Abstract
Background
Chronic inflammation has been linked to memory and other cognitive impairments, as well as Alzheimer’s disease. Here, we investigate the association between inflammatory markers and changes in brain activity measured by regional cerebral blood flow (rCBF) to assess the relationship between inflammation and brain function in older individuals.
Methods
Annual 15O water resting-state positron emission tomography (PET) scans collected over a 5-year period were assessed in 138 cognitively normal older participants (77 males; mean age at baseline = 71.3; mean scans per participant = 3.5) in the Baltimore Longitudinal Study of Aging. Voxel-wise linear mixed models were used to investigate associations between rCBF and C-reactive protein (CRP) and interleukin-6 (IL-6) at the time of scanning. We examined relationships between baseline CRP and IL-6 levels and baseline rCBF, and relationships between baseline and mean inflammatory levels over time and longitudinal rCBF changes.
Results
Higher baseline CRP and IL-6 were each associated with lower baseline rCBF primarily in frontal and occipital regions, with only the lingual gyrus surviving atrophy correction. Higher baseline and mean CRP were also associated with greater rCBF declines over time in anterior cingulate and hippocampal regions, whereas higher baseline and mean IL-6 levels were associated with greater rCBF declines in orbitofrontal and hippocampal regions.
Conclusions
Higher levels of inflammation are associated with longitudinal changes in brain function in regions important for cognition. These results, along with previous studies, suggest that chronic inflammation in older adults may contribute to age-associated declines in cognitive function.
Keywords: Aging, CRP, IL-6, PET, MRI, fMRI, Brain function, Cognition, Health, Alzheimer’s disease
The role of the immune system in aging is an area of increasing interest and investigation, particularly in relation to cognitive decline and dementia (1). In the absence of any overt trigger of inflammation, a number of inflammatory agents, including specific cytokines, influence the regulation of neuronal processes underlying learning and memory, including long-term potentiation, neural plasticity, and neurogenesis (1,2). However, upregulation of the inflammatory response because of infection, injury or aging may disrupt the immuno-regulatory neurobiological processes supporting memory and other functions (2,3). While acute inflammation may lead to temporary disruption of neuronal regulation which is likely reversible, chronic inflammation can lead to neurodegeneration (4,5), including long-lasting deficits in memory processes (6,7).
The role of chronic inflammation in neuronal function is especially important during aging. Levels of many inflammatory markers, including C-reactive protein (CRP) and interleukin-6 (IL-6), increase with age (8) and are chronically elevated in patients with mild cognitive impairment (MCI) and Alzheimer’s disease (9). IL-6 is a proinflammatory cytokine, secreted by macrophages, that stimulates and mediates the acute phase inflammatory response (10,11). CRP is a downstream acute phase reactant that is synthesized in response to IL-6 (12). Both are markers of systemic inflammation, and have been used in large scale studies examining the effects of inflammation on the brain (12–15).
Increased systemic inflammation has been linked to cognitive decline (10,12,16,17) and to structural brain changes (12,15,17,18). Associations with brain structure include greater atrophy, cerebral microstructural disintegration, white matter lesions, lacunar infarcts, and beta amyloid deposition (12,15,17,18). For example, in the Three City-Dijon cohort, IL-6 and CRP levels were associated with greater global brain atrophy and white matter lesion severity (15). High-sensitivity CRP levels were also negatively correlated with fractional anisotropy in another aging cohort (17).
Inflammation has also been associated with alterations in brain function. In young adults, decreases in cerebral glucose metabolism have been shown in the medial temporal lobe and increases have been seen in the subgenual anterior cingulate cortex in conjunction with reductions in mesolimbic connectivity, following an induced inflammatory response (19,20). Other studies have found that higher cytokine levels are related to increased glucose metabolism in insular (21), prefrontal and temporal (22), and temporo-parietal (23) regions of the brain. Studies in older animals have also found that conditions which generate peripheral inflammation trigger an inflammatory response in the microglia, characterized by production of IL-1 (24).Yet, despite the effect of aging on upregulated inflammation and the link between inflammation and brain function, few studies have investigated the association between inflammation and age-related changes in brain function assessed by regional cerebral blood flow, and none have examined the relationship between inflammation and longitudinal changes in brain function that occur over time.
The current study examined the hypothesis that elevated levels of the inflammatory markers CRP and IL-6 are associated with detrimental longitudinal changes in brain function, as older individuals continue to age. Resting-state 15O-water positron emission tomography (PET) scans were used to assess regional cerebral blood flow (rCBF), as an index of brain function (25). Brain activity measured by 15O-PET CBF is based on the principal that local increases in neuronal activity lead to co-localized regional increases in blood flow. Increased cellular activity produces an increased metabolic demand, and the resultant hemodynamic response to neuronal activity provides the additional glucose and oxygen needed by the activated tissue (26). In this way, rCBF is an indirect measure of localized neuronal activity.
We examined rCBF changes associated with CRP and IL-6 over a 5-year period in 138 participants in the Baltimore Longitudinal Study of Aging (BLSA) who were not cognitively impaired. Based on previous findings of associations between inflammatory markers and regional brain metabolism (19–22,27), we hypothesized that CRP and IL-6 would show associations with decreases in brain function over time in an older cohort. Additionally, given prior reports of associations between inflammation and executive and memory function, including cytokine elevations in MCI and Alzheimer’s disease, we expected to see functional alterations in regions associated with executive function and learning and memory, specifically in the medial temporal lobe, cingulate cortex, and medial prefrontal cortex (12,16,17,20).
Methods
Participants
Data on rCBF and inflammatory markers were available for 145 individuals in the neuroimaging substudy (28) of the BLSA (29). Of these, seven individuals with clinical diagnoses of MCI or dementia at any point in the study were excluded from analysis. Thus, the current study included data from 138 cognitively normal older individuals (77 males; mean age at baseline 71.3 [7.7 SD]). Sample characteristics are presented in Table 1. Of the 138 participants, all had baseline inflammatory marker and imaging measures, and 127 had concurrent longitudinal inflammatory and imaging measures.
Table 1.
Participant Demographics
| Participants (n) | 138 |
| Males, n (%) | 77 (55.8%) |
| Baseline Age (years) | 71.3 (7.7) |
| Total PET Scans | 476 |
| Scans Per Participant | 3.5 (1.2) |
| Follow-up Time (years) | 5.3 (2.0) |
| Baseline MMSE | 28.9 (1.2) |
| Hypertension, n (%) | 67 (48.6%) |
| Diabetes, n (%) | 13 (9.4%) |
| Obesity (n %, BMI ≥30) | 43 (31.2%) |
| Baseline CRP (mg/L) | 4.56 (5.34) |
| Baseline IL-6 (pg/mL) | 3.72 (0.78) |
| Mean CRP (mg/L) | 4.60 (5.58) |
| Mean IL-6 (pg/mL) | 3.99 (1.67) |
| Rate of change CRP (mg/L/y) | 0.17 (0.61) |
| Rate of change IL-6 (mg/L/y) | 0.10 (0.09) |
Note: Mean (SD) unless otherwise noted. Baseline values represent the mean of the whole sample of cognitively normal participants (n = 138). Mean and rate of change values were calculated for those with longitudinal imaging measures (>1 PET scan, n = 127). CRP = C-reactive protein; IL-6 = Interleukin-6; MMSE = Mini-Mental State Examination; PET = Positron emission tomography.
Cognitive impairment was determined by consensus diagnosis using Diagnostic and Statistical Manual of Mental Disorders Third Edition, Revised (DSM-III-R) (1987) criteria for dementia, and the National Institute of Neurological and Communication Disorders and Stroke -Alzheimer’s Disease and Related Disorders Association criteria (30), using neuropsychological diagnostic tests and clinical data (31). At neuroimaging study enrollment, participants were free of central nervous system disease (epilepsy, stroke), psychiatric disorders (except depression), severe cardiac disease (myocardial infarction, coronary artery disease requiring angioplasty or bypass surgery), and metastatic cancer.
This study was approved by the local Institutional Review Board. All subjects provided written informed consent prior to each assessment.
Inflammatory Markers
Serum levels of CRP and IL-6 were assessed from samples taken at the visit associated with each PET scan. Stored blood serum samples were used to assess levels of proinflammatory markers, namely CRP (Alpco, Salem, NH) and IL-6 (R&D System, Minneapolis, MN) by enzyme-linked immunosorbent assay (ELISA). Blood samples were drawn from the antecubital vein between 7 and 8 AM after an overnight fast (29). Participants did not smoke, engage in physical activity, or take medications before collection. Samples were immediately processed, cataloged, and stored at −80°C.
All assays were performed according to the kit manufacturers’ instructions. The intra and interassay variations were 5.5%–6.0% and 11.6%–13.8% for CRP and 1.6%–4.2% and 3.3%–6.4% for IL-6, respectively. No significant cross-reactivity or interference was observed in any assays. Samples were obtained at approximately 2-year intervals. Due to the skewed distribution of CRP levels, natural log transformed values were used in the analyses for this measure.
Baseline values were calculated for the whole sample of participants (n = 138). Mean and rates of change values were calculated for those with longitudinal imaging measures (n = 127). Rates of change were calculated using linear mixed effects models. The models included intercept and follow-up time as both fixed effects and random effects. Bayes (EB) estimates for the rates of change were derived from estimates of the random effect of follow-up time. The average interval between first and last visits was 5.3 (2.0 SD) years with an average of 3.5 scans per subject (Table 1).
Neuropsychological Testing
During each neuroimaging visit, participants completed a comprehensive battery of neuropsychological tests. For the current analysis, we evaluated inflammatory markers in relation to five domains of cognition. Standardized scores of each cognitive measure based on the means and standard deviations at the baseline assessments were used to develop composite measures. Verbal memory was defined by the mean of the immediate free recall summary score (five trials) and delayed free recall on the California Verbal Learning Test (CVLT). Verbal fluency/language was defined by the mean of the Letter (ie, FAS) and Category fluency tests. Attention was defined by the mean of Trail-Making A and the Digit Span Forward subtest of the Wechsler Adult Intelligence Scale-Revised. Executive function was based on the mean of Trail-Making B and Digits Backward. The mean of the Card Rotations Test and Clock-to-Command drawing score assessed visuospatial function. Data from evaluations at each imaging visit were used to examine changes in performance over time.
Separate linear mixed effects models were used to analyze the associations between inflammation and longitudinal domain-specific cognitive trajectories. Each cognitive domain measure was used as the dependent variable and baseline or mean levels over time of each inflammatory marker as the main predictor. Each model included the fixed effects of baseline age (mean-centered), sex, regular NSAID use (n = 55), the inflammation measure and interval (follow-up time), as well as all the two-way interactions of each of these terms with interval. The random effects included intercept and interval with unstructured covariance.
PET Scanning
Participants underwent PET scans at baseline and up to eight annual follow-ups. We restricted our analyses to PET scans concurrent with assessments of inflammatory markers. In the present study, we used each participant’s baseline and mean measures of inflammation. Baseline CRP and IL-6 were measured at the participant’s first imaging visit concurrent with a blood draw.
Each PET imaging session included a resting state PET scan in which participants were instructed to keep their eyes open and focused on a computer screen covered by a black cloth. PET measures of rCBF were obtained using [15O] water. For each scan, 75 mCi of [15O] water were injected as a bolus. Scans were performed on a GE 4096+ scanner, which provides 15 slices of 6.5 mm thickness. Images were acquired for 60 seconds from the time the total radioactivity counts in the brain reached threshold level. Attenuation correction was performed using a transmission scan acquired prior to the emission scans.
PET Image Preprocessing
Image preprocessing was performed using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, London, England). For each participant across follow-up sessions, PET images were realigned to the first session, spatially normalized to the MNI (Montreal Neurological Institute) template space with 2 × 2 × 2 mm resolution, and smoothed using a full width at half maximum of 12 mm. Voxel-wise rCBF values for all images were ratio adjusted and scaled to a mean global flow of 50 ml/100 g/min.
Mixed Model Analysis
Voxel-wise correlations were used to examine the relationships between inflammation and rCBF across the whole brain, and follow-up analyses of rCBF trajectories in the significant voxel-based regions were performed using a region of interest approach. In the voxel-based analyses, separate linear mixed effect models were used to analyze the associations between inflammation and rCBF with rCBF as the dependent variable and baseline and mean levels over time (a measure of more chronic inflammation) of each inflammatory marker as the main predictor. The follow-up time was used as the longitudinal time metric (interval). Each model included the inflammation measure and interval as fixed effects, and controlled for baseline age (mean-centered), sex, regular NSAID use (n = 55), as well as all the two-way interactions of each of these terms with interval. The random effects included intercept and interval with unstructured covariance.
The main effect of the inflammation measure estimates the associations between each measure of inflammation, separately, and baseline rCBF. The interaction between the inflammatory marker levels and interval estimates the effect of inflammatory marker levels on the rCBF rates of change over time. Eleven participants had only baseline data, with 127 contributing to the longitudinal analyses.
Whole-brain contrast t-maps of significant associations between CRP and IL-6 levels and rCBF at baseline and between CRP and IL-6 and change in rCBF over time (inflammation × interval interaction) were generated using a statistical threshold of p < .005 as recommended for PET data by the PET Working Group of the NIH/NIA Neuroimaging Initiative (http://www.nia.nih.gov/about/events/2011/positron-emission-tomography-working-group) with an additional cluster size threshold of ≥ 50 voxels (400 mm3).
To further characterize changes in rCBF over time, rCBF values of all significant regions in the voxel-based analysis were extracted from a 4 mm spherical region centered on the local maxima of each area using the Marsbar SPM toolbox (32). The region was consistent across subjects. Using the PET images normalized into standard MNI space, the 4 mm sphere was stereotactically placed on the MNI template and applied to each scan for each local maxima. In these regions of interest analyses, we examined whether age, sex, or NSAID use modified the associations between levels of inflammatory markers and rCBF by determining the interaction between the inflammatory markers and these factors on rCBF at baseline and slope of longitudinal change. The shape of the longitudinal trajectory of rCBF change was also examined. In this analysis, the shape of rCBF change was estimated for each region using linear mixed models adjusted for baseline age, sex, and NSAID use. Nonlinearity of the trajectories was tested by including the quadratic term of time in the model.
Because of the relationship between inflammation and white matter lesions in the brain (15,18), sensitivity analyses were conducted including white matter lesion volume as an additional covariate. Linear mixed models, adjusted for baseline age, sex, NSAID use, and white matter lesion volume were performed to determine if the relationship between CRP and IL-6 and the longitudinal trajectory of rCBF change remained significant after additional adjustment for white matter lesion volume.
The mixed models were implemented using R version 2.11.1 running the packages AnalyzeFMRI version 1.1–12 and lme4 version 0.999375–37.
MRI Scanning
One hundred and twenty-nine of the 138 participants had valid MRI scans. Scanning was performed on a GE Signa 1.5-Tesla scanner. A 3-dimensional T1-weighted spoiled gradient refocused (SPGR) MRI scan (35 ms repetition time; 5 ms echo time; 24 cm field of view; 45° flip angle; 256 × 256 matrix; 0.94 × 0.94 mm voxel size; 1.5-mm slice thickness; 124 slices) was obtained annually at each imaging visit.
MRI Volumes
MRI volume data were used in two follow-up analyses. First, the MRI scans were segmented into gray matter, white matter and cerebrospinal fluid, and spatially normalized into stereotactic space using a high-dimensional elastic warping method (33) and a volume-preserving transformation (34). Binary maps of the clusters showing an association between the inflammatory markers and CBF were generated from the PET analysis, and total volume of gray + white matter was calculated within each cluster for each participant. The total volume of each region was then included as a covariate in the CBF models to control for the effect of tissue loss on the CBF patterns of change.
In the second follow-up analysis, white matter lesion volumes were calculated for each participant. A multi-atlas label fusion-based automated segmentation method was applied for extracting a brain mask on the T1-weighted image of each subject (35). The brain mask included GM, WM, and ventricular and cortical cerebrospinal fluid. WML (WML) volume was segmented using MPRAGE, T2, and FLAIR images based on a support vector machine classifier approach (36,37). Mean WML volumes were calculated for each individual across the study interval, and the mean volumes were then included as a covariate in the follow-up PET analyses.
Results
Inflammatory Markers
At baseline, the mean CRP level was 4.56 mg/L (5.34 SD) and the mean IL-6 level was 3.72 pg/mL (0.78 SD) for all participants. The average levels of inflammatory markers for participants with longitudinal measures (n = 127) were 4.60 mg/L (5.58 SD) for CRP and 3.99 pg/mL (1.67 SD) for IL-6 across all visits. The mean rate of change over time for CRP was 0.17 mg/L/y (0.61 SD), and 0.10 pg/mL/y (0.09 SD) for IL-6 (Table 1). Intraclass correlations (ICC) were used to examine the stability of each marker over time. Log transformed CRP had an ICC of 0.44 and IL-6 had an ICC of 0.34, indicating fair stability over time.
Neuropsychological Testing
The relationship between both CRP and IL-6 and task performance was examined at baseline and in longitudinal change in task performance. Baseline CRP levels showed a relationship with attention at baseline (baseline CRP β = −0.21, p = .04), with mean CRP levels approaching significance with attention at baseline (mean CRP β = −0.18, p = .06). This negative cross-sectional association suggests that higher baseline CRP levels are related to lower baseline performance levels. Higher baseline and mean CRP levels were also associated greater longitudinal decline in performance on visuospatial tasks (baseline CRP β = −0.04, p = .02; mean CRP β = −0.04, p = .02). There was no significant relationship between baseline or mean IL-6 levels and task performance for any cognitive domain.
Inflammation and Baseline rCBF
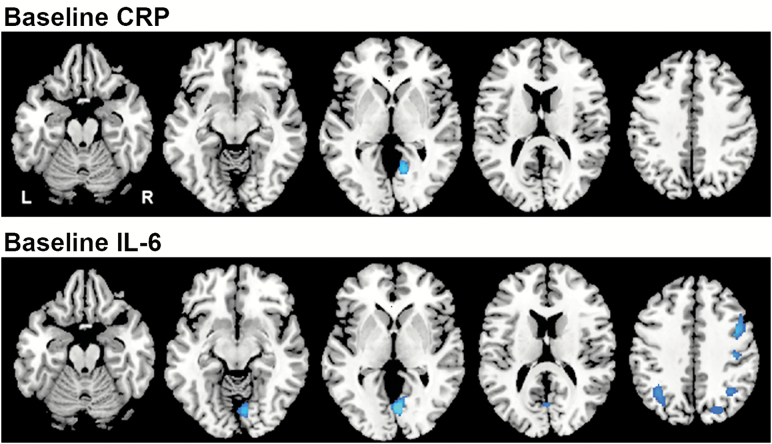
Brain regions showing significant associations between baseline levels of CRP and IL-6 and baseline rCBF are shown in Table 2 and Figure 1.
Table 2.
Associations Between Baseline Levels of Inflammatory Markers and Baseline rCBF
| Coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Region | Side | x | y | z | t Value | p Value | Voxels |
| CRP | Lingual Gyrus (19) | R | 12 | −58 | 2 | −3.36 | .001 | 184 |
| IL-6 | Middle Frontal Gyrus(9)* | R | 42 | 16 | 36 | −3.15 | <.001 | 176 |
| Postcentral Gyrus (4)* | R | 40 | −16 | 40 | −3.16 | .001 | 69 | |
| Lingual Gyrus (18) | R | 10 | −62 | 4 | −3.15 | <.001 | 483 | |
| Superior Occipital Gyrus (19)* | R | 34 | −60 | 42 | −3.16 | .001 | 122 | |
| Superior Occipital Gyrus (19)* | L | −26 | −74 | 24 | −3.15 | <.001 | 395 | |
| Middle Occiptal Cortex (19)* | R | 24 | −86 | 22 | −3.15 | <.001 | 351 | |
Note: Regions where higher levels of CRP and IL-6 were associated lower rCBF. Stereotaxic coordinates are listed, Brodmann areas are indicated in parentheses.
indicates regions that did not survive correction for regional tissue volume. CRP = C-reactive protein; IL-6 = Interleukin-6.
Figure 1.
Associations between baseline levels of inflammatory markers and baseline rCBF. Horizontal sections showing regions with a significant relationship between the inflammatory markers and rCBF at baseline. Blue regions represent areas of decreased activity (rCBF) in relation to higher inflammatory levels. CRP was associated with decreased rCBF in the lingual gyrus and IL-6 with decreases in the middle frontal gyrus, postcentral gyrus, lingual gyrus, superior occipital gyrus, and middle occipital cortex. Of the regions associated with IL-6, only the lingual gyrus survived correction for regional tissue volume. CRP = C-reactive protein; IL-6 = Interleukin-6; rCBF = Regional cerebral blood flow.
Higher baseline CRP levels were associated with lower baseline rCBF in a single region of the right lingual gyrus (Brodmann Area [BA] 19). This relationship did not change when controlling for regional tissue volume in the follow-up analysis. Baseline levels of IL-6 were also inversely associated with baseline rCBF in a number of brain regions. Higher baseline IL-6 levels were associated with lower blood flow in the middle frontal gyrus (BA 9), postcentral gyrus (BA 4), lingual gyrus (BA 18), superior occipital gyri (BA 19), and middle occipital cortex (BA 19). Of note, there were no brain regions where higher levels of baseline CRP or IL-6 were significantly associated with greater brain activity. Of these regions, only the lingual gyrus survived correction for tissue volume. Of note, there were no brain regions where higher levels of baseline CRP or IL-6 were significantly associated with greater brain activity. Additionally, of the covariates included in the models (age, sex, and NSAID use), age (t = 4.22, p < .001) and sex (t = 3.35, p < .001) were independently associated with decreased rCBF only in the lingual gyrus related to IL-6.
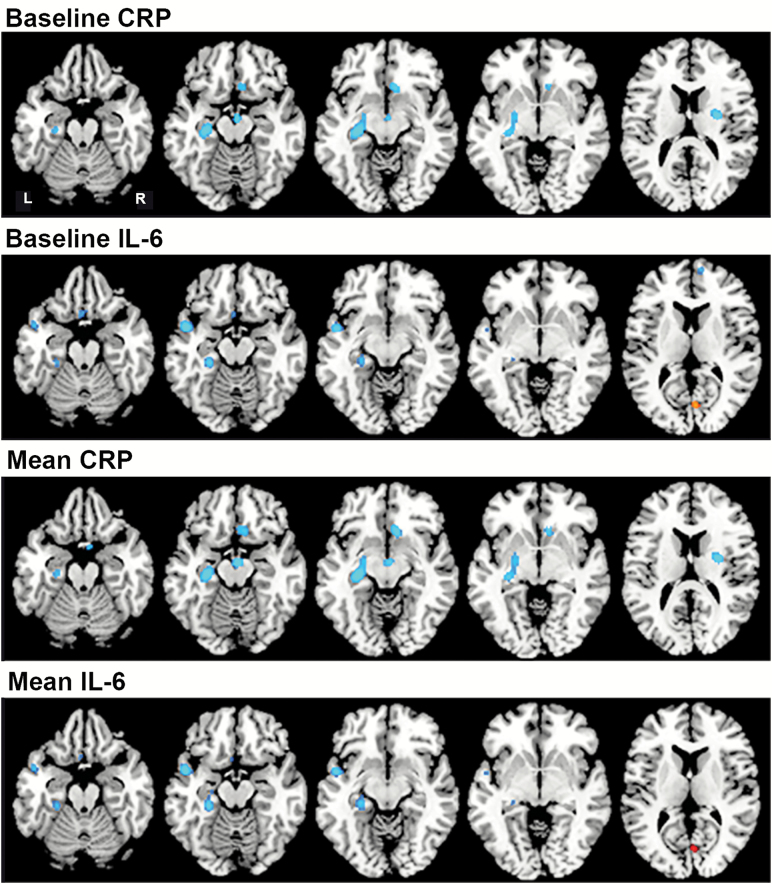
Inflammation and Longitudinal Changes in rCBF
Both baseline and mean levels over time of CRP and IL-6 were assessed in relation to longitudinal change in rCBF (Table 3, Figure 2). Higher baseline CRP levels were associated with greater longitudinal declines in rCBF. Significant associations were observed in the anterior cingulate gyrus (BA 32), hippocampus and parahippocampal gyrus (BA 36), putamen (p < .001), and brainstem (p = .003). These relationships did not change when controlling for regional tissue volume. Analysis of associations between mean CRP and longitudinal change in rCBF showed similar changes over time, with overlapping regions of significance. Higher mean CRP levels were associated with significant longitudinal declines in rCBF in the anterior cingulate cortex (BA 32), entorhinal cortex (BA 28), hippocampus, precuneus (BA 18), cerebellum, putamen, and brainstem. Of these regions, all survived correction for regional tissue volume over time except the precuneus. There were no brain regions showing associations between higher baseline or mean CRP and lower rCBF declines over time.
Table 3.
Associations Between Levels of Inflammatory Markers and Longitudinal rCBF change
| Coordinate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Region | Side | x | y | z | t Value | p Value | Voxels |
| Baseline CRP | Anterior cingulate (32) | R | 4 | 22 | −10 | −2.62 | .001 | 75 |
| Hippocampus/Parahippocampal gyrus (36) | L | −28 | −24 | −20 | −2.62 | <.001 | 283 | |
| Brainstem | R | 6 | −10 | −12 | −2.62 | .003 | 57 | |
| Putamen | R | 28 | −10 | 14 | −2.62 | <.001 | 163 | |
| Mean CRP | Anterior cingulate (32) | R | 10 | 22 | −10 | −3.41 | <.001 | 134 |
| Entorhinal cortex (28) | R | 8 | 6 | −26 | −3.42 | <.001 | 79 | |
| Putamen | R | 28 | −4 | 12 | −3.62 | <.001 | 176 | |
| Hippocampus | L | −30 | −22 | −12 | −3.73 | <.001 | 346 | |
| Precuneus (18)* | R | 16 | −58 | 24 | −3.20 | .001 | 82 | |
| Cerebellum | R | 4 | −44 | −38 | −3.25 | .001 | 65 | |
| Brainstem | R | 2 | −10 | −14 | −3.48 | <.001 | 84 | |
| Baseline IL-6 | Medial frontal cortex(10) | R | 6 | 58 | 16 | −2.62 | .003 | 59 |
| Orbitofrontal cortex (11) | L | -2 | 24 | −24 | −2.61 | <.001 | 185 | |
| Superior temporal gyrus (22) | L | −52 | 0 | −6 | −2.61 | <.001 | 156 | |
| Hippocampus | L | −30 | −30 | −8 | −2.61 | .003 | 108 | |
| Cuneus(17) | R | 4 | −74 | 10 | 3.26 | .004 | 55 | |
| Mean IL-6 | Orbitofrontal cortex (25) | L | -4 | 18 | −16 | −2.62 | .001 | 137 |
| Middle temporal gyrus (21)* | L | -46 | 0 | −12 | −2.62 | <.001 | 159 | |
| Parahippocampal gyrus (36) | L | −24 | −34 | −10 | −2.62 | <.001 | 152 | |
| Cuneus (17) | R | 4 | −74 | 10 | 3.26 | .005 | 58 | |
Note: Regions where higher levels of baseline and mean CRP and IL-6 were associated with longitudinal changes in rCBF. All regions, with the exception of the cuneus, show a negative correlation where higher markers levels were associated with decreases in rCBF. Stereotaxic coordinates are listed, Brodmann areas are indicated in parentheses
indicates regions that did not survive correction for regional tissue volume. CRP = C-reactive protein; IL-6 = Interleukin-6.
Figure 2.
Associations between inflammatory markers and longitudinal regional cerebral blood flow (rCBF) change. Horizontal sections showing the association between inflammatory markers and rCBF change over time. Blue regions represent areas of decreasing rCBF over time in relation to higher inflammatory levels; red region shows increasing rCBF over time in relation to higher IL-6 levels. Baseline and mean levels of each marker showed similar results. CRP was associated with declines in the anterior cingulate cortex, entorhinal cortex, hippocampus and parahippocampal gyrus, and putamen. IL-6 was associated with declines in the medial and orbitofrontal cortex, superior and middle temporal gyrus*, and hippocampus and parahippocampal gyrus, and increased rCBF in the cuneus. *This region was no longer significant after correction for regional tissue volume. CRP = C-reactive protein; IL-6 = Interleukin-6.
Higher baseline and mean IL-6 levels were also associated with steeper rCBF declines in a number of brain regions. Higher baseline IL-6 was associated with steeper declines in rCBF in the medial (BA 10) and orbitofrontal cortex (BA 11), superior temporal gyrus (BA 22), hippocampus, and cuneus (BA 17). These relationships did not change when controlling for regional tissue volume. Similarly, higher mean IL-6 levels were significantly associated with greater longitudinal decreases in rCBF in the orbitofrontal cortex (BA 25), middle temporal gyrus (BA 21), and parahippocampal gyrus (BA 36). An association between higher mean IL-6 and greater increase in rCBF over time was observed in the cuneus (BA 17). Of these regions, all survived correction for regional tissue volume over time except the middle temporal gyrus.
Using the extracted values for the regions of interest, we also examined whether age, sex, or NSAID use modified the associations between levels of inflammatory markers and rCBF. We found no significant interactions between age, sex, or NSAID use and the inflammatory marker associations with rCBF at baseline or in longitudinal change.
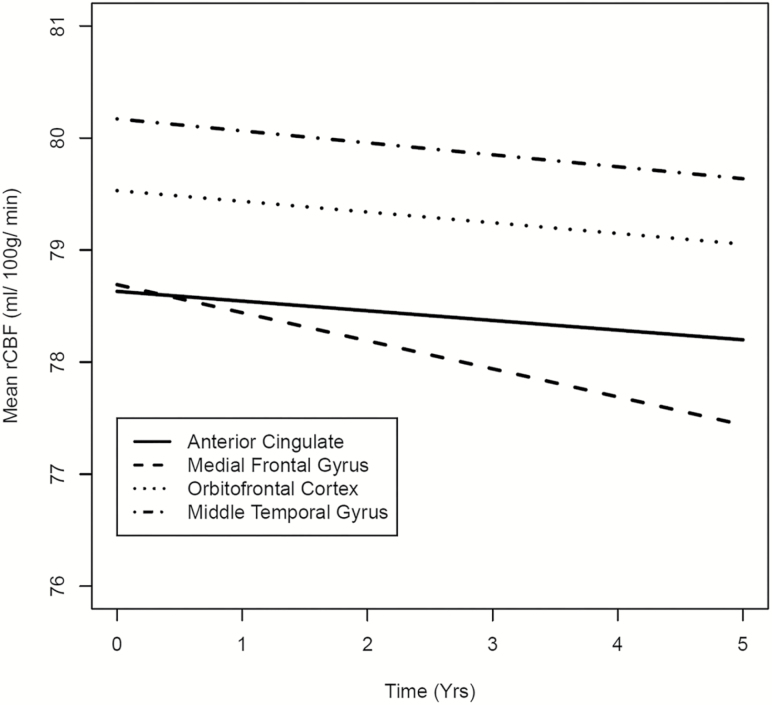
Trajectories of rCBF Change
The tests of nonlinearity of the trajectories did not reach statistical significance, suggesting that the longitudinal rCBF changes are linear in nature (Figure 3). All ROIs of significant longitudinal change showed negative associations between rCBF and the inflammatory markers except the cuneus (BA 17), which showed increased blood flow in association with higher mean IL-6.
Figure 3.
Trajectory of rCBF change over time. The trajectories of regional cerebral blood flow (rCBF) change over time are shown for four regions. Each of these regions was found to be significantly associated with C-reactive protein (CRP) and IL-6. Declining rCBF in the anterior cingulate cortex was associated with higher baseline CRP levels. Declining rCBF in the medial frontal gyrus and orbitofrontal cortex were associated with higher baseline interleukin-6 (IL-6) levels. Declining rCBF in the middle temporal gyrus was associated with higher mean IL-6 levels.
White Matter Lesions and rCBF change
Assessment of white matter lesion volume on the relationship between the inflammatory markers and rCBF showed minor effects for associations with IL-6 only. Associations between baseline IL-6 and rCBF in the middle frontal gyrus (BA 9) and postcentral gyrus (BA 4) were no longer significant after controlling for WML volume. With respect to associations between IL-6 and longitudinal change in rCBF, adjustment for WML only affected the relationship between IL-6 and longitudinal rCBF change in the parahippocampal gyrus (BA 36).
Discussion
Our data reveal a relationship between elevated markers of inflammation and brain function in older individuals. Associations were observed at baseline and in longitudinal change over a 5-year period. Elevated levels of CRP and IL-6 were primarily associated with declines in brain activity as measured by declines in rCBF. These declines were seen in the medial temporal lobe, cingulate cortex, and medial prefrontal cortex, as well as other areas important for learning and memory and executive function.
While CRP and IL-6 are markers of systemic inflammation, both affect neuronal function. IL-6 is an important proinflammatory cytokine, released by macrophages including astrocytes, which stimulates and mediates the acute phase response in a variety of tissues (10,11). Its production within the brain, as well as its ability to cross the blood–brain barrier, makes it especially important for brain and cognitive outcomes (38), since it is associated with synaptic plasticity, long term potentiation, brain atrophy, and white matter lesions (6,15). CRP is a downstream acute phase reactant synthesized in the liver in response to IL-6 (12). While CRP is normally not found in the brain, a previous postmortem study has found evidence of CRP in the plaques and tangles associated with Alzheimer’s disease (AD) (30,39), suggesting that CRP may play a role in some neuropathological processes.
Our findings provide further evidence of a link between elevated CRP and IL-6 and brain function. When examining baseline inflammatory levels in relation to baseline brain activity, higher CRP levels were associated with decreased activity in the lingual gyrus. Higher levels of IL-6 were associated with decreased activity in the lingual gyrus and with decreased activity in the middle frontal, postcentral, lingual, superior occipital, and middle occipital cortex. After controlling for regional tissue volume, however, only the lingual gyrus survived volume correction. Our results, based on voxel-wise analysis of brain activity throughout the brain in older adults, differ from several cross-sectional studies in younger individuals that examined specific a priori regions of the brain after interventions that are associated with heightened immune response. In these studies, associations were observed between an inflammatory response and activity in insula, medial temporal lobe, precuneus, and other temporal and parietal regions (20–22). Our findings, however, show that brain activity is significantly decreased primarily in the occipitotemporal lingual gyrus of older adults in relation to higher levels of circulating inflammatory markers measured at the time of scan.
Our analysis of relationships between the inflammatory markers and longitudinal changes in brain activity reveal two findings. First, both baseline and mean levels of each inflammatory marker were associated with similar patterns of functional decline over time. Baseline and mean CRP were associated with activity declines in the anterior cingulate cortex, hippocampus and medial temporal cortex, putamen, and brainstem, whereas baseline and mean IL-6 were associated with declines in orbitofrontal, and hippocampal and medial temporal cortex, and the majority of these changes survived correction for white matter lesion volume. The similar effect of baseline and mean inflammatory levels on longitudinal functional changes is an interesting finding. Although mean levels of the markers are more likely to capture chronic inflammation over time, our results suggest that a single inflammatory measure may also be predictive of subsequent functional declines despite the modest stability of the inflammatory markers over time.
Second, higher levels of both CRP and IL-6 were associated with functional decline in medial temporal regions over time, specifically in the hippocampus and overlying cortices. These results support previous cross-sectional studies of metabolic (20,22,23) and brain connectivity (27) changes in young individuals in the medial temporal lobe and temporal gyri elicited in response to interventions which increase levels of inflammatory markers. Together, the findings suggest that these areas may be particularly vulnerable to effects of inflammation on the brain. This finding is especially interesting in relation to aging, as these regions are critical components of the temporal lobe memory system (40) and are also areas that show early functional changes in AD and in the prodromal MCI state (41). Although our participants remained cognitively normal during the study, increased functional vulnerability of these regions could contribute to or possibly compound existing factors that lead to the development of cognitive impairment and dementia.
Numerous studies have linked increased inflammation with cognitive decline, MCI, and AD (9,10,12,16), and with brain changes including cerebral glucose metabolism, atrophy, and white matter integrity (14,15,17,18,21–23,27,42). Further, Marsland, et al found a relationship between these cognitive and brain differences, with brain morphological changes mediating the inflammation-cognition relationship (18). The regions exhibiting brain activity changes in our study have all been associated with various aspects of cognition. For example, the orbitofrontal cortex, lingual gyrus, medial temporal lobe, and dorsal striatum are all regions associated with memory processes (40,43–46). Brain regions associated with attention and executive function include the orbitofrontal cortex, cingulate cortex, and lingual gyrus (44,45). Other regions shown here have been linked to language processes, such as the inferior frontal gyrus and the superior temporal gyrus (45,47), and to sensory processing and recognition, including the occipital gyri, and cuneus (24,45,48).
With regard to cognitive performance, our participants showed greater declines in performance over time on the Card Rotations and Clock drawing tests in relation to higher CRP levels. These tests predominantly assess visuospatial function, and the results provide support for the declining activity particularly in the hippocampus and precuneus (45,49). Although clock drawing also taps into executive function, we did not find an association between the inflammatory markers and the other tests of executive function. Thus, we did not find robust evidence for previously reported associations with memory or executive function, or a relationship between IL-6 levels and cognition in this sample. The lack of these associations may be due to the high cognitive performance abilities of the BLSA participants and to the small sample size. Nevertheless, the fact that CRP and IL-6 are associated with declining brain activity in regions known to be involved in various aspects of cognition suggests that these individuals may be more vulnerable to cognitive decline in the future.
While there is strong evidence that upregulated inflammatory processes resulting in increased levels of peripheral CRP and IL-6 may cause neuronal damage in the aging brain (50), there is also evidence to suggest regional vulnerability associated with these markers. For example, the accumulation of beta amyloid and tau protein in the brain is associated with the expression of acute phase proteins and proinflammatory cytokines (51). Proinflammatory cytokine receptor density and tissue expression (9,52) also appear to be upregulated in regions of early neuropathologic change in aging and Alzheimer’s disease such as orbito- and medial frontal, temporal cortices and hippocampus, and precuneus regions (53–55) where declines in brain activity were observed. Although our participants remained cognitively normal through the study interval, it is possible accumulation of neuropathology with advancing age (56) could contribute to the current findings.
There are several limitations to this study. It should be noted that the BLSA is a highly educated, high performing group of older individuals, and as such may not be representative of the general population. Furthermore, although the sample remains cognitively normal to date, as with any longitudinal study it is currently not possible to predict which individuals will develop future cognitive impairment. The study of CRP and IL-6 on brain function in the prodromal stages of Alzheimer’s disease is of key interest due to the link between inflammation and dementia, but we do not have the sample size to examine this link with the current data set.
In summary, our findings provide additional evidence of a link between inflammation and functional changes in the aging brain, which could result in increased vulnerability to age-related cognitive decline. Given the rise of inflammatory markers that occurs with increasing age, the relationship between inflammation and age-related cognitive decline and the underlying neurobiological processes merit further investigation.
Funding
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging, and by Research and Development Contract N01-AG-3-2124.
Conflict of Interest
None reported.
Acknowledgments
We are grateful to the BLSA participants and staff for their dedication to these studies and the staff of the Johns Hopkins PET facility for their assistance. We also thank Drs. Andrea Shafer and Murat Bilgel for their programming assistance in the imaging analyses.
References
- 1. Thambisetty M, Ferrucci L. Soluble interleukin-6 receptor levels and risk of dementia: one more signpost on a long road ahead. J Am Geriatr Soc. 2014;62:772–774. doi:10.1111/jgs.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi:10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 3. Ferrucci L, Corsi A, Lauretani F, et al. . The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi:10.1182/blood-2004-07-2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi:10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi:10.1038/nri2015 [DOI] [PubMed] [Google Scholar]
- 6. Li AJ, Katafuchi T, Oda S, Hori T, Oomura Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 1997;748:30–38. [DOI] [PubMed] [Google Scholar]
- 7. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi:10.1073/pnas.2234031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi:10.1016/j.exger.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 9. Akiyama H, Barger S, Barnum S, et al. . Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi:10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh-Manoux A, Dugravot A, Brunner E, et al. . Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology. 2014;83:486–493. doi:10.1212/WNL.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi:10.1016/j.cyto.2014.05.024 [DOI] [PubMed] [Google Scholar]
- 12. Bettcher BM, Wilheim R, Rigby T, et al. . C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26:103–108. doi:10.1016/j.bbi.2011.07.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bettcher BM, Watson CL, Walsh CM, et al. . Interleukin-6, age, and corpus callosum integrity. PLoS One. 2014;9:e106521 doi:10.1371/journal.pone.0106521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fornage M, Chiang YA, O’Meara ES, et al. . Biomarkers of inflammation and MRI-Defined small vessel disease of the brain: the cardiovascular health study. Stroke. 2008;39:1952–1959. doi:10.1161/STROKEAHA.107.508135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–727. doi:10.1212/WNL.0b013e318248e50f [DOI] [PubMed] [Google Scholar]
- 16. Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi:10.1212/WNL.59.3.371 [DOI] [PubMed] [Google Scholar]
- 17. Wersching H, Duning T, Lohmann H, et al. . Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74:1022–1029. doi:10.1212/WNL.0b013e3181d7b45b [DOI] [PubMed] [Google Scholar]
- 18. Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. doi:10.1016/j.bbi.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi:10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry. 2014;76:585–593. doi:10.1016/j.biopsych.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hannestad J, Subramanyam K, Dellagioia N, et al. . Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012;53:601–607. doi:10.2967/jnumed.111.097014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pomykala KL, Ganz PA, Bower JE, et al. . The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 2013;7:511–523. doi:10.1007/s11682-013-9243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kullmann JS, Grigoleit JS, Wolf OT, et al. . Experimental human endotoxemia enhances brain activity during social cognition. Soc Cogn Affect Neurosci. 2014;9:786–793. doi:10.1093/scan/nst049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114 doi:10.1186/s12974-015-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? implications for PET and fMRI. Neuroimage. 1995;2:148–156. doi:10.1006/nimg.1995.1017 [DOI] [PubMed] [Google Scholar]
- 26. Woolsey TA, Rovainen CM, Wei L, et al. . Dynamic measurements of local cerebral blood flow: examples from rodent whisker barrel cortex. In: Toga AW, Mazziotta JC, eds. Brain Mapping, the Methods. Boston: Academic Press; 1996. [Google Scholar]
- 27. Harrison NA, Cercignani M, Voon V, Critchley HD. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 2015;40:831–838. doi:10.1038/npp.2014.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Resnick SM, Goldszal AF, Davatzikos C, et al. . One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi:10.1093/cercor/10.5.464 [DOI] [PubMed] [Google Scholar]
- 29. Shock N, Greulich R, Andre SR, et al. . Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984. [Google Scholar]
- 30. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 31. Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. doi:10.1002/ana.21031 [DOI] [PubMed] [Google Scholar]
- 32. Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:1140–1141. doi:10.1016/S1053-8119(02)90013-3 [Google Scholar]
- 33. Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi:10.1006/nimg.2001.0937 [DOI] [PubMed] [Google Scholar]
- 34. Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi:10.1109/TMI.2002.803111 [DOI] [PubMed] [Google Scholar]
- 35. Doshi J, Erus G, Ou Y, Gaonkar B, Davatzikos C. Multi-atlas skull-stripping. Acad Radiol. 2013;20:1566–1576. doi:10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lao Z, Shen D, Liu D, et al. . Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi:10.1016/j.acra.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zacharaki EI, Kanterakis S, Bryan RN, Davatzikos C. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11:620–627. [DOI] [PubMed] [Google Scholar]
- 38. Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202 [DOI] [PubMed] [Google Scholar]
- 39. McGeer EG, Yasojima K, Schwab C, McGeer PL. The pentraxins: possible role in Alzheimer’s disease and other innate inflammatory diseases. Neurobiol Aging. 2001;22:843–848. doi:10.1016/S0197-4580(01)00288-3 [DOI] [PubMed] [Google Scholar]
- 40. Straube B. An overview of the neuro-cognitive processes involved in the encoding, consolidation, and retrieval of true and false memories. Behav Brain Funct. 2012;8:35 doi:10.1186/1744-9081-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dickerson BC, Salat DH, Bates JF, et al. . Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi:10.1002/ana.20163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bettcher BM, Yaffe K, Boudreau RM.et al. . Health ABC study. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol Aging. 2015;36:948–954. doi:10.1016/j.neurobiolaging.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:145–156. doi:10.1006/nimg.1999.0527 [DOI] [PubMed] [Google Scholar]
- 44. Petrides M. The orbitofrontal cortex: novelty, deviation from expectation, and memory. Ann N Y Acad Sci. 2007;1121:33–53. doi:10.1196/annals.1401.035 [DOI] [PubMed] [Google Scholar]
- 45. Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. [DOI] [PubMed] [Google Scholar]
- 46. Axmacher N, Schmitz DP, Wagner T, Elger CE, Fell J. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial EEG and functional magnetic resonance imaging study. J Neurosci. 2008;28:7304–7312. doi:10.1523/JNEUROSCI.1778-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mesgarani N, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343:1006–1010. doi:10.1126/science.1245994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–430. doi:10.1093/cercor/9.5.415 [DOI] [PubMed] [Google Scholar]
- 49. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi:10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- 50. Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings?Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:295–303. doi:10.1016/j.pnpbp.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 51. Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357 doi:10.1100/2012/756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. . An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi:10.1038/nature11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 54. Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi:10.1016/0197- 4580(95)00021-6 [DOI] [PubMed] [Google Scholar]
- 55. Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi:10.1016/S0197-4580(97)00056-0 [DOI] [PubMed] [Google Scholar]
- 56. Jack CR Jr, Knopman DS, Chételat G, et al. . Suspected non-Alzheimer disease pathophysiology–concept and controversy. Nat Rev Neurol. 2016;12:117–124. doi:10.1038/nrneurol.2015.251 [DOI] [PMC free article] [PubMed] [Google Scholar]