Abstract
Background
Cognitive impairment and decline may signal the increased risk of incident cardiovascular disease (CVD). We examined associations of global cognitive function, as measured by the Modified Mini-Mental State Examination (3MS) and changes in 3MS over time, with incident CVD, individual CVD outcomes, CVD death, and all-cause mortality.
Methods
A total of 5,596 women (≥ 60) from the Women’s Health Initiative Memory Study free of CVD at baseline were followed for an average of 7.1 years. The 3MS was measured at baseline and annually thereafter. Cox proportional hazards regressions were used to model associations between baseline 3MS and changes in 3MS and time to events.
Results
In the fully-adjusted models for every 5-point lower baseline 3MS score, the risk was 12% greater for incident CVD, 37% for HF, 35% for CVD death, and 24% for all-cause mortality. No significant relationships were found for coronary heart disease (CHD), angina, stroke/transient ischemic attack (TIA), or coronary revascularization. When change in 3MS was added as a time-varying covariate in the fully-adjusted models, for every 1-point/year greater decline in 3MS, the risk was 4% greater for incident CVD, 10% for CHD, 9% for Stroke/TIA, 17% for CVD death, and 13% for all-cause mortality.
Conclusions
In older women free of prevalent CVD at baseline, lower baseline global cognitive function or decline in global cognitive function over time, increased risk of incident CVD, CVD death, and all-cause mortality.
Keywords: Cognitive impairment, Cognitive function decline, CVD, Mortality
Cardiovascular disease (CVD) is the leading cause of death among men and women worldwide. In 2011, nearly 787,000 people in the United States died from CVD, almost one-third of all deaths (1). The pervasive role of vascular brain injury, including stroke, silent infarction, cerebral atrophy, and white matter hyperintensities, in cognitive impairment and decline has been documented and comprehensively reviewed (2,3). Vascular disease in arterial beds outside the brain is associated with increased risk for incidence and progression of cognitive decline (4,5). History of CVD is linked to lower cognitive function and decline in cognitive function (6–9). There is substantial overlap among risk factors for cognitive impairment and CVD (3–5): hypertension, diabetes, obesity, dyslipidemia, smoking, physical inactivity, and high dietary fat and cholesterol intake are associated with increased risk of cognitive impairment (10–12).
Several (13–15), but not all (16,17), studies have found that cognitive impairment is related to higher risk for stroke or stroke death. Others have reported that lower cognitive function may signal increased risk of CVD, CVD death, and all-cause mortality (13,18,19). However, it is unknown whether cognitive decline is associated with CVD risk. To address this, we used repeated cognitive assessments from the Women’s Health Initiative Memory Study (WHIMS) to examine how changes in global cognitive function are associated with incident CVD in older postmenopausal women.
Methods
Study Population
WHIMS (20) was an ancillary study to the Women’s Health Initiative trials of hormone therapy (WHI-HT) (21): two parallel, randomized, double-blind, placebo-controlled clinical trials of conjugated equine estrogens treatment (CEE 0.625 mg/d) for women with a prior hysterectomy (ie, the E-alone trial) or conjugated equine estrogens in combination with medroxyprogesterone acetate (CEE 0.625 mg/d with MPA 2.5 mg/d) for women with an intact uterus (ie, the E+P trial). Recruitment to WHIMS occurred between 1996 and 1999. Eligible women were between ages 65 and 79 and free of dementia, as defined by the WHIMS protocols. A total of 7,479 women consented to participate in WHIMS, 4,532 in the E+P trial, and 2,947 in the E-Alone trial. Study medications in the E+P trial were terminated in 2002 (21) and in the E-alone trial in 2004 (22). WHIMS participants continued to be followed for health outcomes and annual cognitive assessments. Follow-up for the current secondary analysis was conducted through August 2005. All participants provided informed consent prior to participating in the study. The National Institutes of Health and Institutional Review Boards for all participating institutions approved the protocols and consent forms; all study protocols conform to the tenets of the Declaration of Helsinki.
Cognitive Assessment
WHIMS women underwent cognitive screenings by centrally-trained, certified, and masked interviewers for global cognitive functioning with the Modified Mini-Mental State (3MS) examination (23) at enrollment and annually thereafter. The 3MS consists of 15 items that sum from 0 to 100; higher scores reflect better global cognitive functioning. Participants who scored below preestablished cut-points (≤80 for participants with ≤8 years of education and ≤ 88 for participants with ≥9 years of education) were scheduled for a more extensive neurocognitive assessment and neuropsychiatric exam to determine the presence or absence of probable dementia or mild cognitive impairment (MCI), which were centrally adjudicated (24). Regardless of adjudicated dementia status, women continued to be scheduled for annual 3MS assessments.
Outcomes
In this analysis, the primary outcome was incidence of any CVD, which was defined as diagnosis of coronary heart disease (CHD, a combination of hospitalized myocardial infarction [MI], definite silent MI, or coronary death [cause adjudicated as due to definite or possible coronary heart disease]), heart failure (HF), hospitalization for angina, stroke (ischemic and hemorrhagic) and/or transient ischemic attack (TIA), coronary revascularization (documented coronary artery bypass graft, percutaneous transluminal coronary angioplasty, coronary stent placement or atherectomy), peripheral arterial disease (requiring hospitalization, being symptomatic and/or requiring intervention, and located in the abdominal aorta, iliac arteries or lower extremes), carotid artery disease (symptomatic and/or requiring intervention but not included if after a stroke), and other CVD death (adjudicated cause as cerebrovascular disease and other cardiovascular diseases, excluding pulmonary embolism and unknown cardiovascular disease). Other clinical outcomes of interest included the individual CVD outcomes, CVD death (coronary death, and other CVD death as defined above), and all-cause mortality. All potential outcomes initially were identified through self-report at semiannual contacts, and were then adjudicated by trained physician adjudicators after obtaining medical records. The underlying cause of death was classified on the basis of a death certificate, medical records, and other available records such as an autopsy report (25).
Baseline Assessment of Potential Risk Factors for CVD
Baseline demographic characteristics (age, race/ethnicity, and education), health behaviors/lifestyle (smoking status, alcohol intake, physical activity, and dietary patterns), psycho-social behaviors (sleep duration and depressive symptoms), medical history (hypertension, diabetes, and prior CVD), current medication use (statin, aspirin, and antihypertensive medications), and physical measurements (body mass index [BMI]) were collected via self-report and standardized assessments and have been described elsewhere (26). Physical activity was computed as metabolic equivalents per week (27). Dietary patterns were assessed by the Healthy Eating Index (HEI 2010) based on a self-administered food frequency questionnaire (28). Depressive symptoms were measured using an 8-item screening instrument developed for the Medical Outcomes Study that incorporated 6 items about depressive symptoms from the Center for Epidemiological Studies-Depression scale and 2 items from the Diagnostic Interview Schedule, with 0.06 as the cutoff point (29). Hypertension status was based on self-reported history of hypertension and information on current treatment. Diabetes status was based on self-report of diabetes and information on treatment. Treated diabetes was defined by whether the participant had ever been treated for diabetes with pills or shots. Prior CVD was defined as self-reported CHD, HF, coronary artery bypass graft or percutaneous transluminal coronary angioplasty, angina, peripheral arterial disease, carotid artery disease, stroke and/or TIA. BMI (kg/m2) was calculated as weight in kilograms (kg) divided by squared height in meters (m).
Analysis Sample
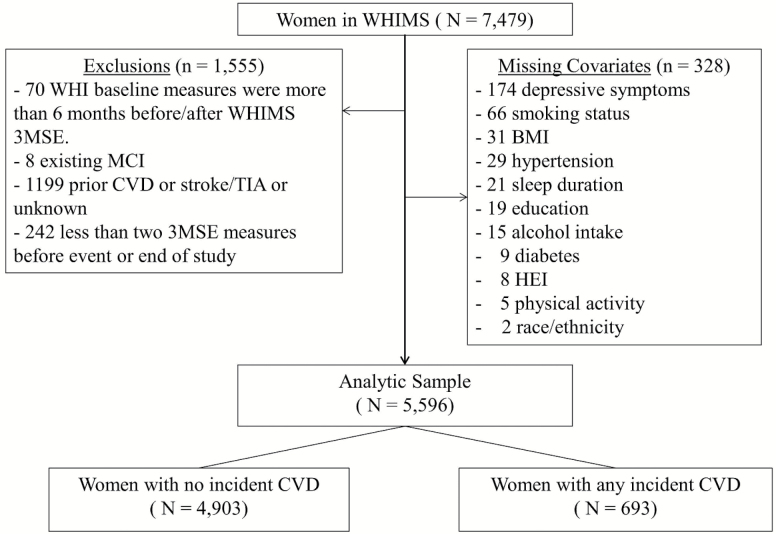
Women whose initial cognitive function assessment was within 6 months of randomization, who had a least two 3MS assessments (to calculate change), who had complete data on covariates, and who did not have prior CVD and MCI at baseline were included in analyses. For those who experienced at least one CVD event, we required two 3MS measurements before the event. This yielded a sample of 5,596 women (Figure 1).
Figure 1.
Study sample. CVD = Cardiovascular disease; HEI = Healthy Eating Index; MCI = Mild cognitive impairment; TIA = Transient ischemic attack; WHIMS = Women’s Health Initiative Memory Study.
Statistical Methods
We compared baseline characteristics for women with and without incident CVD during the follow-up, using chi-square and Student’s t tests. For a given outcome, time to the event was defined as the number of days from enrollment to the first occurrence of the event. Follow-up for events was censored at last contact or death before August, 2005, when the initial phase of WHI ended. For individual CVD outcome analyses, the reference group was women without any incident CVD events during the study. For participants who experienced multiple CVD events, evaluation was limited to their first CVD event, and they were excluded from the analyses of other individual CVD events. Analyses for individual peripheral arterial disease and carotid artery disease were not performed due to the relatively small number of events (24 and 42, respectively).
For each outcome, we performed two sets of analyses to model the relationship between time to event and global cognitive function as measured by the 3MS. First, we fitted Cox proportional hazards regression models to examine whether baseline 3MS predicted time to CVD events or death. Hazard ratios and corresponding 95% confidence intervals (CIs) based on a 5-point lower in 3MS were calculated. A 5-point difference in baseline 3MS was suggested to have a biological gradient, that is, an average decline for older people (70 years and above) over 5 years, and to be more likely clinically meaningful (30). We then fitted time-dependent proportional hazards regression models to explore how the risks of CVD events/death were related longitudinally to cognitive function using rate of change in 3MS score from baseline (1 point/year) as a predictor, in the presence of baseline 3MS. The rate of change in 3MS was calculated from the regression slope of all prior 3MS measurements for each woman and used as a time-dependent variable.
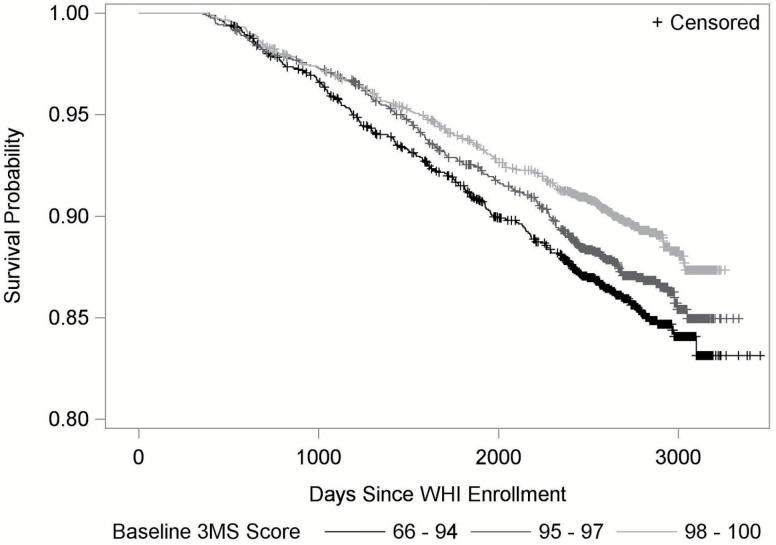
For each set of analyses, we fitted two models: the minimally-adjusted model, which adjusted for age, race/ethnicity, education, and WHI HT treatment assignment; and the fully-adjusted model, which additionally adjusted for baseline smoking status, alcohol intake, physical activity, dietary patterns, sleep duration, depressive symptoms, treated diabetes, hypertension, BMI, and medications use (statin, aspirin, and antihypertensive medicine). Proportional hazards assumptions for baseline 3MS and covariates were examined, using the score test based on scaled Schoenfeld residuals (31). For illustration purpose, we generated a Kaplan–Meier plot by categorizing participants into three groups based on their baseline 3MS score: 66–94, 95–97, and 98–100 (30,32).
Results
The 5,596 women in the study sample were followed up for an average of 7.1 (range: 1.0–9.5) years. During this period, there were 693 incident CVD events, 94 CVD deaths, and 331 all-cause deaths. The annualized CVD incidence was 17.4/1,000 persons per year.
Baseline characteristics are shown in Supplementary Table 1. Women who had an on-study CVD event had slightly lower mean baseline 3MS scores (mean: 95.0; standard deviation [SD]: 4.3) compared with women without an event (mean 95.6; SD: 4.1; p = .0009). Women experiencing events were older, less educated, and less likely to exhibit healthy behaviors and lifestyle at baseline. They also were more likely to have a higher BMI and chronic conditions such as treated diabetes and hypertension. We saw no differences in race/ethnicity, sleep duration, depressive symptoms, and reported use of statins or aspirin between the groups (Supplementary Table 1).
Baseline 3MS as a Predictor of Incident CVD
In minimally-adjusted models, lower baseline 3MS scores at baseline were associated with increased risk of CVD events (Figure 2). Adjustment for health behaviors, medical history, and medication use did not change results materially (Table 1). In fully-adjusted models, for every 5 points lower baseline 3MS score, the risk of incident CVD was 12% greater (hazard ratio (HR): 1.12, 95% confidence interval [CI]: 1.02–1.23, p = .02). Lower baseline 3MS scores were also associated with increased risk of CVD death (HR: 1.35, 95% CI: 1.07–1.69, p = .01) and all-cause mortality (HR: 1.24, 95% CI: 1.09–1.42, p = .001). For individual CVD events, baseline 3MS was only predictive of HF (HR: 1.37, 95% CI: 1.12–1.68, p = .002). Score tests for baseline 3MS and all other covariates did not show violations of the assumption of proportionality.
Figure 2.
Kaplan–Meier plot for time to any cardiovascular disease event by baseline 3MS score.
Table 1.
Associations Between 5-point Lower in Baseline 3MS Score and Incident CVD Events and Death
| Outcome | #Observations | #Events | Minimally-Adjusted Modela | Fully-Adjusted Modela | ||
|---|---|---|---|---|---|---|
| HRb (95% CI) | p Value | HRb (95% CI) | p Value | |||
| Any CVD | 5,596 | 693 | 1.12 (1.02, 1.23) | .02 | 1.12 (1.02, 1.23) | .02 |
| CHD | 5,048 | 145 | 1.06 (0.86, 1.30) | .59 | 1.07 (0.87, 1.31) | .53 |
| HF | 5,046 | 143 | 1.37 (1.13, 1.67) | .002 | 1.37 (1.12, 1.68) | .002 |
| Angina | 5,057 | 154 | 1.14 (0.94, 1.39) | .18 | 1.11 (0.91, 1.36) | .29 |
| Stroke/TIA | 5,095 | 192 | 1.08 (0.90, 1.30) | .39 | 1.09 (0.91, 1.31) | .34 |
| Coronary revascularization | 5,070 | 167 | 0.94 (0.75, 1.17) | .58 | 0.91 (0.73, 1.14) | .42 |
| CVD death | 5,596 | 94 | 1.32 (1.06, 1.65) | .01 | 1.35 (1.07, 1.69) | .01 |
| All-cause mortality | 5,596 | 331 | 1.23 (1.08, 1.40) | .002 | 1.24 (1.09, 1.42) | .001 |
Note: CHD = Coronary heart disease; CI = Confidence interval; CVD = Cardiovascular disease; HF = Heart failure; HR = Hazard ratio; 3MS = Modified Mini-Mental State Exam; TIA = Transient ischemic attack.
aMinimally-adjusted model: adjusted for age, race/ethnicity, education, hormone therapy trial study arm; fully-adjusted model: minimally-adjusted model covariates plus smoking status, alcohol intake, physical activity, HEI 2010 score, sleep duration, depressive symptoms, treated diabetes, hypertension, BMI, statin use, aspirin use and antihypertensive medications. bThe hazard ratios (HR) were for every 5 points lower in 3MS score, p < .05 in bold.
Decline of 3MS as a Predictor of Incident CVD
By the time of any incidence CVD or at end of follow-up, about 1% of the women had 3MS declines of 5 points/year or more, and 6% of the women had declines of 1 point/year or more. We found significant relationships between decline of global cognition and incident CVD, using change in 3MS score as a time-varying covariate (Table 2). The minimally-adjusted and fully-adjusted models had very similar findings. In the fully-adjusted models, the risk for any CVD event was 20% greater for every 1-point greater decline per year in 3MS (HR: 1.04, 95% CI: 1.00–1.08, p = .05). Statistically significant relationships were also found for CHD, stroke/TIA, CVD death, and all-cause mortality. For every 1 point/year greater decline in 3MS, the risk of CHD or stroke/TIA was more than 10% greater, and the risk of CVD death or all-cause mortality was around 15% greater. In these models, the relationships between baseline 3MS and outcomes remained similar as in the models without adding change of 3MS as a time-varying covariate (not shown).
Table 2.
Associations Between 1-Point/year Greater Decline in 3MS Score with Incident CVD Events or Death in WHIMS After Adjusting for Baseline 3MS
| Outcome | # Person years | # Events | Minimally-Adjusted Modela | Fully-Adjusted Modela | ||
|---|---|---|---|---|---|---|
| Decline of 3MS | Decline of 3MS | |||||
| HRb (95% CI) | p Value | HRb (95% CI) | p Value | |||
| Any CVD | 33,502 | 693 | 1.04 (1.00,1.08) | .03 | 1.04 (1.00,1.08) | .05 |
| CHD | 31,518 | 145 | 1.09 (1.03,1.15) | .002 | 1.10 (1.03,1.16) | .002 |
| HF | 31,494 | 143 | 1.00 (0.91,1.10) | .97 | 0.99 (0.90,1.09) | .81 |
| Angina | 31,486 | 154 | 1.01 (0.93,1.10) | .77 | 1.00 (0.92,1.09) | .95 |
| Stroke/TIA | 31,714 | 192 | 1.09 (1.02,1.16) | .01 | 1.09 (1.01,1.16) | .02 |
| Coronary revascularization | 31,604 | 167 | 0.99 (0.90,1.08) | .75 | 0.97 (0.89,1.07) | .59 |
| CVD death | 34,932 | 94 | 1.14 (1.09,1.20) | <.0001 | 1.17 (1.11,1.22) | <.0001 |
| All-cause mortality | 34,932 | 331 | 1.12 (1.08,1.16) | <.0001 | 1.13 (1.09,1.17) | <.0001 |
Note: CHD = Coronary heart disease; CI = Confidence interval; CVD = Cardiovascular disease; HF = Heart failure; HR = Hazard ratio; 3MS = Modified Mini-Mental State Exam; TIA = Transient ischemic attack.
aMinimally-adjusted model: adjusted for age, race/ethnicity, education, hormone therapy trial study arm; fully-adjusted model: minimally adjusted model covariates plus smoking status, alcohol intake, physical activity, HEI 2010 score, sleep duration, depressive symptoms, treated diabetes, hypertension, BMI, statin use, aspirin use and antihypertensive medications. bThe hazard ratios (HR) were for 1 point/year greater decline, p <.05 in bold.
Sensitivity Analysis and Multiple Imputation
Our primary analyses of change in 3MS over time required at least two 3MS measurements for participants. Consequently, we excluded 163 events that occurred between baseline 3MS and the next 3MS measurement. To assess the impact of this approach on the analyses using baseline 3MS as a predictor, we performed a sensitivity analysis to include all of the women with available baseline 3MS data and covariates, and obtained similar results (data not shown). We compared overall incidence CVD rate and survival probability between analysis sample (n = 5,596) and those with missing covariates (n = 328), and did not find the differences to be statistically significant (all p > .05). Under the assumption of missing at random, we imputed the missing covariates using baseline 3MS, all covariates in the full model, outcome censoring status, and logarithm of the survival time through multiple imputation (m = 5) (33,34). Since the pattern of missingness was arbitrary, fully conditional specification methods were applied (35), where discriminant function was used for categorical variables, and predicted mean matching method for continuous variables, since some variable were not normally distributed. The results based on multiple imputation (Supplementary Tables 2 and 3) did not differ materially from those of Tables 1 and 2.
Discussion
In this large prospective study of 5,596 older (≥60 years) women free of prevalent CVD, those with lower baseline global cognitive function and faster decline in global cognitive function, were at greater risk for incident CVD, CVD death, and all-cause mortality.
Baseline Global Cognitive Function as a Predictor of Incident CVD
Our findings that lower global cognitive function is associated with elevated risk for CVD events, CVD death, and all-cause mortality align with observations in general populations, cohorts at increased cardiovascular risk, and patients with type 2 diabetes (13,19,36). Among individual CVD events, we found a similar relationship for HF, but not for stroke/TIA or CHD. In a large high cardiovascular risk population (N = 30,959, mean age 66.5 years) of the combined ONTARGET and TRANSCEND trials, lower Mini-Mental State Examination (MMSE) was associated with greater risks for stroke and hospitalization for HF, but not MI (36) . Other studies presented the mixed results for individual CVD events (36,37). Differences in the sample characteristics, risk factors, and number of events among studies may explain these discrepancies. For example, compared with the combined ONTARGET and TRANSCEND trials, the WHIMS women were healthier, and the numbers of events were substantially lower (143 hospitalized HF vs 1314; 145 CHD vs 1527; 192 stroke/TIA vs 1374). Nonetheless, we did find a significant increased risk in HF. The HRs for CHD, angina, and stroke/TIA were all increased but with large confidence intervals possibly due to the limited number of events. Our findings are in general congruent with other studies even with different number of subjects, types of assessments and definitions of outcomes (38). Other studies demonstrated that domain-specific cognitive measures of frontal function may be more highly associated with stroke risk than global cognitive function (18). Since the WHIMS women had high 3MS scores at baseline and the 3MS scores were skewed with a ceiling effect, we performed the baseline analysis for overall CVD for those women who had 3MS of 90 or higher (n = 5,115). The results based on the fully adjusted model (HR: 1.20; 95% CI: 1.03–1.40, p = .02) were similar to the full sample analyses. Therefore, the results were not driven by those who had 3MS less than 90, which was also supported by Figure 2.
Decline in Global Cognitive Function as a Predictor of Incident CVD
Global cognitive decline independently predicted CHD and stroke/TIA, and was also a strong predictor of any CVD, CVD death, and all-cause mortality, after adjustment for baseline 3MS. To our knowledge, no other studies have examined the relationship between cognitive decline, as a time-varying predictor, and risk of incident CVD. In the combined ONTARGET and TRANSCEND trials, decline in MMSE (from baseline to first follow-up) was associated with increased risk of major CVD, CVD death, non-CVD death and stroke, and a strong trend was observed for MI and HF (36). Another large prospective study of 5,024 older adults (≥70 years) found that 3-year change in global cognitive function predicted incident stroke over 6 years of follow-up (15). Other studies examining global cognitive function or memory functioning trajectories before/after stroke have shown that cognitive or memory decline was common prior to an incident stroke event (39). Importantly, our findings demonstrate that in the absence of prior CVD (including stroke/TIA and CHD), prospective greater cognitive decline signals a 10% greater risk of stroke or CHD, but baseline global cognitive function may not. The latter finding might partly be due to the high cognitive function in WHIMS women. Furthermore, we also explored the effect of 3MS as an immediate change from previous measurement as a time-dependent variable and found that it was neither predictive for any incidence CVD (HR: 1.01; 95% CI: 0.99, 1.03, p = .31), nor any individual CVD event. However, it was predictive for all-cause mortality (HR: 1.05; 95% CI: 1.02–1.08, p = .0001; Supplementary Table 4). To examine whether 3MS decline is more important in those women with higher cognitive function, we tested for the interactions between baseline 3MS and 3MS decline and found them not to be statistically significant (all p > .05).
Potential Mechanisms That May Explain the Association
Associations between cognitive function and risk of future CVD may be explained by different mechanisms. Common risk factors such as age, hypertension, and diabetes could potentially confound the findings. To explore this, we examined interactions that baseline 3MS and 3MS decline had with age, hypertension, and diabetes for CVD events. None were statistically significant. Results from analyses stratified by age, hypertension status, and diabetes status were similar to those for the whole cohort (data not shown). We did not perform interaction tests or stratified analyses for individual CVD events or death due to the relatively small number of events. We were able to adjust for other potential risk factors in our fully-adjusted analyses, and the associations between CVD and cognitive impairment and decline were independent of these CVD risk factors.
It is now widely accepted that vascular aging represented by atherosclerosis and arteriosclerosis is one of the key processes linking cognitive impairment and CVD (4). Development of chronic, low-grade, systemic inflammation with aging, referred to as “inflammaging,” is also independently associated with CVD and cognitive decline (40,41). Therefore, the association between CVD and cognitive impairment and decline could be the sequel of accelerated multi-system aging (42).
Another potential interpretation is that declines in cognitive function lead to suboptimal self-care, for example, adherence to medications. The prevalence of medication nonadherence in cognitively impaired older patients was high (43). Cognitive impairment has been shown to be an important barrier to medication adherence in older adults (44). We do not have direct data on medication adherence; however, when we examined women based on the categories of baseline 3MS, women with lower cognitive function had lower HEI 2010 score, and were less likely to be physically active, or have controlled blood pressure (all p < .05), but they were more likely to take antihypertensive medications, and to have treated diabetes (5.7% vs 3.9% for 64–90 and 98–100 respectively, p = .01), as compare with those with higher cognitive function. Therefore cognitive impairment and decline preceding CVD and mortality could be mediated by nonadherence to medications for CVD risk factors and less healthy lifestyles.
Strengths and Limitations
The strengths of our study include a large sample size, longitudinal design with a long follow-up, repeated measures of the 3MS, confirmed and adjudicated CVD and death outcomes, and availability of many CVD risk factors. Our study is also among the few studies that evaluate the prospective associations between cognitive decline and major CVD events, including a broader spectrum of individual CVD outcomes. Although a wide range of confounding factors was considered, we cannot adjust for potential unmeasured confounding factors. The 3MS is a global cognitive function test, therefore we were unable to pinpoint domain-specific effects; however, it is a widely used cognitive function instrument and easy to administer, making it a strong candidate for cognitive screening in clinical practice. The relatively small number of individual CVD events may have limited power. Extrapolation of the results should be used with care as our study population consists only of relatively healthy postmenopausal women. Brain imaging data would be useful to determine whether vascular disease in the brain explains the findings of the study however such data were only collected at much later time (average 8 years after randomization) in a subset of WHIMS women (45).
Clinical Implications
A rather simple cognitive function test, the 3MS, may serve as an early and relatively cost effective tool for identifying women with increased risk of CVD and have important implications for how health care practitioners monitor cognitive function in aging women to add preventive strategies for CVD, such as improving medication adherence by using simplified medical regimens, assistance from family members and other aids (46).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
Women’s Health Initiative Memory Study is funded by the National Institute on Aging (HHSN271201100004C). The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals, Inc., St. Davids, PA. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services (HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, and HHSN268201100004C).
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi:10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 2. Bilello M, Doshi J, Nabavizadeh SA et al. . Correlating cognitive decline with white matter lesion and brain atrophy magnetic resonance imaging measurements in Alzheimer’s disease. J Alzheimers Dis. 2015;48:987–994. doi:10.3233/JAD-150400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeCarlie C. The role of cerebrovascular disease in dementia. The Neurologist. 2003;9:123–136. [DOI] [PubMed] [Google Scholar]
- 4. Gorelick PB, Scuteri A, Black SE et al. . Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42:2672–2713. doi:10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 2015;12:267–277. doi:10.1038/nrcardio.2014.223 [DOI] [PubMed] [Google Scholar]
- 6. Newman AB, Fitzpatrick AL, Lopez O et al. . Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. J Am Geriatr Soc. 2005;53:1257–1258. doi:10.1111/j.1532-5415.2005.53360.x [DOI] [PubMed] [Google Scholar]
- 7. Haring B, Leng X, Robinson J et al. . Cardiovascular disease and cognitive decline in postmenopausal women: results from the Women’s Health Initiative Memory Study. J Am Heart Assoc. 2013;2:e000369. doi:10.1161/JAHA.113.000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gure TR, Blaum CS, Giordani B et al. . The prevalence of cognitive impairment in older adults with heart failure. J Am Geriatr Soc. 2012;60:1724–1729. doi:10.1111/j.1532-5415.2012.04097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosengart TK, Sweet J, Finnin EB et al. . Neurocognitive functioning in patients undergoing coronary artery bypass graft surgery or percutaneous coronary intervention: evidence of impairment before intervention compared with normal controls. Ann Thorac Surg. 2005;80:1327–34; discussion 1334. doi:10.1016/j.athoracsur.2005.06.052 [DOI] [PubMed] [Google Scholar]
- 10. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi:10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- 11. Memel M, Bourassa K, Woolverton C, Sbarra DA. Body mass and physical activity uniquely predict change in cognition for aging adults. Ann Behav Med. 2016;50:397–408. doi:10.1007/s12160-015-9768-2 [DOI] [PubMed] [Google Scholar]
- 12. Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi:10.1002/ana.410420514 [DOI] [PubMed] [Google Scholar]
- 13. Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. BMJ. 1996;312:608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee M, Saver JL, Hong KS. Cognitive impairment and risk of future stroke: a systematic review and meta-analysis. CMAJ. 2014;186:E536–546. doi:10.1503/cmaj.140147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrucci L, Guralnik JM, Salive ME, Pahor M, Corti MC, Baroni A, Havlik RJ. Cognitive impairment and risk of stroke in the older population. J Am Geriatr Soc. 1996;44:237–241. [DOI] [PubMed] [Google Scholar]
- 16. Skoog I, Lithell H, Hansson L et al. ; SCOPE Study Group. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). Am J Hypertens. 2005;18:1052–1059. doi:10.1016/j.amjhyper.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 17. Wiberg B, Lind L, Kilander L, Zethelius B, Sundelöf JE, Sundström J. Cognitive function and risk of stroke in elderly men. Neurology. 2010;74:379–385. doi:10.1212/WNL.0b013e3181ccc516 [DOI] [PubMed] [Google Scholar]
- 18. Elkins JS, Knopman JS, Yaffe K, Johnston SC. Cognitive function predicts first-time stroke and heart disease. Neurology. 2005;64:1750–1755. doi:10.1212/01.WNL.0000161850.01792.77 [DOI] [PubMed] [Google Scholar]
- 19. De Galan EB, Zoungas S, Chalmers J et al. . Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2009;52:2328–2336. doi:10.1007/s00125-009-1484-7 [DOI] [PubMed] [Google Scholar]
- 20. Shumaker SA, Legault C, Rapp SR et al. ; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi:10.1001/jama.289.20.2651 [DOI] [PubMed] [Google Scholar]
- 21. Rossouw JE, Anderson GL, Prentice RL et al. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 22. Anderson GL, Limacher M, Assaf AR et al. ; Women’s Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi:10.1001/jama.291.14.1701 [DOI] [PubMed] [Google Scholar]
- 23. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 24. Rapp SR, Espeland MA, Shumaker SA et al. ; WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi:10.1001/jama.289.20.2663 [DOI] [PubMed] [Google Scholar]
- 25. Curb JD, McTiernan A, Heckbert SR et al. ; WHI Morbidity and Mortality Committee. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–S128. [DOI] [PubMed] [Google Scholar]
- 26. The Women’s Health Initiative Study Group. Design of the women’s health initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 27. Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. doi:10.1249/MSS.0b013e31818ace55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. George SM, Ballard-Barbash R, Manson JE et al. . Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180:616–625. doi:10.1093/aje/kwu173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wassertheil-Smoller S, Shumaker S, Ockene J et al. . Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI). Arch Intern Med. 2004;164:289–298. doi:10.1001/archinte.164.3.289 [DOI] [PubMed] [Google Scholar]
- 30. Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J Clin Epidemiol. 2007;61:827–831. doi:10.1016/j.jclinepi.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 31. Grambsch PM, Themeau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 32. Bassuk SS, Murphy JM. Characteristics of the Modified Mini-Mental State Exam among elderly persons. J Clin Epidemiol. 2003;56:622–628. [DOI] [PubMed] [Google Scholar]
- 33. Rubin DB. Multiple Imputation for Nonresponse in Surveys.New York: John Wiley & Sons. [Google Scholar]
- 34. van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18: 681–694. [DOI] [PubMed] [Google Scholar]
- 35. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi:10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 36. O’Donnell M, Teo K, Gao P et al. . Cognitive impairment and risk of cardiovascular events and mortality. Eur Heart J. 2012;33:1777–1786. [DOI] [PubMed] [Google Scholar]
- 37. Skoog I, Lithell H, Hansson L et al. ; SCOPE Study Group. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). Am J Hypertens. 2005;18:1052–1059. doi:10.1016/j.amjhyper.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 38. Bressler J, Knopman DS, Sharrett AR et al. . Incident heart failure and cognitive decline: the atherosclerosis risk in communities study. J Card Fail. 2017;23:47–55. doi:10.1016/j.cardfail.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Q, Benjamin CD, Ehntholt A, Glymour MM. Long-term rate of change in memory functioning before and after stroke onset. Stroke. 2012;43:2561–2566. doi:10.1161/STROKEAHA.112.661587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schimidt R, Schimdt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi:10.1002/ana.10265 [DOI] [PubMed] [Google Scholar]
- 41. Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–I10. doi:10.1161/01.CIR.0000129535.04194.38 [DOI] [PubMed] [Google Scholar]
- 42. Franceschi C, Bonafe M, Valensin S et al. . Infamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 43. Husani ZK, Rojas-Fernandez CH. A scoping review on medication adherence in older patients with cognitive impairment or dementia. Res Soc Adm Pharm. 2016;12:815–829. doi:10.1016/j.sapharm.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 44. Cooper L, Carpenter l, Katona C, Schroll M, Wagner C, Fialova D, Livingston G. The AdHOC study of older adults’ adherence to medication in 11 countries. Am J Geriatr Psychiatry. 2005;13:1067–1076. doi:10.1176/appi.ajgp.13.12.1067 [DOI] [PubMed] [Google Scholar]
- 45. Coker LH, Hogan PE, Bryan NR et al. . Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72:125–134. doi:10.1212/01.wnl.0000339036.88842.9e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Braunner DJ. Adherence to medication in patients with dementia: problems and solutions. Geriatrics & Aging. 2009;12:259–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.