rabbit/mouse IgG secondary antibody (SA0001-2, 1:5000; Proteintech, Chicago, USA). Immunoreactive bands were visualized using the WesternBright TM ECL Kit (K-12045-D10; Advansta, Menlo Park, USA) and captured by Molecular Imager ChemiDoc XRS System (731BR02996; Bio-Rad, Shanghai, China). All band intensities were quantified using Quantity One 1-D analysis software (Bio-Rad).
Immunofluorescent staining
The brain tissue sections were immunofluorescently stained according to a previously described method [37]. Briefly, sections were incubated with the primary antibodies anti-Cath-B (D1C7Y, 1:500; Cell Signaling Technology) and anti-LAMP1 (ab24170, 1:500; Abcam) overnight at 4°C. The next day, the samples were incubated with a Cy3-conjugated secondary antibody (P0193, 1:200; Beyotime) for 40 min at 37°C. After immunofluorescent labeling, nuclei were stained with 4,6-diamino-2-phenyl indole (DAPI) (Beyotime). Finally, the slides were covered with coverslips and examined by the fluorescence microscope. NIS-Elements Viewer 4.2 software was used to capture the images.
Statistical analysis
Data were presented as the mean ± SEM. Statistically significant differences were determined by two-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test (for Aβ level) or Student’s t-test (for other comparisons between sham and ovx). All statistical analyses were performed with the SPSS 17.0 software. Differences were considered significant at P < 0.05.
Results
Confirmation of ovarian hormone status
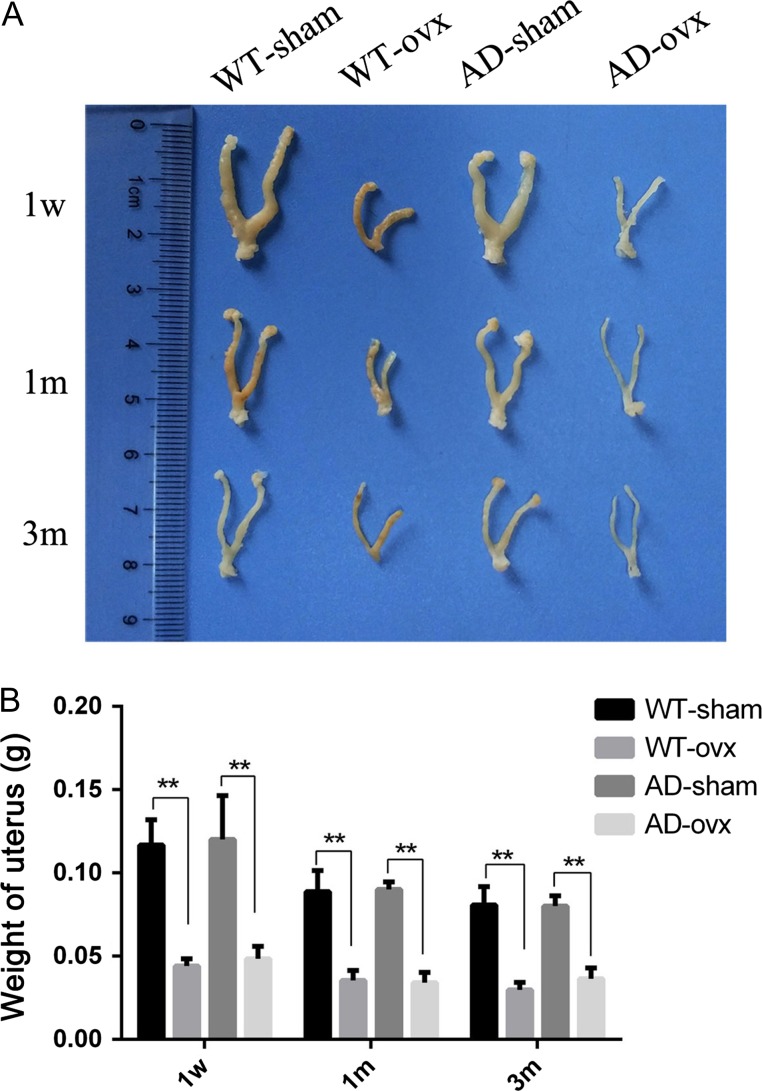
Six days after ovx in mice is equivalent to early post-menopause in humans [38], and 180 days after ovx in mice is similar to 10–20 years after menopause in women [39]. Therefore, we designed three experimental models (1-week, 1-month, and 3-month) to imitate human early post-menopause, middle post-menopause, and late post-menopause, respectively. In the current study, uterine weight was used as a bioassay and an independent indicator to confirm depletion of ovarian hormones in both female wild-type (WT) and transgenic AD (2×TgAD) mice [40]. In our experiment, in both WT and 2×TgAD mice, ovx-induced ovarian hormone depletion led to a significant decrease in uterine weight relative to the sham group (Fig. 1A,B), which suggests that the ovarian hormone loss model was successfully established.
Figure 1.
Ovx-induced decrease in uterine weight (A) Uteri images in all groups. (B) Quantification of the uterine weight. Results showed that the uterine weight of mice in the 1-week, 1-month, and 3-month ovx groups was decreased significantly compared with the corresponding sham groups. **P < 0.01. Data are presented as the mean ± SEM.
Ovarian hormone loss increases neuritic plaque deposition in 2×TgAD mice
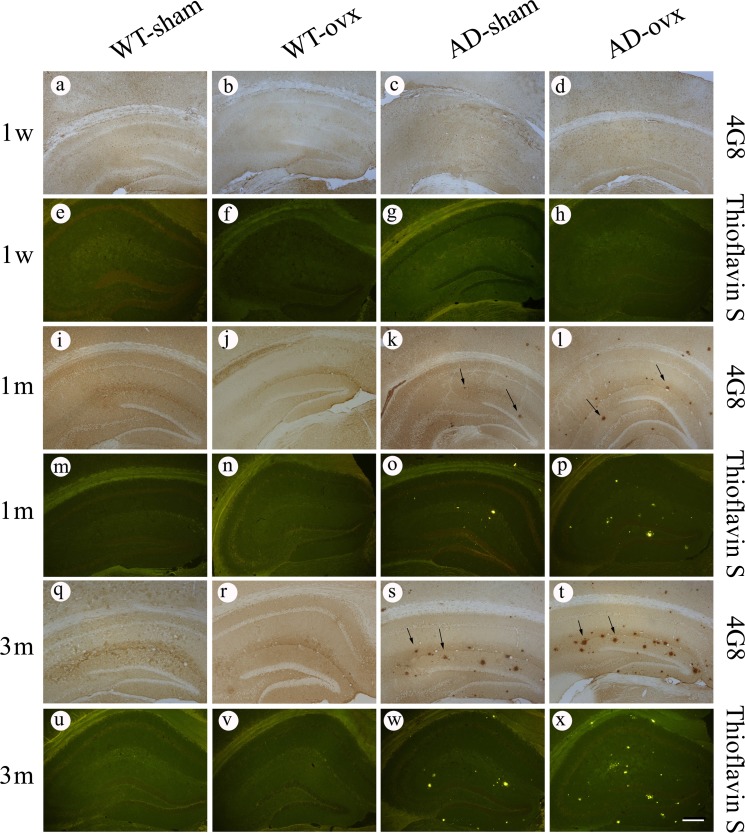
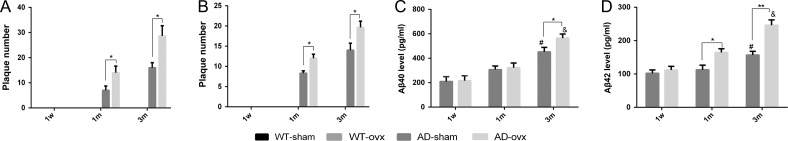
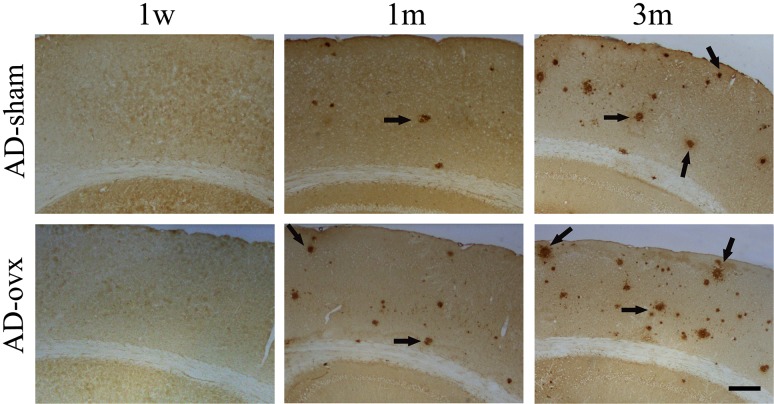
To investigate whether ovariectomy could result in the changes in Aβ deposition in vivo, 4G8 immunostaining and thioflavin S staining were used to detect Aβ-containing neuritic plaques in the brain (Fig. 2). No plaques were observed in any of the mouse brains in the WT groups. However, plaques were observed in 2×TgAD mice at 1 month and 3 months following the ovx operation as indicated by the 4G8 immunostaining (Fig. 2, k,l,s,t) and thioflavin S staining (Fig. 2, o,p,w,x). Quantification results showed that the number of plaques was significantly increased in the hippocampus of 1-month and 3-month AD-ovx mice compared with that in the AD-sham group (Fig. 3A,B). The same results were obtained in the cortex of 2×TgAD mice as in the hippocampus by 4G8 immunostaining (Fig. 4). These results revealed that middle and long-term ovarian hormone deprivation can increase Aβ neuritic plaque deposition in the brains of 2×TgAD mice.
Figure 2.
Effect of ovx on neuritic plaque deposition in the hippocampus of 2×TgAD mice 4G8 immunostaining (a–d, i–l, and q–t) and thioflavin S staining (e–h, m–p, and u–x) were used to detect neuritic plaques in the hippocampus. Black arrows point to plaques. In the WT mice, no plaques were observed, and ovx did not affect neuritic plaque deposition. However, a few neuritic plaques were observed in the brain sections of 1-month and 3-month AD groups. Scale bar: 200 μm.
Figure 3.
Aβ level in the brains of 2×TgAD mice (A) Quantification of the number of neuritic plaques immunostained by 4G8. (B) Quantification of the number of neuritic plaques stained by thioflavin S. (C) Aβ40 level in the transgenic brain tissues. (D) Aβ42 level in the transgenic brain tissues. *P < 0.05 and **P < 0.01. ***P < 0.05 vs. 1-month-AD-sham. ****P < 0.05 vs. 1-month-AD-ovx. A two-way ANOVA and a post hoc Bonferroni adjustment were used. Data are presented as the mean ± SEM.
Figure 4.
The effect of ovx on neuritic plaque deposition in the cortex of 2×TgAD mice 4G8 immunostaining was used to detect neuritic plaques in the cortex. In these 2×TgAD mice, similar results were obtained in the hippocampus. No plaques were observed in the 3-month-old mice (equal to 1 week after ovariectomy). Neuritic plaques were observed in the brain sections of 4-month and 6-month-old mice (equal to 1 month and 3 months after ovariectomy, respectively). Scale bar = 200 μm.
To further confirm the effect of ovarian hormone loss on AD pathogenesis, an ELISA assay was also performed to measure Aβ40 and Aβ42 levels in transgenic brain tissues (Fig. 3C,D). The results indicated that the ovx induced an increased level of Aβ40 in AD mouse brain, especially in 3m-ovx group. Meanwhile, ovx also led to an elevated Aβ42 level in AD mouse brain, starting from 1m-ovx group mice, and more obviously in 3m-ovx group mice.
Taken together, these data indicate that middle-term and long-term ovarian hormone deprivation can result in overproduction and deposition of Aβ in 2×TgAD brain tissues.
Ovariectomy-induced changes in autophagy-related proteins
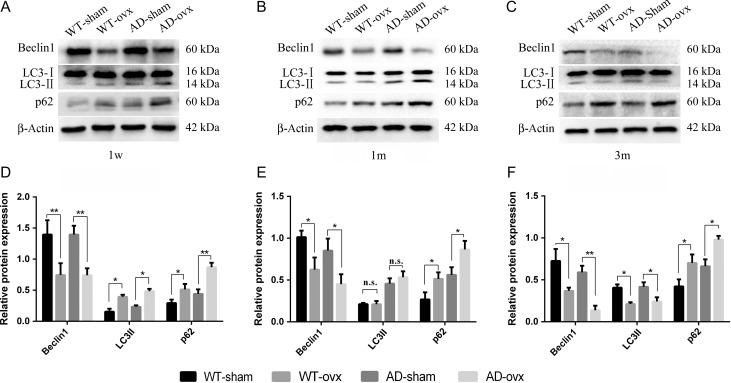
To further investigate the underlying autophagic function within ovarian hormone deprivation, we first examined its effect on the expressions of autophagy-related proteins. The hippocampus and cortex tissues were collected from both WT and 2×TgAD female mice. Three important proteins of autophagosome formation, i.e. Beclin1, LC3-II, and p62, were examined. In the 1-week group, ovx mice exhibited lower Beclin1 protein level relative to the sham mice both in WT and 2×TgAD groups (Fig. 5A,D). More importantly, relative to the expression in the sham group, LC3-II and p62 were significantly increased following the ovx operation in both the WT and 2×TgAD groups (Fig. 5A,D). In the 1-month group, ovx induced a significant decrease in the level of Beclin1 and an increase in the level of p62 (Fig. 5B,E). However, there was no significant difference in the LC3-II expression (Fig. 5B,E). In the 3 months post-ovx mouse brains, lower expression levels of Beclin1 and LC3-II proteins and higher expression level of p62 protein were observed, when compared with the sham mouse brains in both the WT and 2×TgAD groups (Fig. 5C,F). These results suggest that autophagy is impaired in the hormone deprivation condition.
Figure 5.
Ovx-induced change in autophagy The hippocampus and cortex tissue collected from brains of WT-sham, WT-ovx, AD-sham, and AD-ovx groups were assessed for Beclin1, LC3, and p62 expression. Expressions of Beclin1, LC3, and p62 proteins were determined by western blot analysis. (A,D) ovx induced a significant decrease in Beclin1 expression in the 1-week group as well as a significant increase in LC3-II expression and increase in p62 expression in both the WT and 2×TgAD mice. (B,E) ovx induced a significant decrease in Beclin1 expression, increase in p62 expression, and no significant difference in LC3-II expression in the 1-month group. (C,F) ovx induced a significant decrease in Beclin1 expression, decrease in LC3-II expression and increase in p62 expression in the 3-month group. Data are presented as the mean ± SEM. Beclin1 and p62 protein expressions were relative to β-actin. LC3-II expression was relative to LC3-I. *P < 0.05 and **P < 0.01. n.s., not significant.
Ovariectomy-induced changes in Cath-B expression in the mouse brain
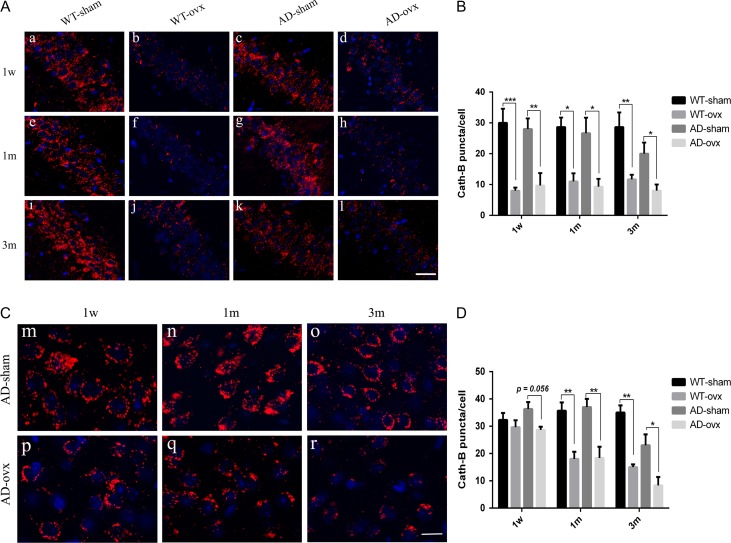
To investigate the impact of ovariectomy on lysosomal function, we assessed the enzymatic activity of Cath-B (a key lysosome aspartic protease) using immunofluorescent staining. As shown in Fig. 6A,B, the appearance of Cath-B-positive puncta in cells was fine and granular. We found that there were no changes of Cath-B-positive puncta in cells in the hippocampal CA1 region between WT-sham group (Fig. 6A, a,e) and AD-sham group (Fig. 6A, c,g), which is the same as WT-ovx group and AD-ovx group in either 1-week and 1-month mice (Fig. 6A). However, in the hippocampus of 3-month group AD-sham mice (Fig. 6A, k), Cath-B-positive puncta in cells were greatly decreased as compared with the corresponding WT-sham mice (Fig. 6A, i), meanwhile, ovx caused a more significant reduction of Cath-B-positive puncta in cells in 2×TgAD than that of WT mice, when compared the ovx group with the corresponding sham group. We found the similar results in the cortex. As shown in Fig. 6C,D, the number of Cath-B puncta in cells was notably decreased in 3-month group 2×TgAD mice (Fig. 6C, o), as compared with those in 1-week (Fig. 6C, m) and 1-month (Fig. 6C, n) group 2×TgAD mice. And ovx led to a significant reduction of Cath-B puncta in cells, especially in 3-month group mice. These results indicate that the activity of Cath-B is decreased by ovarian hormone deprivation.
Figure 6.
Expression of Cath-B in the mouse brains (A) Expression of Cath-B in the CA1 of the hippocampus of mice in all groups. Upper panels represent 1 week after ovariectomy (a–d). Middle panels represent 1 month after ovariectomy (e–h). Lower panels represent 3 months after ovariectomy (i–l). Cath-B-positive puncta were observed (red puncta). (B) Quantification of Cath-B in the CA1 region of the hippocampus showed a significant decrease in the ovx model compared with that in the sham in both WT and 2×TgAD mice. (C) Expression of Cath-B in the cortex of AD mice (m–r). (D) Quantification of Cath-B in the cortex showed a significant decrease in the ovx model compared with that in the sham in both WT and 2×TgAD mice. Nuclei were stained blue with DAPI. Scale bar in picture A: 20 μm; Scale bar in picture C: 10 μm. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Ovariectomy-induced decrease in the expression of LAMP1 in the mouse brain
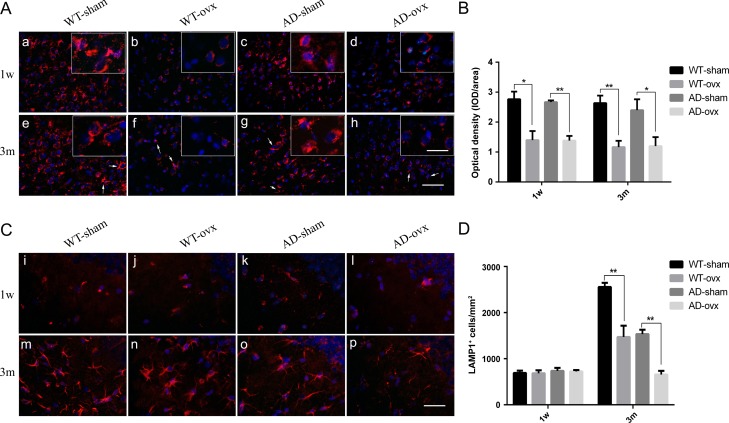
To further investigate lysosomal function, we focused on the expression of LAMP1. Immunofluorescent staining revealed that ovx led to a decrease of LAMP1 expression level in the cortex of the ovx group of both WT and 2×TgAD mice, as compared with the corresponding sham group (Fig. 7A). However, there was no significant change of LAMP1 expression between WT-sham group and AD-sham group, the same as WT-ovx group and AD-ovx group, in either 1-week or 3-month mice. In the hippocampal region, ovx had little effect on LAMP1 expression in 1-week WT and 2×TgAD mice (Fig. 7C, i–l). However, in 3-month group mice, ovx induced a significant decrease of LAMP1 expression in hippocampus, especially in 2×TgAD mice, as compared with the corresponding sham group (Fig. 7C, m–p). Taken together, these results suggest that ovarian hormone loss impairs the degradation capacity of lysosomes.
Figure 7.
Expression of LAMP1 in the mouse brain (A) Expression of LAMP1 in the cortex of mice 1 week and 3 months after ovariectomy. Upper panels represent 1 week after ovariectomy (a–d). The other panels represent 3 months after ovariectomy (e–h). (B) Quantification of LAMP1 in the cortex showed a significant decrease in both ovariectomized WT and 2×TgAD mice compared with that in the sham mice. (C) Expression of LAMP1 in the hippocampus 1 week and 3 months after ovariectomy. Upper panels represent 1 week after ovariectomy (i–l). The other panels represent 3 months after ovariectomy (m–p). (D) Quantification of LAMP1 in the hippocampus showed a significant decrease in LAMP1 expression in both ovariectomized WT and 2×TgAD group mice compared with that in sham mice. Nuclei are stained blue with DAPI. Arrows indicate filamentous patterns for LAMP1 in the cortex. Scale bar in picture A: 50 μm; inset: 20 μm; picture C: 30 μm. Data are presented as the mean ± SEM. *P < 0.05 and **P < 0.01.
Discussion
AD is a multifactorial neurodegenerative disease with three characteristics: production of neurofibrillary tangles through intracellular hyper-phosphorylation, formation of SP from extracellular Aβ, and development of neuronal loss [41]. Among these, Aβ deposits are the primary pathological feature that affects brain structure in preclinical AD. In addition, familial Alzheimer’s disease is caused by mutations in APP, PS1, and PS2, resulting in spontaneously age-related dysmnesia and Aβ deposition [42]. APP/PS1 double-transgenic mice exhibit gradually increased levels of Aβ and decreased learning and memory ability with the development of age [43]. Furthermore, amyloid plaques in the neocortex and hippocampus have been observed as early as 4–6 months of age [44,45]. Such a transgenic mouse is considered to be a reliable animal model for the study of the pathological mechanisms of AD.
In recent years, it has been demonstrated that female sensitivity to the development of AD is primarily associated with a drop in plasma ovarian hormone concentrations during menopause [46]. However, the fundamental mechanisms of this phenomenon remain unclear. In this study, we investigated the effect of ovarian hormone deficiency on brain Aβ deposition and autophagy in two mouse models, normal WT and 2×TgAD female mice. In primates, 30 days of estrogen deprivation led to a permanent >30% loss of substantial nigra dopaminergic neurons, and 10-day estrogen treatment restored the density of tyrosine hydroxylase-immunoreactive cells in the brain of 30-day ovariectomized animal model [47]. In this experiment, we designed a mouse model of 1 week, 1 month, and 3 months after bilateral ovariectomy to simulate different stages of menopause, i.e. early stage, mid-stage, and late-stage. Our results indicated that the weights of the uteri in both WT and 2×TgAD mice were decreased when ovarian hormone deficiency was induced by ovx. In mammals, the uterus is the main source of estrogen and progesterone, which coordinate cell proliferation and differentiation. Furthermore, when neuritic plaques occurred, ovx induced an increase in Aβ levels and neuritic plaque deposition in 2×TgAD mice. These data indicate that the increase in Aβ levels, associated with reproductive senescence, is due to ovarian hormones loss, which is consistent with a previous study [48]. Recent studies have demonstrated that Aβ is closely related to autophagy [49]. Autophagy is essential for maintaining cellular metabolism homeostasis and, as a sweeper for toxic materials such as Aβ, which has provided a new perspective on the pathophysiology of neurodegenerative diseases [50].
Several decades ago, it was reported that steroid sex hormones could be linked to autophagy [51]. Hormone deprivation was identified as a potent inducer of autophagy in the mouse uterus. Both E2 and P4 are negative regulators of autophagic activation in the mouse uterus [52]. In addition to the uterus, the ovary, bone, mammary glands, and male reproductive organs are other sex steroid hormone-responsive organs in which sex steroid hormones regulate autophagic response [53]. Kimura et al. [54] reported that estrogen has a negative impact on the development of NaAsO2 nephrotoxicity through its suppression of the autophagic flux. However, the effect of ovarian hormone on autophagy in the AD brain is not clear.
Autophagy is a dynamic process that is involved in the synergistic effect of autophagy-related genes such as Beclin1, LC3, p62, Cath-B, and LAMP1. Autophagosome clearance is initiated upon fusion with late endosomes and/or lysosomes, which introduces into the resulting autolysosome the acidification machinery necessary and the dozens of lysosomal acidic hydrolases for substrate digestion and enzyme activation [55]. Lysosomes are the final destination for autophagic cargo degradation. Low efficiency of autophagic cargo degradation is induced by lysosomal dysfunction [56]. In the present study, we found that ovx could downregulate the expression of Beclin1 in both the WT-ovx and AD-ovx group, with more reduction in 3-month group AD-ovx mice, the same trend as that in microglia isolated from human AD brains postmortem [57], and Caspase-3 cleavage activated in the brains of AD patients may decrease Beclin1 protein levels [58]. These findings indicated that the deprivation of ovarian hormones may impair Aβ phagocytic capacity, while Beclin1 is the essential factor for the activation of autophagy. In addition, we found that the expression level of LC3-II was increased in the WT-ovx and AD-ovx groups one week after ovariectomy, while decreased greatly in the 3-month AD-ovx mice. LC3-II levels elevate with an increase in autophagosomes but decrease with lysosomal degradation. We then examine the degradation of p62, a marker of lysosomal protein. We found that p62 was kept increasing in both WT-ovx and AD-ovx groups, with the higher level in the 3-month AD-ovx mice. The increases in both LC3-II and p62 suggested impaired autophagic flux [59]. Furthermore, diminished Cath-B and LAMP1 also affect lysosomal degradation function, which is one of the key pathogenic factors in AD. It has been suggested that selectively restoring lysosomal proteolytic function in mouse models of AD can ameliorate diverse pathological, synaptic, and cognitive deficits [60]. Lysosomal dysfunction results in LC3-II accumulation, while ovariectomy may have no effect on Aβ level or neuritic plaque deposition, since brain reserve capacity serves as a stronger counterweight to neurodegeneration at the early stage of ovarian hormone deprivation [61]. One month or 3 months after ovariectomy in 2×TgAD mice, Aβ levels and neuritic plaque deposition were increased, which might be due to the decreased Aβ clearance capability because lysosomal dysfunction can cause accumulation of nonfunctional organelles and incomplete clearance of Aβ [55,62]. Three months after ovariectomy, the LC3-II level was decreased in 2×TgAD mice, indicating that long-term ovarian hormone deprivation can reduce autophagosome formation and maturation, and affect autophagic function as well.
In summary, by evaluating double-transgenic ovariectomized mice, we found that ovarian hormone deprivation affects Aβ levels and autophagic flux, resulting in decreased autophagic activity and lysosomal function. Correspondingly, Aβ degradation was blocked, and Aβ deposition was increased. Nevertheless, while the mechanism by which ovarian hormone deficiency affects autophagic flux and AD remains to be determined, our study moves the field further towards an understanding of post-menopausal patients with dementia, and, depending on the duration of ovarian hormone loss, the regulation of autophagic flux might be a new treatment for female AD patients during the post-menopausal period.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81671257, 81371221, and 31600825), the Natural Science Foundation Project of Chongqing (No. cstc2016jcyjA0069), and the Program for Science and Technology Projects of Yuzhong District in Chongqing, China (No. 20130132).
References
- 1. Alzheimer’s Association 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 2016, 12: 459–509. [DOI] [PubMed] [Google Scholar]
- 2. World Alzheimer Report 2015 : The Global Impact of Dementia. Alzheimers’s Disease International (ADI) 2015
- 3. Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and gender differences in Alzheimer’s disease: recommendations for future research. J Womens Health (Larchmt) 2012, 21: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 4. Grimm A, Mensah-Nyagan AG, Eckert A. Alzheimer, mitochondria and gender. Neurosci Biobehav Rev 2016, 67: 89–101. [DOI] [PubMed] [Google Scholar]
- 5. Chapman RM, Mapstone M, Gardner MN, Sandoval TC, Mccrary JW, Guillily MD, Reilly LA, et al. . Women have farther to fall: gender differences between normal elderly and Alzheimer’s disease in verbal memory engender better detection of Alzheimer’s disease in women. J Int Neuropsychol Soc 2011, 17: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, Laferla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 2007, 27: 13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol 2012, 34: 989–998. [DOI] [PubMed] [Google Scholar]
- 8. Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer’s disease risk and therapy. Prog Brain Res 2010, 182: 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, De Andrade M, Melton LJ 3rd. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2008, 70: 200–209. [DOI] [PubMed] [Google Scholar]
- 10. Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis 2010, 7: 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pike CJ, Carroll JC, Rosario ER, Barron A. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 2009, 30: 239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engler-Chiurazzi EB, Singh M, Simpkins JW. Reprint of: from the 90s to now: a brief historical perspective on more than two decades of estrogen neuroprotection. Brain Res 2015, 1633: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh M, Su C. Progesterone and neuroprotection. Horm Behav 2013, 63: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, et al. . Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 2002, 161: 1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol 2010, 119: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki K, Terry RD. Fine structural localization of acid phosphatase in senile plaques in Alzheimer’s presenile dementia. Acta Neuropathol 1967, 8: 276–284. [DOI] [PubMed] [Google Scholar]
- 17. Raquel SV, Laura TE, Elisabeth SM, Manuel T, David BV, Ines MG, Vanessa DC, et al. . Abnormal accumulation of autophagic vesicles correlates with axonal and synaptic pathology in young Alzheimer’s mice hippocampus. Acta Neuropathol 2012, 123: 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Omata Y, Lim YM, Akao Y, Tsuda L. Age-induced reduction of autophagy-related gene expression is associated with onset of Alzheimer’s disease. Am J Neurodegener Dis 2014, 3: 134–142. [PMC free article] [PubMed] [Google Scholar]
- 19. Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, et al. . Macroautophagy—a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 2005, 171: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohta K, Mizuno A, Ueda M, Li S, Suzuki Y, Hida Y, Hayakawa-Yano Y, et al. . Autophagy impairment stimulates PS1 expression and gamma-secretase activity. Autophagy 2010, 6: 345. [DOI] [PubMed] [Google Scholar]
- 21. Correia SC, Resende R, Moreira PI, Pereira CM. Alzheimer’s disease-related misfolded proteins and dysfunctional organelles on autophagy menu. DNA Cell Biol 2015, 34: 261–273. [DOI] [PubMed] [Google Scholar]
- 22. Yoon SY, Kim DH. Alzheimer’s disease genes and autophagy. Brain Res 2016, 1649: 201–209. [DOI] [PubMed] [Google Scholar]
- 23. Mizushima N. Autophagy: process and function. Genes Dev 2007, 21: 2861–2873. [DOI] [PubMed] [Google Scholar]
- 24. Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest 2015, 125: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman LG, Qureshi YH, Yu WH. Promoting autophagic clearance: viable therapeutic targets in Alzheimer’s disease. Neurotherapeutics 2015, 12: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 2005, 64: 113–122. [DOI] [PubMed] [Google Scholar]
- 27. Yu ZQ, Ni T, Hong B, Wang HY, Jiang FJ, Zou S, Chen Y, et al. . Dual roles of Atg8−PE deconjugation by Atg4 in autophagy. Autophagy 2012, 8: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Streeter A, Menzies FM, Rubinsztein DC. LC3-II Tagging and Western Blotting for Monitoring Autophagic Activity in Mammalian Cells. Methods Mol Biol 2016, 1303: 161–170. [DOI] [PubMed] [Google Scholar]
- 29. Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. Autophagy 2010, 29: 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shvets E, Fass E, Scherz-Shouval R, Elazar Z. The N-terminus and Phe52 residue of LC3 recruit p62/SQSTM1 into autophagosomes. J Cell Sci 2008, 121: 2685–2695. [DOI] [PubMed] [Google Scholar]
- 31. Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, et al. . Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem 2008, 283: 22847–22857. [DOI] [PubMed] [Google Scholar]
- 32. Bjrky G, Lamark T, Brech A, Outzen H, Perander M, Vervatn A, Stenmark H, et al. . p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 2005, 171: 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, et al. . Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131: 1149–1163. [DOI] [PubMed] [Google Scholar]
- 34. Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, et al. . Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron 2006, 51: 703–714. [DOI] [PubMed] [Google Scholar]
- 35. Fuentealba RA, Liu Q, Zhang J, Kanekiyo T, Hu X, Lee JM, Ladu MJ, et al. . Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Abeta42 uptake and lysosomal trafficking. PLoS One 2010, 5: e11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cermak S, Kosicek M, Mladenovic-Djordjevic A, Smiljanic K, Kanazir S, Hecimovic S. Loss of Cathepsin B and L leads to lysosomal dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer’s proteins. PLoS One 2016, 11: e0167428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeng Q, Zheng M, Zhang T, He G. Hippocampal neurogenesis in the APP/PS1/nestin-GFP triple transgenic mouse model of Alzheimer’s disease. Neuroscience 2016, 314: 64–74. [DOI] [PubMed] [Google Scholar]
- 38. Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence In: Conn M (ed). Handbook of Models for Human Aging. Cambridge, MA: Academic Press, 2006, 533–552. [Google Scholar]
- 39. Wang JM, Hou X, Adeosun S, Hill R, Henry S, Paul I, Irwin RW, et al. . A dominant negative ERβ splice variant determines the effectiveness of early or late estrogen therapy after ovariectomy in rats. PLoS One 2012, 7: e33493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging 2012, 33: 1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zimmermann R, Beck G, Knispel S, Maler JM, Weih M, Wiltfang J, Kornhuber J, et al. . Intrathecal IgG synthesis in patients with alterations in the neurochemical dementia diagnostics. J Alzheimers Dis 2010, 19: 1199–1203. [DOI] [PubMed] [Google Scholar]
- 42. Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, et al. . FAD mutant PS-1 gene-targeted mice: increased A beta 42 and A beta deposition without APP overproduction. Neurobiol Aging 2002, 23: 335–348. [DOI] [PubMed] [Google Scholar]
- 43. Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol 2004, 55: 801–814. [DOI] [PubMed] [Google Scholar]
- 44. Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, et al. . Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 2006, 24: 516–524. [DOI] [PubMed] [Google Scholar]
- 45. Selkoe DJ. The ups and downs of Abeta. Nat Med 2006, 12: 758–759. [DOI] [PubMed] [Google Scholar]
- 46. Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation and neurodegeneration. Endocr Rev 2016, 37: 372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci 2000, 20: 8604–8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamson DK, Roes MM, Galea LA. Sex hormones and cognition: neuroendocrine influences on memory and learning. Compr Physiol 2016, 6: 1295–1337. [DOI] [PubMed] [Google Scholar]
- 49. Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, et al. . Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 2017, 93: 1015–1034. [DOI] [PubMed] [Google Scholar]
- 50. Lim Y, Cho H, Kim EK. Brain metabolism as a modulator of autophagy in neurodegeneration. Brain Res 2016, 1649: 158–165. [DOI] [PubMed] [Google Scholar]
- 51. Zhan L, Li J, Wei B. Autophagy in endometriosis: friend or foe? Biochem Biophys Res Commun 2018, 495: 60–63. [DOI] [PubMed] [Google Scholar]
- 52. Choi S, Shin H, Song H, Lim HJ. Suppression of autophagic activation in the mouse uterus by estrogen and progesterone. J Endocrinol 2014, 221: 39–50. [DOI] [PubMed] [Google Scholar]
- 53. Park J, Shin H, Song H, Lim HJ. Autophagic regulation in steroid hormone-responsive systems. Steroids 2016, 115: 177–181. [DOI] [PubMed] [Google Scholar]
- 54. Kimura A, Ishida Y, Nosaka M, Kuninaka Y, Hama M, Kawaguchi T, Sakamoto S, et al. . Exaggerated arsenic nephrotoxicity in female mice through estrogen-dependent impairments in the autophagic flux. Toxicology 2016, 339: 9–18. [DOI] [PubMed] [Google Scholar]
- 55. Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med 2013, 19: 983–997. [DOI] [PubMed] [Google Scholar]
- 56. Liu B, Fang M, Hu Y, Huang B, Li N, Chang C, Huang R, et al. . Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy 2014, 10: 416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lucin Kurt M, O’Brien Caitlin E, Bieri G, Czirr E, Mosher Kira I, Abbey Rachelle J, Mastroeni Diego F, et al. . Microglial Beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer’s disease. Neuron 2013, 79: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bordi M, Berg MJ, Mohan PS, Peterhoff CM, Alldred MJ, Che S, Ginsberg SD, et al. . Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy 2016, 12: 2467–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bustos V, Pulina MV, Kelahmetoglu Y, Sinha SC, Gorelick FS, Flajolet M, Greengard P. Bidirectional regulation of Aβ levels by Presenilin 1. Proc Natl Acad Sci USA 2017, 114: 7142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, et al. . Therapeutic effects of remediating autophagy failure in a mouse model of Alzheimer disease by enhancing lysosomal proteolysis. Autophagy 2011, 7: 788–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malpetti M, Ballarini T, Presotto L, Garibotto V, Tettamanti M, Perani D. Gender differences in healthy aging and Alzheimer’s dementia: a 18F-FDG-PET study of brain and cognitive reserve. Hum Brain Mapp 2017, 8: 4212–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 2017, 40: 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]