Abstract
Objectives
Benzhydrocodone is a hydrocodone prodrug that has been combined with acetaminophen (APAP) in a novel immediate-release analgesic. This study evaluated the relative bioavailability, intranasal abuse potential, and safety of benzhydrocodone/APAP compared with commercially available hydrocodone bitartrate (HB)/APAP.
Design
Single-center, randomized, double-blind, double-dummy, two-part study comprising a Dose Selection (Part A) phase and a Main Study (Part B) phase.
Setting
Clinical research site.
Subjects
Healthy adult, nondependent, recreational opioid users with a history of intranasal abuse.
Methods
Subjects (N = 42) in Part B received five in-clinic treatments consisting of intranasal and oral benzhydrocodone/APAP (13.34/650 mg), intranasal and oral hydrocodone/APAP (15/650 mg), and placebo, with four or more days of washout between treatments. Pharmacodynamic assessments included subjective effects of Drug Liking, Overall Drug Liking, and Take Drug Again (assessed on visual analog scale [VAS]), as well as nasal irritation. Pharmacokinetics and safety were also assessed.
Results
Hydrocodone Cmax was 11% lower for intranasal benzhydrocodone/APAP vs intranasal HB/APAP (P = 0.0027). Early cumulative hydrocodone exposures for intranasal benzhydrocodone/APAP through 0.5, 1, and 2 hours were reduced by approximately 50%, 29%, and 15%, respectively (P ≤ 0.0024). Correspondingly, Drug Liking VAS values up to two hours postdose were significantly lower for intranasal benzhydrocodone/APAP vs intranasal HB/APAP (P ≤ 0.0079), although peak Drug Liking VAS (Emax) scores were not different (P = 0.2814). Adverse nasal effects were more frequent for intranasal benzhydrocodone/APAP vs intranasal HB/APAP.
Conclusions
Reduced hydrocodone exposure and drug liking at early time intervals, coupled with adverse nasal effects, can be expected to provide a level of deterrence to the intranasal route of abuse for benzhydrocodone/APAP.
Keywords: Abuse Potential, Benzhydrocodone, Hydrocodone, Intranasal, Pharmacokinetics, Bioavailability
Introduction
Pain is among the most common reasons individuals seek medical care. In a large survey from 2012, an estimated 126.1 million adults in the United States reported at least some pain in the prior three months, with 23.4 million of these adults reporting “a lot” of pain [1]. Opioid analgesics are well recognized as effective therapy for appropriately selected patients with moderate to severe pain [2,3]. Prescribing of opioids has increased dramatically in the last two decades, with an estimated 259 million prescriptions dispensed in 2012 [4]. This increase in opioid prescribing has been mirrored by a well-documented and concerning increase in opioid abuse, diversion, and overdose deaths [5]. In 2014, for example, the Centers for Disease Control estimated that nearly 19,000 overdose deaths in the United States were associated with prescription opioids [5]. Among approved prescription opioids, immediate-release (IR) hydrocodone combination products are the most commonly prescribed, with approximately 90 million prescriptions dispensed in 2015 [6]. Not surprisingly, IR hydrocodone combination products are also subject to the highest level of abuse and diversion among all the opioid products [7].
Abuse of IR hydrocodone combination products is most prevalent via the oral route of administration, with more than 80% of individuals being evaluated for substance abuse treatment endorsing this route [8]. However, 23% of adults and 43% of adolescents reported abuse of these products by the intranasal (IN) route within the last 30 days [8]. Hydrocodone combination products appear to have been the first opioid abused by many individuals being evaluated for substance abuse treatment, and initial abuse of such products is associated with progression to more potent opioids and riskier, nonoral routes of administration [9]. Frequent intranasal abuse of IR hydrocodone combination products has been associated with significant morbidity that can include perforated septa, serious fungal and bacterial infections, and, in some instances, oronasal fistulas [10–13]. Opioid products are manipulated for abuse to obtain a more intense and faster onset of effect, as reflected by an increase in peak plasma concentration (Cmax) and/or a shorter time to Cmax (Tmax). In human abuse potential studies, these altered pharmacokinetics are associated with subjective reports of higher drug liking and greater desire to take the drug again [14–18].
Attempts to reduce abuse of hydrocodone-containing combination analgesics have included restricting access by rescheduling these controlled substances from schedule III to the more restrictive schedule II classification, changing product labeling by adding warnings for prescribers, creating new guidelines that recommend shorter courses of therapy (usually ≤3 days and rarely >7 days), and implementing prescription monitoring plans [19–21]. The development of abuse-deterrent forms of IR hydrocodone combination products would afford an additional strategy to reduce abuse and diversion of these products. In the extended-release (ER) class of opioids, which account for only approximately 10% of all opioid prescriptions, there are a number of hydrocodone-, oxycodone-, and morphine-containing formulations that have been approved with abuse-deterrent product labeling [21–28]. Given the widespread prescribing and availability of IR opioids and that abusers report a preference for IR over ER opioids due to their fast onset and ease of product manipulation [29], there is an unmet need for IR opioids with abuse-deterrent features.
Benzhydrocodone is a hydrocodone prodrug that has been combined with acetaminophen (APAP) in a novel IR compound, benzhydrocodone/APAP. Chemically, benzhydrocodone is a new molecular entity formed by a covalent bond between hydrocodone and benzoic acid. The pharmacologically inactive prodrug benzhydrocodone is designed to quickly and effectively release its active hydrocodone component through esterase metabolism in the intestinal tract following oral administration. However, in vitro stability and human pharmacokinetic studies indicate that bypassing presystemic metabolism, as occurs with nonoral administration, results in significantly reduced conversion to hydrocodone [30,31]. While this means that benzhydrocodone is still susceptible to oral abuse, it may be a poor candidate for nonoral routes of abuse.
Here we report results of a trial to assess the relative bioavailability, IN abuse potential, and safety of benzhydrocodone/APAP compared with commercially available hydrocodone/APAP in nondependent recreational opioid users.
Methods
This study was conducted in accordance with the 2015 FDA guidance on the evaluation of abuse-deterrent opioids [32]. This was a single-center, randomized, double-dummy, double-blind, two-part study comprising a Dose Selection phase (Part A) and a Main Study (Part B) to assess the IN abuse potential for benzhydrocodone/APAP. Part A was designed to establish the maximum tolerated dose (MTD) of crushed benzhydrocodone/APAP and crushed hydrocodone/APAP to be used in Part B.
The study was conducted in accordance with Good Clinical Practice, as outlined in the International Conference of Harmonisation Guidelines governing protection of human subjects and the obligations of clinical investigators, as well as in compliance with the FDA Code of Federal Regulations and Health Canada regulations. The study complied with the World Medical Association Declaration of Helsinki. Before study start, the protocol was reviewed and approved by an institutional review board (IRB). Prior to entry into the study, each subject was required to read, sign, and date an IRB-approved informed consent form.
Subject Eligibility
Screening
At screening, subjects were eligible if they were experienced opioid users with a history of opioid use for nontherapeutic reasons on 10 or more occasions within the past year and at least once in the 12 weeks prior to the study. Subjects were eligible if they were male or female, 18 to 55 years of age, and were not currently physically dependent on opioids, per the criteria listed in the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision [33]. Eligible subjects were required to be experienced with IN insufflation (defined as ≥3 IN uses in the year before screening).
Subjects were ineligible if they had chronic respiratory disease, clinically significant systemic disease, an anatomic nasal deformity (e.g., deviated or perforated septum), or ongoing rhinorrhea or nasal infection. Subjects with a history of drug or alcohol dependence or who had participated in, were currently participating in, or were seeking treatment for substance-related disorders (excluding nicotine and caffeine) were ineligible. Identical screening procedures were implemented for Part A and Part B, and subjects who participated in Part A were allowed to join Part B.
Qualification Phase
Eligible subjects in either Part A or Part B underwent an in-clinic naloxone challenge (to confirm the absence of physical opioid dependence) [30] and a Drug Discrimination Test (to ensure that the subject could identify active drug effects). In the Drug Discrimination Test, subjects received double-blind single IN doses of active drug and placebo, separated by at least 24 hours. The active drugs administered during the Drug Discrimination Test were hydrocodone bitartrate powder (40 mg) and crushed hydrocodone/APAP tablets (at the MTD) in Part A and Part B, respectively. Weight-matched microcrystalline-cellulose powder was given as placebo in both parts.
Study Procedures
Part A was a randomized, double-blind, dose escalation (or reduction) trial in cohorts of eight subjects. Subjects of each cohort were assigned to one of two dose escalation sequences (four subjects each) testing IN administration of either crushed benzhydrocodone/APAP or hydrocodone/APAP vs placebo, administered on consecutive days, separated by approximately 24 hours. Subjects were not permitted to blow their noses for two hours following dosing. After completion of each cohort’s dosing, the resulting data were unblinded to evaluate the need for further cohorts.
The following three criteria were considered for the selection of the dose to be used in Part B: 1) The dose must be safe, well tolerated, and be completely insufflated by at least two of four subjects in each subcohort. 2) There must be a peak difference of 15 or more points vs placebo on the bipolar visual analog scale (VAS) for Drug Liking in at least two of the four subjects. 3) There must be no treatment-related, moderate to severe adverse events (AEs) that would pose a significant safety/tolerability concern and no clinically significant respiratory depression.
In Part B, all enrolled subjects received five in-clinic treatments (Table 1) separated from each other by a minimum 96-hour washout period and administered in one of 10 crossover sequences; each subject was assigned treatments by a computer-generated randomization scheme with a Williams design. Each treatment included a crushed IN dose and an oral intact capsule in a double-dummy design. Subjects were not permitted to blow their noses for two hours following dosing.
Table 1.
Crushed IN and intact oral doses for Part B
| TreatmentSequence | CrushedIN Dose | Oral IntactDose (Capsules) |
|---|---|---|
| A | Placebo powder | Placebo capsules |
| B | Placebo powder | Benzhydrocodone/APAP (at MTD) |
| C | Benzhydrocodone/APAP (at MTD) | Placebo capsules |
| D | Hydrocodone/APAP(at MTD) | Placebo capsules |
| E | Placebo powder | Hydrocodone/APAP(at MTD) |
APAP = acetaminophen; MTD = maximum tolerated dose.
Assessments
At screening, medical history (including drug use), demographic information, baseline laboratory values, urinalysis (including drug screening), and physical examination were collected for each subject. Safety assessments included examination of subjects’ nasal cavities before and after each insufflated dose. Other safety assessments included continuous monitoring for AEs and pulse oximetry before and up to eight hours after IN dosing.
Nasal Effects Assessments were performed in each dosing period predose and at 1.5, 2, 4, 6, 8, 12, and 24 hours postdose using a four-point Likert scale, with possible scores of 0 (none), 1 (mild), 2 (moderate), and 3 (severe). Six parameters were assessed: nasal burning, facial pain/pressure, need to blow nose, nasal irritation, nasal congestion, and runny nose/nasal discharge.
Pharmacokinetic Analyses
During Part B, plasma hydrocodone concentrations were determined from blood samples obtained predose and at five minutes, and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 hours postdose. Descriptive statistics were calculated for parameters including peak plasma hydrocodone concentration (Cmax), time to maximum concentration (Tmax), and area under the plasma hydrocodone concentration-time curve from time 0 to 0.5 hours (AUC0–0.5), 1 hour (AUC0–1), 2 hours (AUC0–2), 4 hours (AUC0–4), 8 hours (AUC0–8), and 24 hours (AUC0–24). A linear mixed-effects model was used to analyze the natural log-transformed pharmacokinetic (PK) parameters (Cmax and AUCs). The least squares (LS) geometric mean ratios (test/control) along with the corresponding 90% confidence intervals (CIs) were calculated. An additional post hoc measure of the abuse quotient (AQ = Cmax/Tmax) was calculated for each active treatment arm [34].
Pharmacodynamic Analyses
During Part B, Drug Liking was assessed at five minutes and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours postdose. At each of these time points, subjects were asked, “Do you like the drug effect you are feeling now?” Subjects responded on a 100-point, bipolar VAS anchored at 0 by “strong disliking,” at 50 by “neither like nor dislike,” and at 100 by “strong liking.” Ease of insufflation (“snorting”) was assessed at within five minutes postdose. For this rating, subjects utilized a 100-point, unipolar VAS anchored at 0 by “very easy” and at 100 by “very difficult.”
The study’s primary pharmacodynamic (PD) end point was Drug Liking peak effect (Emax). Secondary end points included time to peak effect (TEmax) and area under the Drug Liking effect curve (AUE), which was calculated for time 0 to 0.5 hours (AUE0–0.5), 1 hour (AUE0–1), 2 hours (AUE0–2), 4 hours (AUE0–4), 8 hours (AUE0–8), and 24 hours (AUE0–24). In subjects completing all treatments, results were tested by analysis of variance (ANOVA) for statistically significant differences between treatments (P < 0.05). The primary comparison was between IN benzhydrocodone/APAP and IN hydrocodone/APAP. Ease of insufflation results were tested using the same methodology.
Overall Drug Liking and Take Drug Again were also assessed as secondary endpoints using a bipolar VAS scale at 12 and 24 hours after study drug administration.
Determination of Sample Size
A total of 40 completed subjects were expected to provide at least 90% power at the one-sided significance level of 0.025 to detect treatment differences of 10.6 or more points in Emax for the bipolar Drug Liking VAS, assuming a correlation of 0.5 and a standard deviation of differences of 20 points.
Statistical Methods
In both study parts, descriptive statistics (N, arithmetic mean, SD, median, minimum, maximum, first and third quartile limits, and coefficient of variation [CV]) were calculated for all PD parameters collected in the randomized populations. For Part B, statistical analysis of the PD primary end point Drug Liking Emax was performed using a mixed-effects model of ANOVA for the completer and the per-protocol populations. The model utilized SAS PROC MIXED to perform the analysis and included treatment (five levels), period (five levels), and sequence (10 levels) as fixed effects, and subject-nested-in-sequence as random effect. The five treatment levels were placebo, IN crushed benzhydrocodone/APAP, oral intact benzhydrocodone/APAP, IN crushed hydrocodone/APAP, and oral intact hydrocodone/APAP.
Based on the analysis of the ANOVA model, pairwise comparisons of LS means between individual treatments were conducted at the significance level of 0.05 (two-sided) for consistency, using a model-based t test. The differences of pairwise LS means were reported for each comparison. Based on equivalent dose, the primary null hypotheses of no difference in Drug Liking Emax between the reference drug and test drug were tested for the following comparisons: IN hydrocodone/APAP vs IN benzhydrocodone/APAP and oral intact hydrocodone/APAP vs oral intact benzhydrocodone/APAP. Abuse quotients were calculated for individual subjects, and the mean AQ for each treatment was compared using descriptive statistics.
Results
Subject Disposition
Of 110 subjects admitted to the qualification phase of Part A, 51 were randomized and received study drug during the dose selection phase, and 49 completed Part A. During Part A, seven cohorts received IN doses of benzhydrocodone/APAP and placebo, and six cohorts received IN doses of hydrocodone/APAP and placebo. Treatment doses included one to four crushed tablets. For benzhydrocodone/APAP, two-tablet doses were the highest that could be reliably insufflated (Table 2). As a result, two-tablet doses (totaling 13.34/650 mg of benzhydrocodone/APAP and 15/650 mg of hydrocodone bitartrate/APAP) providing equimolar dosages of hydrocodone were administered in Part B.
Table 2.
Study drug insufflation during Part A
| Dose (Number of Crushed Tablets) | Benzhydrocodone/APAP |
Hydrocodone/APAP |
||
|---|---|---|---|---|
| Total Number of Subjects | Number Completing Insufflations (%)* | Total Number of Subjects | Number Completing Insufflations (%)* | |
| 1 | 8 | 7 (88) | 8 | 8 (100) |
| 2 | 7 | 6 (86)† | 8 | 6 (75)† |
| 3 | 6 | 3 (50) | 4 | 4 (100) |
| 4 | 4 | 1 (25) | 4 | 1 (25) |
APAP = acetaminophen.
Each IN treatment to be completed within 10 or fewer minutes.
Maximum tolerated dose, per protocol.
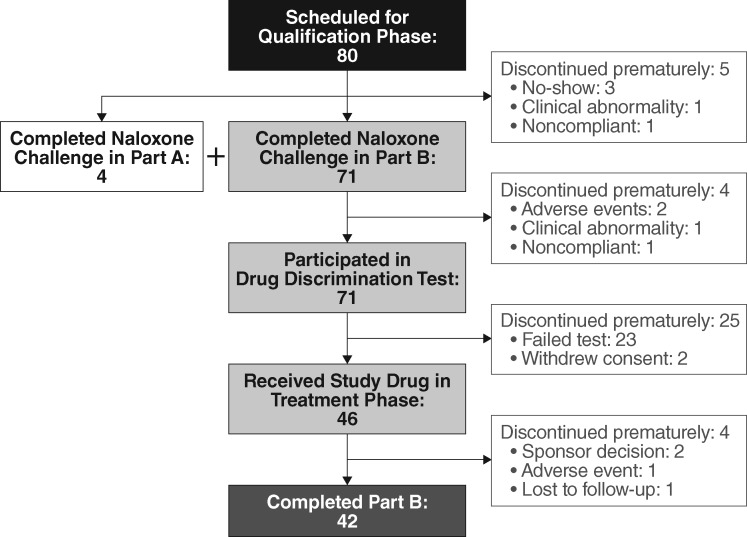
Of 80 subjects admitted to the qualification phase of Part B (41 from Part A, including four not requiring naloxone rechallenge and 39 newly recruited), 46 were randomized and received study drug during the treatment phase, and 42 completed Part B. Subject disposition during Part B is summarized in Figure 1. Baseline subject characteristics for both parts of the study are summarized in Table 3.
Figure 1.
Subject disposition in Part B.
Table 3.
Demographic and drug abuse characteristics in randomized subjects
| Characteristic | Part A (N = 51) | Part B (N = 46) | |
|---|---|---|---|
| Age, y | Mean (SD) | 39.3 (9.2) | 37.6 (9.3) |
| Median (range) | 38 (21–55) | 37 (19–54) | |
| Sex, No. (%) | Male | 39 (76.5) | 35 (76.1) |
| Female | 12 (23.5) | 11 (23.9) | |
| Race, No. (%) | White | 43 (84.3) | 31 (67.4) |
| Black/African American | 6 (11.8) | 11 (23.9) | |
| Asian | 0 | 2 (4.3) | |
| Other | 2 (3.9) | 2 (4.3) | |
| Weight, kg | Mean (SD) | 78.8 (12.7) | 79.5 (11.8) |
| Median (range) | 75.1 (56.0–116.7) | 77.7 (61.6–105.0) | |
| BMI, kg/m2 | Mean (SD) | 25.5 (2.8) | 25.9 (3.1) |
| Median (range) | 25.3 (19.9–31.9) | 25.7 (20.9–32.0) | |
| Maximum COWS Total score | Mean (SD) | 0.4 (0.5) | 0.5 (0.6) |
| Median (range) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) | |
| Most often used drug class in last 12 mo | |||
| Opioids/morphine derivatives, No. (%) | 12 (23.5) | 13 (28.3) | |
| Stimulants, No. (%) | 39 (76.5) | 33 (71.7) | |
| Number of times abusing drugs in last 12 wk | |||
| Mean (SD) | 41.1 (34.4) | 45.1 (57.2) | |
| Median (range) | 35.0 (8.0–215) | 33.0 (5.0–330.0) | |
| Number of times IN abuse in last 12 wk | |||
| Mean (SD) | 11.5 (7.8) | 11.8 (11.9) | |
| Median (range) | 10.0 (2.0–30.0) | 8.0 (1.0–70.0) | |
BMI = body mass index; COWS = clinical opiate withdrawal scale; IN = intranasal.
There were 42 randomized subjects who completed all five periods of Part B, completed at least one postdose time point of the Drug Liking VAS from each period, and contributed at least one postdose PK time point from each period. This population served as the primary population for the PD analysis.
Relative Bioavailability
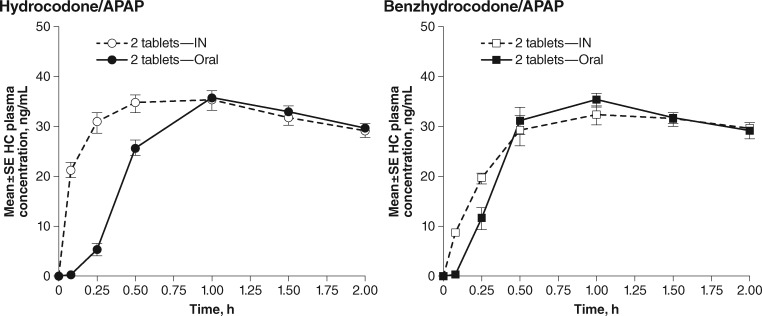
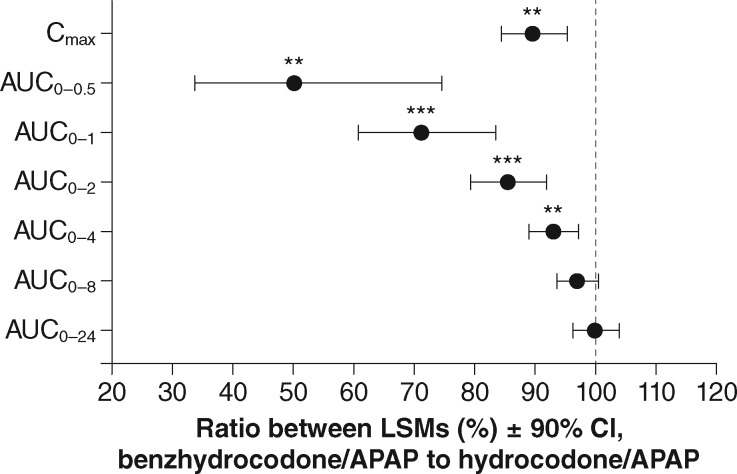
Mean hydrocodone plasma concentrations for subjects on active treatments (Part B) up to two hours postdose for oral vs IN hydrocodone/APAP and for oral vs IN benzhydrocodone/APAP are shown in Figure 2. The corresponding PK parameters are listed in Table 4. Relative to oral hydrocodone/APAP, IN administration produced a more rapid rise in hydrocodone plasma concentration with no alteration in Cmax. In contrast, for benzhydrocodone/APAP, IN administration produced hydrocodone plasma concentrations over time that were similar to oral administration but with a 13% lower Cmax (P = 0.0004). For the comparison of the IN routes of administration, Cmax was reduced by approximately 11% (P = 0.0027) for IN benzhydrocodone/APAP relative to IN hydrocodone/APAP, with no statistical difference in AUClast or AUCinf. Early cumulative systemic hydrocodone exposure was reduced by approximately 50% (AUC0-0.5), 29% (AUC0-1), and 15% (AUC0-2) for IN benzhydrocodone/APAP vs IN hydrocodone/APAP (P < 0.01 for all comparisons) (Figure 3). The slower rate of rise for IN benzhydrocodone/APAP relative to IN hydrocodone/APAP yielded an AQ value that was approximately 33% lower for IN benzhydrocodone/APAP (Table 4).
Figure 2.
Mean hydrocodone concentrations after active treatment dosing during part B. APAP = acetaminophen; IN = intranasal.
Table 4.
Pharmacokinetic parameters for active treatment arms
| Oral Benzhydrocodone/APAP | IN Benzhydrocodone/APAP | IN Hydrocodone/APAP | Oral Hydrocodone/APAP | |
|---|---|---|---|---|
| (N = 42) | (N = 43) | (N = 43) | (N = 42) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Cmax, ng/mL | 40.4 (12.1) | 34.7 (8.7) | 39.1 (11.5) | 39.9 (13.8) |
| Tmax, h | 1.21 (0.44) | 1.29 (0.47) | 0.97 (0.48) | 1.42 (0.59) |
| AUClast | 241.2 (71.6) | 265.2 (67.8) | 265.1 (60.6) | 243.3 (71.6) |
| AUCinf | 252.7 (77.7) | 278.3 (75.1) | 276.6 (65.3) | 254.5 (78.4) |
| AQ (Cmax/Tmax) | 38.6 | 31.9 | 56.5 | 34.5 |
APAP = acetaminophen; AQ = abuse quotient; Cmax = maximum concentration; IN = intranasal; Tmax = time to maximum concentration.
Figure 3.
Ratios of log-transformed geometric least squares mean values of pharmacokinetic parameters for IN benzhydrocodone/APAP and IN hydrocodone/APAP. **P<0.01; ***P<0.001, linear mixed-effects model. APAP = acetaminophen; AUC0-0.5, AUC0-1, AUC0-2, AUC0-4, AUC0-8, AUC0-24 = area under the plasma concentration time curve from time 0 to the specified time point, in hours; Cmax = maximum observed concentration; CI = confidence interval.
IN Abuse Potential
Drug Liking Emax was significantly greater for the positive controls of IN hydrocodone/APAP and oral intact hydrocodone/APAP compared with placebo (P < 0.0001 each comparison), thereby confirming study validity. Mean Drug Liking Emax was close to neutral or 50 (“neither like nor dislike”) for placebo, at 53.0 points (SD = 7.7 points).
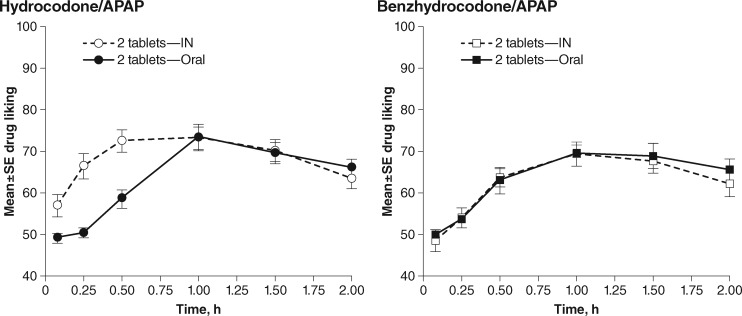
For the primary end point of Drug Liking Emax, mean values were comparable across the active treatments, ranging from 75.9 points (SD = 15.1 points) for IN benzhydrocodone/APAP to 79.0 points (SD = 17.6 points) for IN hydrocodone/APAP (LSM difference [95% CI] between IN treatments: 3.1 [-2.5, 8.7], P = 0.2814). Trends in Drug Liking over the first two hours paralleled the PK results (Figure 4). Drug Liking VAS at early time intervals (AUE0–0.5, AUE0–1, and AUE0–2) was significantly lower for IN benzhydrocodone/APAP than for IN hydrocodone/APAP (P < 0.0001, P < 0.0001, and P = 0.0079, respectively). Drug Liking over time was essentially identical for oral and IN benzhydrocodone/APAP, with no significant differences over any of these early time intervals (AUE0–0.5: P = 0.5583, AUE0–1: P = 0.8770, and AUE0–2: P = 0.6594, respectively). In contrast, the Drug Liking LS mean scores were significantly greater for IN hydrocodone/APAP compared with the oral route at these time points (P ≤ 0.0001, P ≤ 0.0001, and P = 0.0250, respectively).
Figure 4.
Mean Drug Liking ratings* after active treatment dosing during the treatment phase of Part B. *On a 100-point bipolar visual analog scale anchored at 0 by “strong disliking,” at 50 by “neither like nor dislike,” and at 100 by “strong liking.” APAP = acetaminophen; IN = intranasal.
Among the four treatments, IN hydrocodone/APAP produced the shortest mean TEmax of 1.0 hours (SD = 1.1 hours), followed by IN benzhydrocodone/APAP (mean = 1.8 hours, SD = 3.7 hours), oral hydrocodone/APAP (mean = 2.0 hours, SD = 3.6 hours), and oral benzhydrocodone/APAP (mean = 2.0 hours, SD = 3.7 hours). Despite seemingly meaningful delays in mean TEmax for IN benzhydrocodone/APAP vs IN hydrocodone/APAP, and for oral vs IN hydrocodone/APAP, none of these differences reached statistical significance.
Similar to results for Drug Liking, mean scores at 12 hours were similar for Overall Drug Liking VAS between IN hydrocodone/APAP and IN benzhydrocodone/APAP (mean = 69.9, SD = 25.6, and mean = 69.2, SD = 23.4, respectively; P = 0.9101); the same was true for the 24-hour results (mean = 71.5, SD = 24.5, and mean = 68.9, SD = 24.9, respectively; P = 0.4688). All other comparisons between active treatment arms showed no statistically significant differences.
The mean Take Drug Again VAS results were also similar between IN hydrocodone/APAP and IN benzhydrocodone/APAP at 12 hours (mean = 72.1, SD = 27.9, and mean = 68.1, SD = 25.6, respectively) and 24 hours (mean = 71.1, SD = 26.8, and mean = 67.4, SD = 27.3, respectively). LS mean differences between the two IN treatments were not significant at either 12 hours (P = 0.3979) or 24 hours (P = 0.4020).
The VAS score for Ease of Insufflation was significantly higher (ie, more difficult) for benzhydrocodone/APAP than for IN hydrocodone/APAP, (P = 0.0100) at a mean of 57.0 (SD = 35.7) vs 43.3 (SD = 32.5). Also, for all six individual parameters and for the parameters’ average of the Nasal Effects Assessment, subjects reported that IN benzhydrocodone/APAP produced greater adverse nasal effects than IN hydrocodone/APAP (Table 5).
Table 5.
Nasal effects assessment parameters in randomized patients during Part B*
| Parameter | IN Benzhydrocodone/APAP | IN Hydrocodone/APAP | P† |
|---|---|---|---|
| (N = 44) | (N = 43) | ||
| Mean Emax (SD) | Mean Emax (SD) | ||
| Average | 1.5 (0.8) | 0.9 (0.8) | <0.0001 |
| Nasal burning | 1.6 (1.0) | 0.7 (0.7) | <0.0001 |
| Facial pain/pressure | 1.0 (1.0) | 0.5 (0.8) | <0.0001 |
| Need to blow nose | 1.5 (0.9) | 1.0 (0.9) | <0.0001 |
| Nasal irritation | 1.5 (1.0) | 0.7 (0.7) | <0.0001 |
| Nasal congestion | 1.5 (1.0) | 1.0 (0.8) | 0.0009 |
| Runny nose/nasal discharge | 1.4 (1.0) | 0.8 (0.9) | <0.0001 |
APAP = acetaminophen; Emax = maximum effect rating; IN = intranasal; LS = least squares.
Nasal effects were determined using a four-point Likert scale where 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
Statistically significant difference in LS means for the comparison of IN benzhydrocodone/APAP vs IN hydrocodone/APAP.
Safety
No deaths or serious AEs were reported during this study. Overall, the most common AEs were typical of opioids, with few differences between the two treatments. All 46 subjects randomized to treatment in Part B experienced at least one AE. The most common AEs were euphoric mood and somnolence, reported by 74% and 31% of subjects receiving oral benzhydrocodone/APAP, respectively, 77% and 34% who received IN benzhydrocodone/APAP, 67% and 37% who received IN hydrocodone/APAP, and 73% and 39% who received oral hydrocodone/APAP. One subject discontinued the study due to an AE (supraventricular extrasystoles and ventricular extrasystoles) that resolved on the same day as onset after the study drug was withdrawn. Respiratory, thoracic, and mediastinal AEs reported after IN dosing in the treatment phase of Part B are summarized in Table 6. Subjects who received IN benzhydrocodone/APAP reported these AEs more frequently than those who received IN hydrocodone/APAP, particularly with regard to AEs of nasal discomfort, nasal congestion, rhinorrhea, and throat irritation.
Table 6.
Respiratory, thoracic, and mediastinal AEs during treatment Part B
| AE | IN Benzhydrocodone/APAP, | IN Hydrocodone/APAP, | Placebo* |
|---|---|---|---|
| (N = 44) | (N = 43) | (N = 42) | |
| No. (%) | No. (%) | No. (%) | |
| Any AE of interest | 29 (65.9) | 9 (20.9) | 10 (23.8 |
| Nasal discomfort | 16 (34.4) | 2 (4.7) | 2 (4.8) |
| Nasal congestion | 7 (15.9) | 2 (4.7) | 6 (14.3) |
| Rhinorrhea | 7 (15.9) | 4 (9.3) | 3 (7.1) |
| Throat irritation | 6 (13.6) | 3 (7.0) | 0 |
| Oropharyngeal pain | 1 (2.3) | 1 (2.3) | 1 (2.4) |
| Dry throat | 1 (2.3) | 0 | 0 |
| Upper airway cough syndrome | 0 | 1 (2.3) | 0 |
AE = adverse event; APAP = acetaminophen; IN = intranasal.
Placebo was administered both IN and orally. Each IN active treatment was co-administered with oral placebo.
Discussion
The objective of this study was to examine the intranasal abuse potential of the novel prodrug combination benzhydrocodone/APAP relative to hydrocodone/APAP in recreational opioid abusers. While there were no significant differences in the primary end point of Drug Liking Emax, there were notable pharmacodynamic and pharmacokinetic differences that are likely to provide less incentive for IN abuse of benzhydrocodone/APAP vs hydrocodone/APAP. For example, pharmacokinetic data demonstrate an approximate 11% decrease in Cmax for IN benzhydrocodone/APAP compared with IN hydrocodone/APAP and a 15% to 50% reduction in cumulative hydrocodone exposure at early time intervals up to two hours. Correspondingly, subjects receiving IN benzhydrocodone/APAP showed significant reductions in Drug Liking VAS over the same early time intervals. Nasal irritant effects of IN benzhydrocodone/APAP were markedly greater compared with IN hydrocodone/APAP across several different nasal effect measures, a finding that may also contribute to a lower abuse potential of this novel prodrug combination.
The lack of differences in Drug Liking Emax between benzhydrocodone/APAP and hydrocodone/APAP warrants further consideration. Due to the large volume of powder insufflated (1,100 mg for benzhydrocodone/APAP and 850 mg for hydrocodone/APAP) and in light of the fact that subjects were not permitted to blow their nose for the first two hours after administration, it is very likely that a significant portion of the intranasal dose was swallowed for both treatments. The swallowed portion of the benzhydrocodone dose was rapidly converted to hydrocodone in the intestinal tract and was therefore orally bioavailable. The portion of the benzhydrocodone dose that was absorbed via the nasal mucosa, however, was converted much less efficiently, and thus did not significantly contribute to early systemic hydrocodone plasma concentrations and Drug Liking effects. Consistent with this interpretation are findings from a study in which pure benzhydrocodone and hydrocodone active pharmaceutical ingredient (API) were administered intranasally. Under these conditions, there were marked reductions in all indices of hydrocodone exposure and Drug Liking for IN benzhydrocodone API relative to IN hydrocodone API [30]. These collective findings confirm that benzhydrocodone retains its prodrug properties when absorbed via the nasal mucosa, regardless of whether it is derived from crushed tablets or the pure API.
With respect to the slower rise in drug plasma concentrations and Drug Liking observed in the current study for IN benzhydrocodone/APAP relative to hydrocodone/APAP, a longstanding and established literature suggests that this profile would correspond to a potential product that is less abusable. Across a range of psychoactive drugs, including opioids [15,16], stimulants [14], benzodiazepines [18], and barbiturates [17], the rate of rise of drug concentrations has been found to be a critical determinant of abuse potential. Specifically, a more rapid rate of rise in drug concentration corresponds to greater abuse potential. In a study by Comer et al., oxycodone engendered high levels of Drug Liking when infused intravenously over a two-minute interval but was not differentiated from placebo when infused over 15-, 30-, 60-, or 90-minute intervals [15]. Such findings indicate that relatively small differences in rate of onset—on the order of minutes—can have marked effects on the overall abuse potential of an opioid formulation. It is important to note that differences in time to peak concentration (Tmax) have also shown to correspond to different Drug Liking scores between two formulations, even when peak concentrations (Cmax) were comparable [17,18].
These above-referenced findings are also consistent with a large behavioral economics literature documenting that drug abusers display impulsive behaviors relative to non–drug abusers. That is, abusers steeply discount future rewards, generally preferring smaller, immediate rewards relative to larger, delayed rewards [35–37]. This inability to delay rewards has been described as a core feature of substance abusers. As such, an opioid formulation such as benzhydrocodone/APAP that significantly delays the onset of positive subjective effects can be expected, over time, to be a less preferred drug compared with similar IR opioids with rapid onset after intranasal administration.
To the extent that the abuse-related effects of opioids are a composite of both positive and negative pharmacodynamic effects, the finding of greater nasal effects for benzhydrocodone/APAP may also contribute to lower abuse potential relative to hydrocodone/APAP. At odds with this interpretation are Take Drug Again data indicating that, despite these aversive nasal effects, subjects reported similar willingness to take both opioid combinations again. A more comprehensive understanding of the abuse potential of these two drug combinations could be gleaned from additional studies that utilize drug choice as the dependent measure rather than relying solely on verbal reports of Drug Liking and Take Drug Again after a single drug administration. Experimental designs such as the multiple choice procedure, in which abusers make a choice between two drugs or between a drug vs money, can provide additional information on a drug’s reinforcing effects that is pertinent to predicting real-world abuse [38–40]. Choice procedures have proven valuable for identifying differences in the relative abuse potential of opioids even under conditions where few or no differences in positive subjective effects were observed [40]. For immediate-release opioids that must be orally bioavailable within a very short period of time to provide analgesia, such study designs may prove to be more informative than Drug Liking paradigms.
This study has a number of limitations. Subjects in this study were asked to insufflate relatively large volumes of the powdered drug combination within a short period of time. The exact proportions of nasal vs oral absorption under these conditions are not known, and it is therefore not possible to fully disentangle the relative contributions of each route to the pharmacokinetic and pharmacodynamic effects. Anecdotal reports indicate that some individuals may prefer to insufflate small portions of powder in discrete intervals over time, a practice that may limit the volume of powder that is swallowed. It is also possible that abusers combine routes of administration within a single episode, insufflating one tablet of crushed hydrocodone/APAP while also taking one or more intact tablets orally. Understanding these abuse patterns may inform the development of future abuse potential studies of IR hydrocodone combinations. Subjects in this study were nondependent, recreational opioid abusers who administered opioids for nonmedical purposes 10 or more times in the past year and were experienced with the intranasal route of administration. These results, therefore, may not be generalizable to other populations such as naïve abusers or opioid-dependent subjects.
In summary, benzhydrocodone/APAP showed lower systemic exposure to hydrocodone and lower Drug Liking in early time intervals (up to 2 hours) compared with hydrocodone/APAP. Benzhydrocodone/APAP also produced greater adverse nasal effects than hydrocodone/APAP. This profile can be expected to provide a level of deterrence to the intranasal route of abuse for this combination.
Acknowledgments
The authors thank Beatrice Setnik, PhD, of INC Research, for contributions to the design and conduct of the study. Editorial assistance in formatting, proofreading, copyediting, and fact-checking was provided by The Curry Rockefeller Group, LLC. KemPharm, Inc., provided funding to CRG for all editorial support.
References
- 1. Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015;168:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65(RR-1):1–49. [DOI] [PubMed] [Google Scholar]
- 4. Paulozzi LJ, Mack KA, Hockenberry JM.. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines—United States, 2012. MMWR 2014;6326:563–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Califf RM, Woodcock J, Ostroff S.. A proactive response to prescription opioid abuse. N Engl J Med 2016;37415:1480–5. [DOI] [PubMed] [Google Scholar]
- 6. IMS Institute. Medicines Use and Spending in the US: A Review of 2015 and Outlook to 2020. Parsippany, NJ: IMS Institute for Healthcare Informatics; 2016. [Google Scholar]
- 7. Drug Enforcement Administration. Drug Fact Sheet. Hydrocodone. Available at: www.dea.gov (accessed July 12, 2016).
- 8. Cassidy TA, Oyedele N, Mickle TC, Guenther S, Budman SH.. Patterns of abuse and routes of administration for immediate-release hydrocodone combination products Pharmacoepi Drug Saf 2017; doi: 10.1002/pds.4249. [DOI] [PMC free article] [PubMed]
- 9. Cassidy TA, Oyedele N, Beaumont J, Guenther S, Mickle TC, Budman SH Progression of non-medical use of hydrocodone combination products: results from an Internet survey of recreational drug abusers. Presented at International Conference on Opioids. Boston, MA; 2016.
- 10. Alexander D, Alexander K, Valentino J.. Intranasal hydrocodone-acetaminophen abuse induced necrosis of the nasal cavity and pharynx. Laryngoscope 2012;12211:2378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Houlton JJ, Donaldson AM, Zimmer L, Seiden A.. Intranasal drug-induced fungal rhinopharyngitis. Int Forum Allergy Rhinol 2012;22:130–4. [DOI] [PubMed] [Google Scholar]
- 12. Vosler PS, Ferguson BJ, Contreras JI et al. , Clinical and pathologic characteristics of intranasal abuse of combined opioid-acetaminophen medications. Int Forum Allergy Rhinol 2014;410:839–44. [DOI] [PubMed] [Google Scholar]
- 13. Sloan PA, Klimkina O.. Intranasal abuse of prescription hydrocodone/acetaminophen results in oronasal fistula: A case report. J Opioid Manag 2009;56:383–5. [DOI] [PubMed] [Google Scholar]
- 14. Abreu ME, Bigelow GE, Fleisher L, Walsh SL.. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;1541:76–84. [DOI] [PubMed] [Google Scholar]
- 15. Comer SD, Ashworth JB, Sullivan MA et al. , Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J Opioid Manag 2009;54:203–12. [DOI] [PubMed] [Google Scholar]
- 16. Marsch LA, Bickel WK, Badger GJ et al. , Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther 2001;2993:1056–65. [PubMed] [Google Scholar]
- 17. de Wit H, Bodker B, Ambre J.. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl) 1992;107(2–3):352–8. [DOI] [PubMed] [Google Scholar]
- 18. Mumford GK, Evans SM, Fleishaker JC, Griffiths RR.. Alprazolam absorption kinetics affects abuse liability. Clin Pharmacol Ther 1995;573:356–65. [DOI] [PubMed] [Google Scholar]
- 19. Drug Enforcement Administration. Schedules of controlled substances: Rescheduling of hydrocodone combination products from schedule III to schedule II. Federal Register 2014;79163:49661–82. [PubMed] [Google Scholar]
- 20.FDA Announces Enhanced Warnings for Immediate-Release Opioid Pain Medications Related to Risks of Misuse, Abuse, Addiction, Overdose and Death. US Department of Health and Human Services. Silver Spring, MD: US Food and Drug Administration; 2016. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491739.htm (accessed June 15, 2016).
- 21. Volkow ND, McLellan AT.. Opioid abuse in chronic pain–misconceptions and mitigation strategies. N Engl J Med 2016;37413:1253–63. [DOI] [PubMed] [Google Scholar]
- 22.Embeda [package insert]. Bristol, TN: King Pharmaceuticals, Inc.; 2009.
- 23.Targiniq [package insert]. Stamford, CT: Purdue Pharma L.P.; 2014.
- 24.Hysingla [package insert]. Stamford, CT: Purdue Pharma L.P.; 2015.
- 25.MorphaBond [package insert]. Valley Cottage, NY: Inspirion Delivery Technologies LLC; 2015.
- 26.Oxycontin ER [package insert]. Stamford, CT: Purdue Pharma L.P.; 2015.
- 27.Xtampza [package insert]. Cincinnati, OH: Patheon Pharmaceuticals; 2016.
- 28.Troxyca ER [package insert]. New York: Pfizer, Inc.; 2016.
- 29. Cicero TJ, Ellis MS, Kasper ZA.. Relative preferences in the abuse of immediate-release versus extended-release opioids in a sample of treatment-seeking opioid abusers. Pharmacoepi Drug Saf 2017;261:56–62. [DOI] [PubMed] [Google Scholar]
- 30. Mickle T, Guenther S, Roupe KA, Zhou J, Dickerson D, Webster L. Pharmacokinetics and abuse potential of benzhydrocodone, a novel prodrug of hydrocodone, after intranasal administration in recreational drug users. Presented at the International Conference on Opioids. Boston, MA; 2016. [DOI] [PMC free article] [PubMed]
- 31.Data on File. Coralville, IA: KemPharm, Inc.; 2014.
- 32.Food and Drug Administration. US Department of Health and Human Services. Guidance for Industry: Abuse-Deterrent Opioids—Evaluation and Labeling. Final Guidance. 2015.
- 33. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 34. Moorman-Li R, Motycka CA, Inge LD et al. , A review of abuse-deterrent opioids for chronic nonmalignant pain. P T 2012;377:412–8. [PMC free article] [PubMed] [Google Scholar]
- 35. Madden GJ, Petry NM, Badger GJ, Bickel WK.. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: Drug and monetary rewards. Exp Clin Psychopharmacol 1997;53:256–62. [DOI] [PubMed] [Google Scholar]
- 36. Bickel WK, Marsch LA.. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction 2001;961:73–86. [DOI] [PubMed] [Google Scholar]
- 37. Johnson MW. An efficient operant choice procedure for assessing delay discounting in humans: Initial validation in cocaine-dependent and control individuals. Exp Clin Psychopharmacol 2012;203:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Griffiths RR, Troisi JR, Silverman K, Mumford GK.. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behav Pharmacol 1993;41:3–13. [PubMed] [Google Scholar]
- 39. Greenwald MK, Hursh SR.. Behavioral economic analysis of opioid consumption in heroin-dependent individuals: Effects of unit price and pre-session drug supply. Drug Alcohol Depend 2006;851:35–48. [DOI] [PubMed] [Google Scholar]
- 40. Comer SD, Metz VE, Cooper ZD et al. , Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behav Pharmacol 2013;24(5–6):504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]