Abstract
Background
Strategies used to predict fracture in community-dwellers may not be useful in the nursing home (NH). Our objective was to develop and validate a model (Fracture Risk Assessment in Long-term Care [FRAiL]) to predict the 2-year risk of hip fracture in NH residents using readily available clinical characteristics.
Methods
The derivation cohort consisted of 419,668 residents between May 1, 2007 and April 30, 2008 in fee-for service Medicare. Hip fractures were identified using Part A diagnostic codes. Resident characteristics were obtained using the Minimum Data Set and Part D claims. Multivariable competing risk regression was used to model 2-year risk of hip fracture. We validated the model in a remaining 1/3 sample (n = 209,834) and in a separate cohort in 2011 (n = 858,636).
Results
Mean age was 84 years (range 65–113 years) and 74.5% were female. During 1.8 years mean follow-up, 14,553 residents (3.5%) experienced a hip fracture. Fifteen characteristics in the final model were associated with an increased risk of hip fracture including dementia severity, ability to transfer and walk independently, prior falls, wandering, and diabetes. In the derivation sample, the concordance index was 0.69 in men and 0.71 in women. Calibration was excellent. Results were similar in the internal and external validation samples.
Conclusions
The FRAiL model was developed specifically to identify NH residents at greatest risk for hip fracture, and it identifies a different pattern of risk factors compared with community models. This practical model could be used to screen NH residents for fracture risk and to target intervention strategies.
Keywords: Nursing home, Hip fracture, Prediction model, Mortality
Nearly 10% of hip fractures in the United States occur among nursing home (NH) residents (1). Thirty-six percent of NH residents with hip fracture will die within 6 months, and another 17.3% of ambulatory residents will become completely disabled (2). Among survivors, infections and pressure ulcers are common (3), leading to functional decline and a diminished quality of life (4). In addition to the physical suffering experienced by residents with hip fracture, the financial cost of fractures in the NH is high: receipt of skilled nursing facility services, utilization of emergency department visits, hospital readmissions, and even civil and criminal litigation are common in the months following a hip fracture in the NH (5,6).
During a recent Forum on Aging and Skeletal Health, scientists concluded that there is a real knowledge gap with regards to identifying who is at greatest risk for fracture in NHs (7). Existing tools used to screen community dwellers cannot simply be applied in the NH for a number of reasons. First, most screening tools rely on technology, such as dual energy x-ray absorptiometry (DXA) and vertebral radiographs, which may not be feasible in the NH (8). Second, existing clinical prediction models were not developed for use in frail, institutionalized persons (9–11). The most widely used clinical model, the Fracture Risk Assessment Tool (FRAX) (10), does not include the contribution of falls or functional status, which are important predictors of fracture in the NH (12). Alternative models consider falls (9,11), but they fail to account for the high competing risk of mortality. Finally, existing clinical models estimate the 5- or 10-year absolute risk of fracture: an inappropriately long time frame given the mean 2.4-year life expectancy of long-stay residents (13).
It is therefore important to identify new strategies that will accurately estimate hip fracture risk in long-stay residents in an effort to inform interventions and national policies aimed at ameliorating this serious problem. Existing interventions, including calcium and vitamin D supplementation (14) and bisphosphonates (15–18), likely prevent fracture in at least a subset of NH residents. At the same time, long-stay residents with extreme cognitive and functional limitations have a low risk of fracture (12) and high mortality (19) such that they may not benefit. An ideal screening tool would identify residents with reasonable life expectancy and a high risk for fracture who could potentially benefit from intervention.
Our objective was to develop and validate the Fracture Risk Assessment in Long term care (FRAiL) model to estimate the 2-year absolute risk of hip fracture in a large, nationwide sample of long-stay residents. Predictor variables are derived from readily available clinical information including the Minimum Data Set (MDS) and pharmacy claims data, with no additional diagnostic testing required.
Methods
Subjects and Design
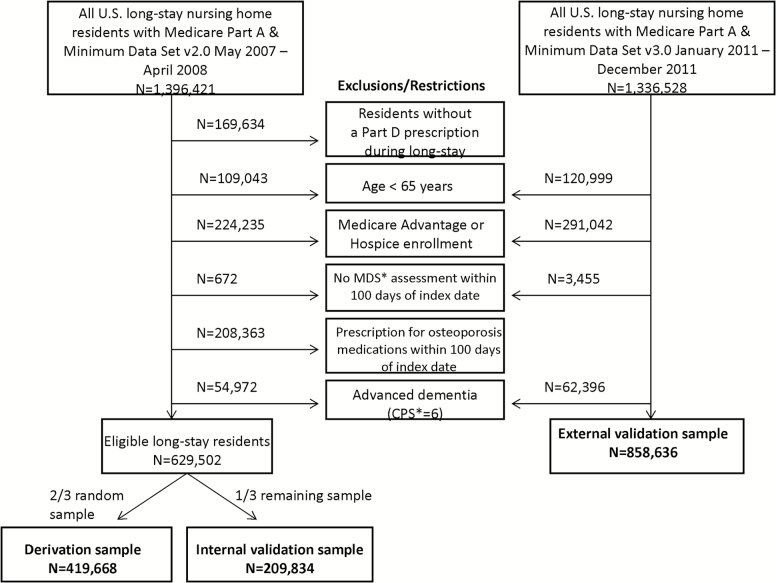
For the source population, a 100% sample of Medicare Part A claims from 2007 to 2011 was linked to NH assessments for all residents enrolled in a fee-for-service Medicare program (20). Details of our selection criteria have been previously published (1). Figure 1 shows the selection of long-stay residents in the derivation sample and the internal and external validation samples. We developed our model in the earlier cohort using MDS v.2.0 because we did not have complete drug ascertainment on the external validation sample. We used the same exclusion criteria in the external validation sample except that we did not exclude osteoporosis drug users because we were missing drug information. This study was approved by the Institutional Review Board at Hebrew SeniorLife.
Figure 1.
Diagram of the selection of long-stay nursing home residents included in the derivation sample and the internal and external validation samples of the FRAiL model. * CPS = Cognitive performance scale; MDS = Minimum data set.
Follow-up
Follow-up began on the index date: first day of the study period that a resident qualified as long-stay (ie, 100 days in the same nursing facility). Residents were followed from the index date until the first event of incident hip fracture, death, or 2 years follow-up. All residents had the opportunity for 2-years of follow-up.
Hip Fracture
Incident hip fractures were ascertained using Medicare Part A claims. A hip fracture was defined as a hospitalization with the primary or secondary International Classification of Diseases, 9th edition (ICD-9) diagnosis of 820.xx or 733.14 with or without an accompanying procedural code. The estimated positive predictive value using a similar definition is 98%, with sensitivity of 96% (21). To be sure that we were excluding encounters for the follow-up care of a hip fracture, we only counted the first hip fracture after the index date without a hospitalization for hip fracture in the previous 100 days.
Model Characteristics
The MDS is a federally mandated needs assessment performed on all NH residents at the time of admission and quarterly thereafter. Information on 34 characteristics from 7 domains (demographic, cognitive/function, neuropsychiatric, falls, pain, nutrition, and comorbidities) was obtained using the Medicare Enrollment File and the MDS assessment closest to and preceding the index date. For characteristics, “prior hip fracture” and “hospitalization,” we ascertained information using the MDS and Part A claims.
In 2010, certified NHs in the U.S. switched from using MDS version 2.0 to version 3.0. Among the differences in the newer MDS version, residents are queried for assessment of pain and cognition whenever possible, rather than relying on nurse assessment. Presently, version 3.0 is used throughout the United States, whereas version 2.0 is still used in many countries (eg, Finland, Canada). We did not consider any characteristic in our model that was not available in both versions. In the development cohort, we included residents with a valid MDS version 2.0, whereas in the external validation sample, we used version 3.0. Supplementary Appendix Table 1 includes the full list of characteristics considered and the distribution of characteristics according to whether the resident experienced a hip fracture or died during follow-up.
Prescription drug information was ascertained from Medicare Part D claims. Residents were categorized as users of each medication class if the resident had an active prescription on the index date based on the amount and frequency of drug dispensed (Supplementary Appendix Table 2). Because benzodiazepines are infrequently covered under Medicare Part D, we considered users according to Part D claims (if available) or an MDS indicator of anxiety/hypnotic drug use. We additionally considered high risk medication characteristics including total prescription drug count and number of potentially inappropriate drugs as defined by the recent Beers criteria (22).
Statistical Analysis
Competing risk proportional hazards regression using the Fine and Gray method (23) was used. This model provides a hazard ratio to describe the association between each characteristic and risk of hip fracture, adjusting for observed mortality (24). We modeled risk in men and women combined, and also separately by sex. We first modeled the unadjusted association between each characteristic and hip fracture risk in the derivation sample. We considered a broad list of characteristics associated with risk of falls or fracture (Supplementary Appendix 1). Many of these characteristics have also been associated with mortality in NH residents (25,26). Characteristics associated with fracture (p ≤ .05) in unadjusted models were entered into nine domain-specific models (demographics, cognitive/function, neuropsychiatric, falls, pain, nutrition, comorbidities, medication associated with falls, and medications associated with bone mineral density [BMD]). Characteristics that were significant (p ≤ .05) in domain-adjusted models were entered into a final stepwise selection model. We considered a number of biologically plausible interactions including medications and falls, age and comorbidities, and cognition and transfers. In a sensitivity analysis, we removed residents who were totally dependent in transfers because their immobility confers a very low risk of fracture.
We assessed discrimination using the concordance index (C-index) in the model for men and the model for women, and in each of the 9 U.S. census tracks separately. Because medications were the only characteristics not included in the MDS, we repeated the C-index after excluding medications. Calibration was assessed by comparing the observed versus expected frequency of hip fracture across deciles of predicted risk, and calibration was tested with the Nam-D’Agostino chi-square test (27). We calculated sensitivity, specificity, positive and negative predictive value, and the likelihood ratio using thresholds of risk that corresponded to the 5th, 7th, 8th, and 9th deciles of predicted risk in women.
Validation
We validated the separate model for men and women in the internal and external samples. We used the same techniques to assess discrimination and calibration. All analyses were performed using SAS (v9.3) and R (Prediction Error Curves [PEC] pkg) (28).
Results
Among 419,668 residents in the derivation sample, mean age was 83.9 years (range 65–113 years) and 71.4% were female (Table 1). Over a mean follow-up of 1.8 years, 14,553 residents (3.5%) were hospitalized with hip fracture and 176,192 residents (42.0%) died without hip fracture. Participants with hip fracture were more likely to be female, independent in activities of daily living and transfers, to have fallen, and wander as compared to residents without hip fracture (Supplementary Appendix Table 1).
Table 1.
Characteristics Associated With the 2-Year Risk of Hip Fracture in the Final Combined FRAiL Model for Men and Women, as Shown in the Derivation Cohort (N = 419,668)
| Resident Characteristics | Mean* (SD) or Number (%) of Residents | β Coefficient (SE) | HR (95% CI) |
|---|---|---|---|
| Age, years* (per 5 y increment) | 83.9 (8.3) | 0.025 (0.006) | 1.03 (1.02, 1.04) |
| Female | 299,794 (71.4) | 0.21 (0.02) | 1.24 (1.19, 1.29) |
| Race | |||
| White | 349,500 (83.3) | REF | REF |
| Black | 53,669 (12.8) | −0.57 (0.03) | 0.56 (0.53, 0.60) |
| Hispanic | 7,375 (1.8) | −0.038 (0.07) | 0.96 (0.85, 1.10) |
| Asian | 3,881 (0.9) | −0.46 (0.11) | 0.63 (0.52, 0.78) |
| Native American | 1,735 (0.4) | 0.18 (0.12) | 1.21 (0.96, 1.51) |
| Other/Unknown | 3,508 (0.8) | −0.18 (0.10) | 0.84 (0.69, 1.02) |
| Cognitive Performance Score* (per 1 point increase in CPS) | 2.5 (1.4) | 0.029 (0.007) | 1.03 (1.02, 1.04) |
| ADL Hierarchy Scale | |||
| Independent or Mild Dependence (0–2) | 132,670 (31.6) | REF | REF |
| Extensive assistance 1†(3) | 112,492 (26.8) | 0.0068 (0.02) | 1.01 (0.96, 1.06) |
| Extensive assistance 2‡(4) | 79,098 (18.8) | −0.15 (0.03) | 0.86 (0.81, 0.92) |
| Dependent (5) | 80,226 (19.1) | −0.30 (0.04) | 0.74 (0.69, 0.81) |
| Total Dependence (6) | 15,176 (3.6) | −0.51 (0.0.10) | 0.60 (0.50, 0.73) |
| Locomotion in Room | |||
| Independent or supervision | 132,228 (31.5) | REF | REF |
| Limited Assistance | 69,023 (16.4) | −0.22 (0.03) | 0.80 (0.76, 0.85) |
| Extensive Assistance | 49,324 (11.8) | −0.37 (0.04) | 0.69 (0.63, 0.75) |
| Total Dependence | 169,077 (40.3) | −0.73 (0.04) | 0.48 (0.45, 0.52) |
| Bladder continence | |||
| Mostly continent | 209,119 (49.8) | REF | REF |
| Frequent incontinence | 85,363 (20.3) | −0.16 (0.02) | 0.85 (0.81, 0.89) |
| Total incontinence | 125,170 (29.8) | −0.28 (0.03) | 0.75 (0.71, 0.80) |
| Previous fall | 167,381 (39.9) | 0.25 (0.02) | 1.28 (1.24, 1.33) |
| Transfer Performance | |||
| Independent or supervision | 124,021 (29.6) | REF | REF |
| Limited Assistance | 83,644 (19.9) | −0.044 (0.03) | 0.96 (0.90, 1.01) |
| Extensive Assistance | 144,313 (34.4) | −0.28 (0.04) | 0.76 (0.70, 0.82) |
| Total Dependence | 67,686 (16.1) | −0.52 (0.06) | 0.60 (0.53, 0.67) |
| Easily distracted | 74,316 (17.7) | 0.07 (0.02) | 1.08 (1.03, 1.13) |
| Wandering | 36,875 (8.9) | 0.28 (0.03) | 1.32 (1.26, 1.39) |
| Osteoarthritis | 73,738 (17.6) | −0.04 (0.02) | 0.96 (0.93, 0.99) |
| BMI* (per kg/m2) | 26.3 ± 6.2 | −0.056 (0.002) | 0.95 (0.94, 0.95) |
| Pressure ulcer (any stage vs none) | 27,690 (6.7) | −0.17 (0.05) | 0.84 (0.77, 0.92) |
| Diabetes | 119,490 (28.5) | 0.08 (0.02) | 1.09 (1.05, 1.13) |
| Medications | |||
| Acetylcholinesterase inhibitors | 105,838 (25.2) | 0.091 (0.02) | 1.10 (1.05, 1.14) |
| Alpha blockers | 31,362 (7.5) | −0.11 (0.04) | 0.90 (0.83, 0.97) |
| Antidepressants—SSRI | 113,047 (26.9) | 0.092 (0.02) | 1.10 (1.06, 1.14) |
| Benzodiazepines | 81,356 (19.4) | 0.11 (0.02) | 1.11 (1.07, 1.16) |
Note: ADL = Activities of daily living; BMI = Body mass index; CPS = Cognitive Performance Scale; kg = Kilograms; m2 = Meters squared; REF = Reference; SD = Standard deviation; SSRI = Selection serotonin reuptake inhibitor.
*Mean (SD). †At least extensive assistance in personal hygiene or toilet use, and less than extensive in both eating and locomotion. ‡At least extensive assistance, but not total dependence, in eating or locomotion
Supplementary Appendix Table 3 presents the hazard ratio (HR) and 95% confidence interval (CI) of resident characteristics that were significant predictors of hip fracture in the unadjusted and domain specific models. Fifteen characteristics remained significant predictors of hip fracture in the fully adjusted model: older age, white race, female, impaired cognition, activities of daily living independence, locomotion independence, urinary continence, previous falls, transfer independence, easily distracted, wandering, absence of osteoarthritis, absence of pressure ulcer, low body mass index, and diabetes. The final model coefficients are shown in Table 1. In addition, four classes of medications were associated with hip fracture: acetylcholinesterase inhibitors, alpha blockers, selective serotonin reuptake inhibitor antidepressants, and benzodiazepines. Previous falls (HR 1.28, 95% CI 1.24, 1.33) and wandering (HR 1.32, 95% CI 1.26, 1.39) were the factors associated with the greatest increase in fracture risk. Locomotion dependence (HR 0.48, 95% CI 0.45, 0.52), activities of daily living dependence (HR 0.60, 95% CI 0.50, 0.73), transfer dependence (HR 0.60, 95% CI 0.53, 0.67), and urinary incontinence (HR 0.75, 95% CI 0.71, 0.80) were associated with a decreased risk of hip fracture. Interaction terms were not significant, and thus, not included in the full model. Supplementary Appendix Table 4 provides a numerical example of the FRAiL model calculation in two different female NH residents using the combined model.
In separate models with men and women, associations were similar with the exception of diabetes: diabetes was associated with an increased risk of hip fracture in women (HR 1.12, 95% CI 1.07, 1.17), but not in the model for men (HR 0.99, 95% CI 0.92, 1.07; p for interaction = .002). In a sensitivity analysis that removed residents who were totally dependent on transfers, the results were similar (not shown).
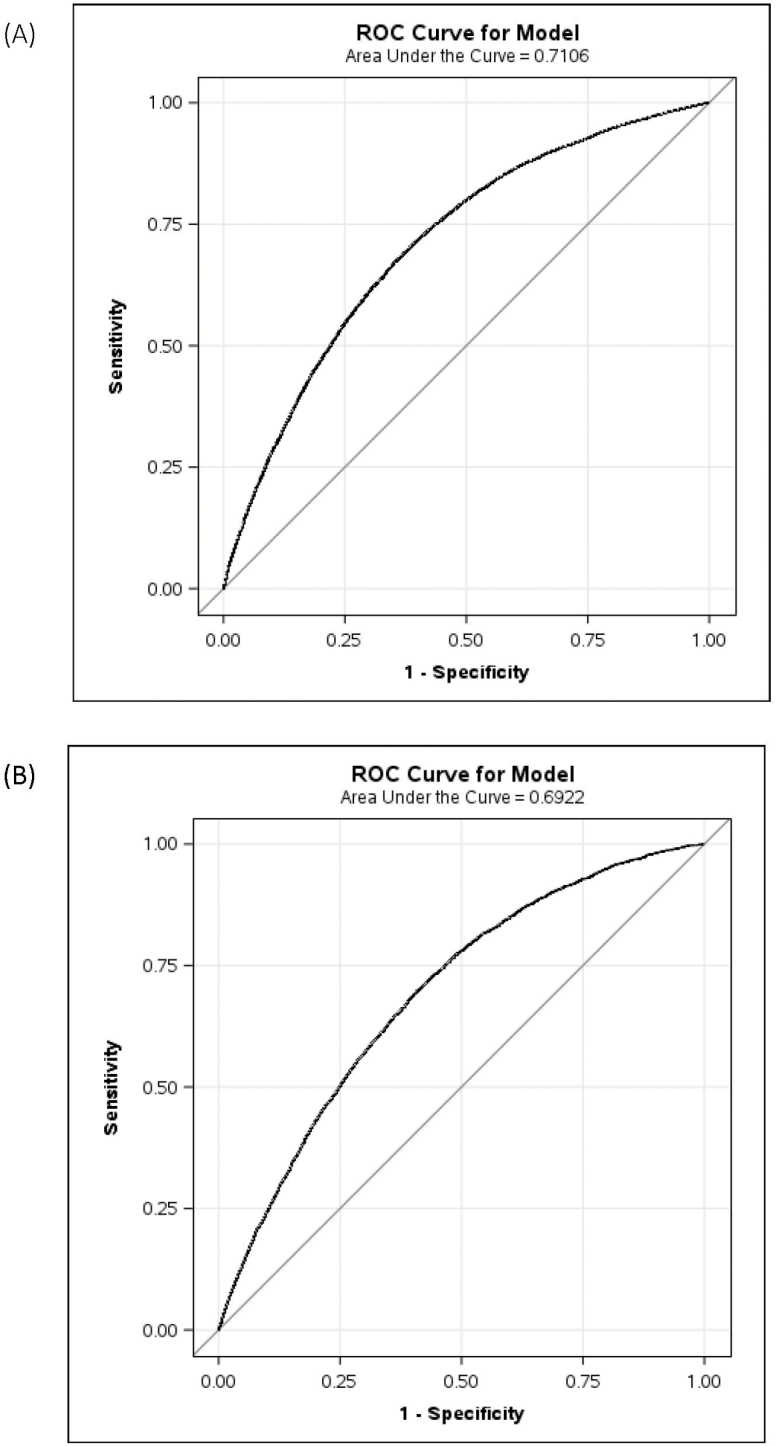
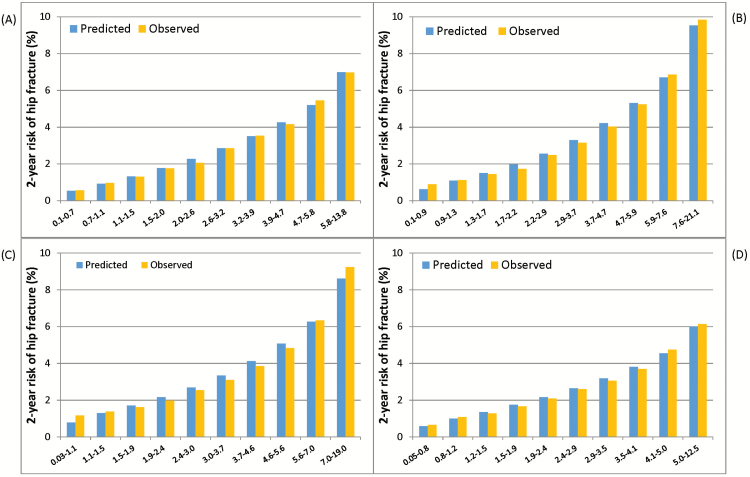
In the derivation sample, the C-index was 0.71 in the model for women and 0.69 in the model for men (Figure 2), and it was similar across all nine geographic regions (range 0.68–0.73). Removing the four medication classes reduced the C-index < 1%, and so these medications were not included in the calibration plots or subsequent validation models. Figure 3 shows the results of the calibration plot in men and women (chi-square for women 14.5, p = .11; men 4.7, p = .86).
Figure 2.
Receiver operating characteristic curve of (A) the FRAiL model for women, and (B) the FRAiL model for men in the derivation sample.
Figure 3.
Calibration plots comparing the observed to expected probabilities of hip fracture in long stay nursing home residents across deciles of predicted risk: (A) Women in the derivation sample, (B) Men in the derivation sample, (C) Women in the external validation sample, and (D) Men in the external validation sample.
In the internal validation sample (n = 209,834), 7,207 residents (3.4%) were hospitalized with hip fracture and 94,882 residents (45.2%) died during follow-up. In the external validation sample (n = 858,636), 28,050 residents (3.3%) were hospitalized with hip fracture and 372,041 residents (43.3%) died during follow-up. The associations between resident characteristics and hip fracture risk in the validation samples were similar to the derivation sample except that easily distracted, osteoarthritis, and pressure ulcers were no longer significant in the full model (Supplementary Appendix Table 5). In the external validation sample, the C-index was 0.69 for women and 0.67 for men. Calibration remained excellent (Figure 2).
Sensitivity and specificity are shown in the derivation sample in Supplementary Appendix Table 6. A threshold of ≥ 6% (corresponding to the top two deciles of predicted risk in women) had a sensitivity of 81.4% and a specificity of 44.8%.
Conclusions
The FRAiL model is the first prediction model to estimate the 2-year risk of hip fracture in long-stay NH residents specifically. It has moderate discrimination and excellent calibration in men and women across geographic regions. Importantly, this is a practical model that only requires information from the MDS that is routinely being used in many countries to collect information on NH residents throughout their stay. Thus, the FRAiL model could be automated and systematically used to screen residents at risk for fracture at the time of MDS completion. This is congruent with the current NH practice of allowing the MDS to trigger a Clinical Assessment Protocol (CAP) or Care Area Assessment (CAA), a prompt for providers to weigh appropriate intervention options in at-risk patients.
Few studies have evaluated strategies to predict fracture in NH residents. BMD as measured by dual energy x-ray absorptiometry is commonly used in community dwellers, and low BMD has been shown to predict fracture in NH residents as well (29,30). Nonetheless, central DXA is not available in most U.S. NHs. Sending residents out of the facility to obtain such testing is costly and may be contrary to patient preferences (8). Even when available this technology may not be useful, as 82% of white, female long-stay residents have a BMD T-score ≤ −2.5 (ie, below the usual cutpoint for pharmacologic treatment eligibility) (30).
A more practical approach to screen long-stay residents for fracture is a fracture risk algorithm, like FRAX, but one that is specifically tailored to the NH setting. Unlike FRAX, that models 10-year risk of fracture in individuals aged 40–90 years, our tool estimates the 2-year risk of fracture in long-stay residents between the ages of 65–113 years. Similar to FRAX, the FRAiL model identified white race, female sex, and low body mass index as important predictors of hip fracture. The association between advanced age and risk of hip fracture in NH residents was very modest (HR per 5 years of age, 1.03; 95% CI 1.02, 1.04) as compared with community based studies (HR or RR per 5 years of age, 1.40–1.65) (31,32). The modest association with age and fracture risk is striking despite the wide range of ages included in our study. Overall, discrimination in FRAiL was similar to FRAX without BMD (Women: C-index in FRAiL-0.71; FRAX without BMD-0.61–0.75) (33).
The FRAiL model confirms that residents with dependence in care and transfers have a lower risk of hip fracture as compared with higher functioning residents. Residents who were unable to walk in their room were 42% less likely to fracture their hip as compared with residents who walked independently. Similarly, residents who were totally incontinent of urine were 25% less likely to fracture their hip as compared with continent residents. The association between functional dependence and decreased fracture risk has been described in some (12,34,35), but not all (36), studies of NH residents. This association differs from community based studies, where functional impairment may serve as a marker of frailty, and it is associated with an increased risk of fracture (31,37,38). The unique relationship between functional characteristics and fracture risk in the NH emphasizes the importance of using a different screening strategy in this setting.
In addition to functional characteristics, the FRAiL model found neurobehavioral characteristics, like wandering, were associated with a 32% increased risk of hip fracture. Residents who wander likely have more opportunity to fall, and associated behavioral symptoms and medications may also contribute to fracture risk. Resistance to care and wandering were described as risk factors for fracture in a previous study in the NH (35).
We found that diabetes was associated with a modest increased risk of hip fracture in women, but not in men. We do not believe the differential sex effect could be explained by obesity differences, as body mass index was similar in male and female diabetics with hip fracture (mean 26.6 kg/m2 in both groups). We did not have information on the severity of disease, and further exploration is warranted to understand these sex differences in risk.
Of the 28 medication classes that we considered, only three were associated with an increased risk of hip fracture in the fully adjusted model: acetylcholinesterase inhibitors, benzodiazepines/sedatives, and selective serotonin reuptake inhibitor antidepressants. Alpha blockers were associated with a slight decreased risk of hip fracture in adjusted models (HR 0.90, 95% CI 0.83, 0.97). This may be due to chance, or due to confounding by indication. Despite the association between medications and hip fracture, medication use minimally improved the ability of the FRAiL model to discriminate hip fracture (<1%). Even so, medication use is highly modifiable, and medication reduction is still likely an effective means to prevent falls (39), and probably fractures, in this population.
Another strategy to screen residents at risk for fracture is based on history of hip or vertebral fracture. Interestingly, prior history of hip fracture, as determined by the MDS or Part A claims, was not a predictor of subsequent fracture in our fully adjusted model. A meta-analysis of six studies of NH residents concluded that prior fracture was a predictor of osteoporotic fracture (RR = 1.71, 95% CI = 1.30–2.24) (40); however, only a few of these studies adjusted for functional status (34–36) or falls (29,35,41), and none used a competing risk approach, which may explain the differences in our results. The lack of association between prior fracture is strikingly different from community based models, such as FRAX (10), and it highlights the importance of using a separate screening strategy in the NH.
Strengths of the study include a large, nationally representative sample of long-stay residents with Medicare claims linked with the MDS. Missing data was rare and unlikely to be related to the outcome fracture. We validated our results in a contemporary cohort of long-stay residents with clinical characteristics obtained from MDS v3.0. In the future, we plan to test whether we can improve model discrimination by adding characteristics specific to MDS v3.0, such as presence of trouble sleeping or balance issues while toileting. The current model considered many related characteristics, including hypnotic medication use and transfer dependence, but it is possible that these additional variables would improve model performance.
Stratifying residents at risk for fracture with the FRAiL model might then be a successful way to reduce polypharmacy and target interventions to prevent fracture. If the FRAiL model was used to screen the 1 million long-stay residents in the United States, 20% of female and 9% of male residents (n = 172,500) would be classified as “high risk” with a 2-year probability of hip fracture of ≥ 6%. Using our calibration plots, we estimate that 13,575 “high risk” residents will fracture their hip within 2 years. If osteoporosis medications and fall prevention strategies were employed in high-risk residents to reduce the risk of hip fracture by 30% (14–18,39), using this tool to target interventions could prevent more than 2,000 hip fractures annually with their ensuing costs. Further, this tool could be used to identify residents at low risk of fracture whereby stopping osteoporosis medication may be appropriate. Future studies should refine the proposed threshold by identifying the absolute level of risk whereby prevention strategies reduce the risk of fracture and are cost-effective.
There are also some limitations of our study. First, the current model requires manual calculation in order to estimate a resident’s risk of fracture. We have provided the model coefficients and a numerical example in Supplementary Appendix 5 in order that others can use and replicate our model. Although it is possible to develop a scoring sheet or a website to facilitate implementation, we believe that uptake of manual risk scoring systems in the NH setting has been low. Instead, we recommend developing automated processes to implement screening with the FRAiL model. This is possible because all model characteristics are already collected as part of the MDS, and all facilities, regardless of whether they have adapted electronic medical records, must regularly report MDS data to the Centers for Medicare & Medicaid Services. Second, we did not have information on calcium and nonprescription forms of Vitamin D. Supplement use may serve as a marker of quality for the facility (42), and thus, if we had included supplement use in our models, we may have biased results. Third, a small proportion of subjects (2%) enrolled in Medicare Advantage for one or more months during follow-up, and we may not have complete fracture ascertainment on these individuals. Fourth, characteristics such as functional status are likely to change over time in a NH population. Although modeling the risk of fracture with time varying characteristics would likely improve model performance, it would have prohibited the use of the model as a screening tool in clinical practice. Finally, we did not have information on disease severity or every clinical characteristic that could be associated with fracture, such as orthostatic blood pressure, intensity of treatment, or bone mineral density. Adding these and other characteristics may improve our model performance, but it compromises the ease of use of the present tool and the potential for automated calculation during routine MDS assessments.
The current NH practice of treating residents with osteoporosis drugs to prevent fracture varies widely (range 0%–85%, mean = 12%–36%,) (42,43). Most of the differences in treatment variation are explained by individual provider preferences, rather than patient or facility characteristics, or even shared practice patterns suggesting that a more standardized approach to screening and treatment is needed (43,44). The FRAiL tool would be an important first step in overcoming this key barrier to preventing fractures in the NH setting.
Supplementary Material
Supplementary data is available at Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institute of Health (NIH), National Institute on Aging (NIA), #1R01AG045441 and #5P01AG027296-05. A.R.Z. is supported by an Agency for Healthcare Research and Quality (AHRQ) award (5K12HS022998).
Conflicts of Interest
S.D.B. receives grant funding from Amgen, and D.P.K. receives grant funding from Merck Sharp & Dohme and Policy Analysis, Inc and consulting fees from Merck Sharp & Dohme and Roivant unrelated to the current project. V.M. holds stock of unknown value in PointRight, Inc. an information services company providing advice and consultation to various components of the long-term care and post-acute care industry, including suppliers and insurers. In addition, V.M. chairs the Independent Quality Committee for HRC Manor Care, Inc., a nursing home chain, and serves as chair of a Scientific Advisory Committee for NaviHealth, a post-acute care service organization.
Supplementary Material
Acknowledgments
These analyses were presented in part as an abstract on October 12, 2015 and September 16–17, 2016 at the American Society for Bone and Mineral Research annual meetings in Seattle, WA and Atlanta, GA.
References
- 1. Berry SD, Lee Y, Zullo AR, Kiel DP, Dosa D, Mor V. Incidence of hip fracture in U.S. Nursing Homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230–1234. doi:10.1093/gerona/glw034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273–1280. doi:10.1001/jamainternmed.2014.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berry SD, Samelson EJ, Bordes M, Broe K, Kiel DP. Survival of aged nursing home residents with hip fracture. J Gerontol A Biol Sci Med Sci. 2009;64:771–777. doi:10.1093/gerona/glp019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaupre LA, Jones CA, Johnston DW, Wilson DM, Majumdar SR. Recovery of function following a hip fracture in geriatric ambulatory persons living in nursing homes: prospective cohort study. J Am Geriatr Soc. 2012;60:1268–1273. doi:10.1111/j.1532-5415.2012.04033.x [DOI] [PubMed] [Google Scholar]
- 5. Zimmerman S, Chandler JM, Hawkes W et al. . Effect of fracture on the health care use of nursing home residents. Arch Intern Med. 2002;162:1502–1508. [DOI] [PubMed] [Google Scholar]
- 6. Hoverman C, Shugarman LR, Saliba D, Buntin MB. Use of postacute care by nursing home residents hospitalized for stroke or hip fracture: how prevalent and to what end?J Am Geriatr Soc. 2008;56:1490–1496. doi:10.1111/j.1532-5415.2008.01824.x [DOI] [PubMed] [Google Scholar]
- 7. Khosla S, Bellido TM, Drezner MK et al. . Forum on aging and skeletal health: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2011;26:2565–2578. doi:10.1002/jbmr.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenspan S, Nace D, Perera S et al. . Lessons learned from an osteoporosis clinical trial in frail long-term care residents. Clin Trials. 2012;9:247–256. doi:10.1177/1740774511430516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ. 2012;344:e3427. [DOI] [PubMed] [Google Scholar]
- 10. Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi:10.1007/s00198-007-0543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporos Int. 2007;18:1109–1117. doi:10.1007/s00198-007-0362-8 [DOI] [PubMed] [Google Scholar]
- 12. Girman CJ, Chandler JM, Zimmerman SI et al. . Prediction of fracture in nursing home residents. J Am Geriatr Soc. 2002;50:1341–1347. [DOI] [PubMed] [Google Scholar]
- 13. Jones AL, Dwyer LL, Bercovitz AR, Strahan GW. National Nursing Home Survey: 2004 overview. Vital Health Stat. 2009;13:1–155. [PubMed] [Google Scholar]
- 14. Chapuy MC, Arlot ME, Duboeuf F et al. . Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–1642. doi:10.1056/NEJM199212033272305 [DOI] [PubMed] [Google Scholar]
- 15. Greenspan SL, Schneider DL, McClung MR et al. . Alendronate improves bone mineral density in elderly women with osteoporosis residing in long-term care facilities. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;136:742–746. [DOI] [PubMed] [Google Scholar]
- 16. Iwamoto J, Matsumoto H, Takeda T. Efficacy of risedronate against hip fracture in patients with neurological diseases: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2008;24:1379–1384. doi:10.1185/030079908X297321 [DOI] [PubMed] [Google Scholar]
- 17. Prieto-Alhambra D, Judge A, Arden NK, Cooper C, Lyles KW, Javaid MK. Fracture prevention in patients with cognitive impairment presenting with a hip fracture: secondary analysis of data from the HORIZON Recurrent Fracture Trial. Osteoporos Int. 2014;25:77–83. doi:10.1007/s00198-013-2420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med. 2015;175:913–921. doi:10.1001/jamainternmed.2015.0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell SL, Teno JM, Kiely DK et al. . The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi:10.1056/NEJMoa0902234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories(*). Health Serv Res. 2011;46(1 Pt 1):120–137. doi:10.1111/j.1475-6773.2010.01194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rigler SK, Ellerbeck E, Whittle J, Mahnken J, Cook-Wiens G, Shireman TI. Comparing methods to identify hip fracture in a nursing home population using Medicare claims. Osteoporos Int. 2011;22:57–61. doi:10.1007/s00198-010-1264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–2246. [DOI] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi:10.1111/j.1532-5415.2010.02767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flacker JM, Kiely DK. Mortality-related factors and 1-year survival in nursing home residents. J Am Geriatr Soc. 2003;51:213–221. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell SL, Miller SC, Teno JM, Kiely DK, Davis RB, Shaffer ML. Prediction of 6-month survival of nursing home residents with advanced dementia using ADEPT vs hospice eligibility guidelines. JAMA. 2010;304:1929–1935. doi:10.1001/jama.2010.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D’Agostino RN, Nam BH. Evaluation of the Performance of Survival Analysis Models: Discrimination and Calibration Measures, Handbook of Statistics. Amsterdam: Elsevier; 2004. [Google Scholar]
- 28. Wolbers M, Blanche P, Koller MT, Witteman JC, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. doi:10.1093/biostatistics/kxt059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broe KE, Hannan MT, Kiely DK, Cali CM, Cupples LA, Kiel DP. Predicting fractures using bone mineral density: a prospective study of long-term care residents. Osteoporos Int. 2000;11:765–771. doi:10.1007/s001980070055 [DOI] [PubMed] [Google Scholar]
- 30. Chandler JM, Zimmerman SI, Girman CJ et al. . Low bone mineral density and risk of fracture in white female nursing home residents. JAMA. 2000;284:972–977. [DOI] [PubMed] [Google Scholar]
- 31. Cummings SR, Nevitt MC, Browner WS et al. . Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi:10.1056/NEJM199503233321202 [DOI] [PubMed] [Google Scholar]
- 32. Jackson RD, Donepudi S, Mysiw WJ. Epidemiology of fracture risk in the Women’s Health Initiative. Curr Osteoporos Rep. 2008;6:155–161. [DOI] [PubMed] [Google Scholar]
- 33. Leslie WD, Lix LM. Comparison between various fracture risk assessment tools. Osteoporos Int. 2014;25:1–21. doi:10.1007/s00198-013-2409-3 [DOI] [PubMed] [Google Scholar]
- 34. Nakamura K, Oyama M, Takahashi S et al. . Fracture incidence in nursing homes in Japan. Osteoporos Int. 2010;21:797–803. doi:10.1007/s00198-009-1015-x [DOI] [PubMed] [Google Scholar]
- 35. Colón-Emeric CS, Biggs DP, Schenck AP, Lyles KW. Risk factors for hip fracture in skilled nursing facilities: who should be evaluated?Osteoporos Int. 2003;14:484–489. doi:10.1007/s00198-003-1384-5 [DOI] [PubMed] [Google Scholar]
- 36. Dobnig H, Piswanger-Sölkner JC, Obermayer-Pietsch B et al. . Hip and nonvertebral fracture prediction in nursing home patients: role of bone ultrasound and bone marker measurements. J Clin Endocrinol Metab. 2007;92:1678–1686. doi:10.1210/jc.2006-2079 [DOI] [PubMed] [Google Scholar]
- 37. Wihlborg A, Englund M, Åkesson K, Gerdhem P. Fracture predictive ability of physical performance tests and history of falls in elderly women: a 10-year prospective study. Osteoporos Int. 2015;26:2101–2109. doi:10.1007/s00198-015-3106-1 [DOI] [PubMed] [Google Scholar]
- 38. Brown JS, Vittinghoff E, Wyman JF et al. . Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721–725. [DOI] [PubMed] [Google Scholar]
- 39. Ray WA, Taylor JA, Meador KG et al. . A randomized trial of a consultation service to reduce falls in nursing homes. JAMA. 1997;278:557–562. [PubMed] [Google Scholar]
- 40. Khatib R, Santesso N, Pickard L et al. . Fracture risk in long term care: a systematic review and meta-analysis of prospective observational studies. BMC Geriatr. 2014;14:130. doi:10.1186/1471-2318-14-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyles KW, Schenck AP, Colón-Emeric CS. Hip and other osteoporotic fractures increase the risk of subsequent fractures in nursing home residents. Osteoporos Int. 2008;19:1225–1233. doi:10.1007/s00198-008-0569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colón-Emeric C, Lyles KW, Levine DA et al. . Prevalence and predictors of osteoporosis treatment in nursing home residents with known osteoporosis or recent fracture. Osteoporos Int. 2007;18:553–559. doi:10.1007/s00198-006-0260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Curtis JR, Arora T, Xi J et al. . Do physicians within the same practice setting manage osteoporosis patients similarly? Implications for implementation research. Osteoporos Int. 2009;20:1921–1927. doi:10.1007/s00198-009-0900-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parikh S, Brookhart MA, Stedman M, Avorn J, Mogun H, Solomon DH. Correlations of nursing home characteristics with prescription of osteoporosis medications. Bone. 2011;48:1164–1168. doi:10.1016/j.bone.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.