Lymph node and adipose tissue fibrosis may limit CD4+ T-cell recovery and contribute to comorbidities in treated human immunodeficiency virus (HIV) infection. We demonstrate that fibrosis decreases with continued suppressive antiretroviral therapy; telmisartan, an angiotensin receptor blocker and peroxisome proliferator-activated receptor γ agonist, confers no additional benefit.
Keywords: HIV, ART, lymph node fibrosis, adipose tissue fibrosis, TGF-beta
Abstract
Background
Fibrosis in lymph nodes may limit CD4+ T-cell recovery, and lymph node and adipose tissue fibrosis may contribute to inflammation and comorbidities despite antiretroviral therapy (ART). We hypothesized that the angiotensin receptor blocker and peroxisome proliferator-activated receptor γ agonist telmisartan would decrease lymph node or adipose tissue fibrosis in treated human immunodeficiency virus type 1 (HIV) infection.
Methods
In this 48-week, randomized, controlled trial, adults continued HIV–suppressive ART and received telmisartan or no drug. Collagen I, fibronectin, and phosphorylated SMAD3 (pSMAD3) deposition in lymph nodes, as well as collagen I, collagen VI, and fibronectin deposition in adipose tissue, were quantified by immunohistochemical analysis at weeks 0 and 48. Two-sided rank sum and signed rank tests compared changes over 48 weeks.
Results
Forty-four participants enrolled; 35 had paired adipose tissue specimens, and 29 had paired lymph node specimens. The median change overall in the percentage of the area throughout which collagen I was deposited was −2.6 percentage points (P = 0.08) in lymph node specimens and −1.3 percentage points (P = .001) in adipose tissue specimens, with no between-arm differences. In lymph node specimens, pSMAD3 deposition changed by −0.5 percentage points overall (P = .04), with no between-arm differences. Telmisartan attenuated increases in fibronectin deposition (P = .06). In adipose tissue, changes in collagen VI deposition (−1.0 percentage point; P = .001) and fibronectin deposition (−2.4 percentage points; P < .001) were observed, with no between-arm differences.
Conclusions
In adults with treated HIV infection, lymph node and adipose tissue fibrosis decreased with continued ART alone, with no additional fibrosis reduction with telmisartan therapy.
Antiretroviral therapy (ART) has dramatically improved clinical outcomes in human immunodeficiency virus (HIV)–infected individuals [1]. However, HIV-associated immune activation persists despite suppressive ART, and diseases of inflammation, including cardiovascular disease, malignancies, and hepatic disease, cause substantial morbidity and mortality [2]. Circulating concentrations of inflammation, coagulation, and fibrosis biomarkers independently predict all-cause mortality in HIV infection [3, 4].
Starting in acute infection, inflammation in HIV infection may propagate and be propagated by transforming growth factor β1 (TGF-β1)–mediated fibroblast activation and subsequent lymphoid tissue fibrosis [5]. Lymphoid tissue fibrosis may impair CD4+ T-cell recovery during ART [6] and facilitate local HIV replication and persistence, inflammation, and comorbidities [7].
Adipose tissue fibrosis can be a normal response to limit adipose tissue expansion but may also propagate a proinflammatory environment and facilitate ectopic fat deposition [8], which has been associated with increased inflammation, cardiovascular disease burden, and mortality in HIV-infected persons [9–13]. In nondiabetic persons, severity of subcutaneous adipose tissue fibrosis positively correlates with insulin resistance [14]. Lipopolysaccharide, which is increased in HIV-infected persons because of gut barrier breach, triggers adipose tissue inflammation and fibrosis [15]. Finally, activated immune cells in adipose tissue surrounding lymph nodes may contribute to or reflect local inflammation [16], perpetuating fibrosis. Thus, an intervention to decrease lymph node and adipose tissue fibrosis may improve clinical outcomes in HIV infection.
Angiotensin II signaling through the angiotensin II type 1 receptor likely enhances TGF-β production and signaling [17–20]. To date, the effects of angiotensin receptor blockers (ARBs) on TGF-β–mediated tissue fibrosis have not been studied in HIV-infected persons receiving ART. Telmisartan is an ARB and a partial peroxisome proliferator-activated receptor γ (PPAR-γ) agonist, with potential antiinflammatory and antifibrotic effects [21–25].
In this randomized, open-label, 2-arm, pilot study, we added telmisartan or no study drug to continued ART to test the hypothesis that 48 weeks of telmisartan treatment will decrease lymph node and/or adipose tissue fibrosis in HIV-infected adults receiving suppressive ART.
METHODS
Institutional review boards at each site approved the study. All participants provided written informed consent prior to initiation of study procedures.
Study Design
AIDS Clinical Trials Group protocol A5317 (clinical trials registration 01928927) was a 48-week, 2-step, open-label, randomized, controlled trial of the effects of continued ART plus telmisartan versus continued ART alone on lymph node and/or adipose tissue fibrosis. Eligibility criteria included HIV infection, age ≥18 years, virologic suppression during ART for ≥48 weeks before study entry, HIV RNA load of <50 copies/mL at screening, no change in ART for 12 weeks before study entry, body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared) of 20–35, systolic blood pressure of ≤160 mm Hg, and diastolic blood pressure of ≤100 mm Hg. Exclusion criteria included known renal artery stenosis, cirrhosis or advanced liver disease, decompensated congestive heart failure, unstable coronary artery disease, bleeding disorder/coagulopathy, heritable connective tissue disorders, history of angiotensin-converting enzyme inhibitor or ARB intolerance, use of anticoagulants except aspirin, need for regular potassium supplementation, or use of thiazolidinedione, ARB, or angiotensin-converting enzyme inhibitor in the 24 weeks before study entry.
In step 1, participants who met eligibility criteria underwent inguinal lymph node and subcutaneous abdominal adipose tissue biopsies. Participants with sufficient adipose and/or lymph node biopsy specimens entered step 2 and were randomized 2:1 to receive telmisartan or no study drug. A 40-mg daily dose of telmisartan was given during weeks 0–4, and an 80-mg daily dose was given during weeks 5–48. Participants in the control arm received no study drug but underwent all study evaluations. All participants were instructed to continue ART throughout the study period. Follow-up inguinal lymph node and subcutaneous abdominal adipose tissue biopsies were performed at week 48. Clinical and safety evaluations were performed at weeks 0, 4, 12, 24, 36, and 48.
Lymph Node Tissue Specimen Analysis
Collagen I (mouse anti-human collagen I [clone COL-1]; Sigma-Aldrich, St. Louis, MO), fibronectin (mouse anti-fibronectin [Ab-11]; Thermo Fisher Scientific, Waltham, MA), and phosphorylated SMAD3 (pSMAD3; rabbit anti-Smad3 [phospho S423 + S425; EP823Y]; Abcam, Cambridge, MA) immunohistochemical staining was performed on formalin-fixed, paraffin-embedded lymph node tissue specimens [26]. Slides were scanned at 400× using the Aperio AT2 System (Leica Biosystems, Vista, CA). Representative regions of interest (0.25 mm2) were identified, and high-resolution images were extracted from whole-tissue scans. The percentage of the lymph node T-cell zone area staining for collagen I, fibronectin, or pSMAD3 was quantified using Photoshop (CS5 and CS6; Adobe Systems, San Jose, CA), Noiseware 5 (Imagenomic, Alexandria, VA; for noise reduction), and Fovea Pro beta 5 (Reindeer Graphics, Asheville, NC) image analysis tools.
Adipose Tissue Specimen Analysis
Formalin-fixed, paraffin-embedded adipose tissue slides were stained for collagens I and VI (Santa Cruz Biotechnology, Dallas, TX) and fibronectin (Abcam). Slides were digitized on a ScanScope AT (Leica Biosystems). Morphometric analysis was performed with Tissue Studio (Definiens, Parsippany, NJ) to determine the percentage of the area stained by collagen I, collagen VI, and fibronectin, using a nonbiased method. A stain-specific algorithm was created using the predefined marker area detection module and classification tool. Thresholds were set to classify individual stains over the entire tissue area.
All staining and analysis for lymph node and adipose tissue were performed with investigators blinded to the study group.
Statistical Analysis
The co–primary outcome measures were between-group 48-week changes in the percentage of the areas of lymph node and adipose tissue specimens in which collagen I was deposited (hereafter, the “percentage deposition”). An absolute decrease in lymph node fibrosis of 4% is expected to correlate with an increase of 75 CD4+ T cells/mm3 (based on an estimated baseline CD4+ T-cell count of 200 cells/mm3 and 10% lymph node fibrosis) [6, 27] and was the predicted effect size for this study. The assessment of subcutaneous adipose tissue fibrosis, which has not been described in HIV infection outside of lipodystrophy, was exploratory, with the same desired effect size (4 percentage points) used. Thirty-six participants in the telmisartan arm and 18 in the control arm provided 90% power to detect an absolute between-group difference of ≥4% in collagen I percentage deposition over 48 weeks, allowing for ≤25% loss to follow-up and/or missing data (2-sided α = 0.05).
The primary analysis was per protocol and limited to participants who had paired biopsy data, continued to receive study treatment until ≤5 days of the week 48 biopsy (in the telmisartan arm only), and did not change ART or have virologic failure. All analyses comparing changes in continuous outcome measures between study arms at a postbaseline time point(s) used an equivalent version of the Wilcoxon rank sum statistic. We estimated the probability (θ) that the likelihood an outcome in the telmisartan arm was less than or equal to that in the control arm [28] by using the θ estimate (with 95% confidence intervals [CIs]) and corresponding P values. The null hypothesis was a θ of 0.5, with a θ of >0.5 indicating a less likely outcome in the telmisartan arm. Measurements below (above) assay limits were analyzed as the lowest (highest) rank. To assess differential treatment effects on the co–primary outcome measures, θ was estimated within independent participant subgroups and compared across subgroups, using linear contrasts.
The safety analysis compares the highest-grade primary event between study arms, using the Wilcoxon rank sum test. All statistical tests were 2-sided, with a nominal α level of 0.05 and no adjustment for multiple testing.
RESULTS
Participant Characteristics
Enrollment occurred during January 2014–April 2015 at 11 US sites. Fifty-eight participants entered step 1 and underwent biopsy (Figure 1). Forty-four participants had adequate lymph node and/or adipose tissue biopsy specimens and entered step 2. Of these, 93% were men, 48% were black or Hispanic, the median age was 48 years, the BMI was 25, and the median CD4+ T-cell count was 588 cells/mm3 (Table 1). Eighty-nine percent received nucleoside reverse transcriptase inhibitors (NRTIs; 70% received tenofovir), 39% received non-NRTIs, 55% received protease inhibitors (PIs), and 32% received integrase strand transfer inhibitors (INSTIs). All INSTI recipients were randomized to the telmisartan arm by chance, but ART use was otherwise balanced between arms. Eight had an HIV-1 RNA load of >40 copies/mL at entry (median, 151 copies/mL) despite an undetectable HIV-1 RNA load (<50 copies/mL) at screening. All but 1 participant with detectable HIV RNA at entry experienced resuppression of the viral load to <200 copies/mL throughout the follow-up period. This participant was removed from the analysis. Prohibited medications were used by 2 participants in the telmisartan arm (1 used deserpidine during weeks 3–38 and methylprednisolone acetate once in week 44, and the other used deserpidine starting in week 37) and 3 in the control arm (1 used lisinopril during weeks 28–36, 1 used lisinopril starting in week 11, and 1 used deserpidine once in week 44).
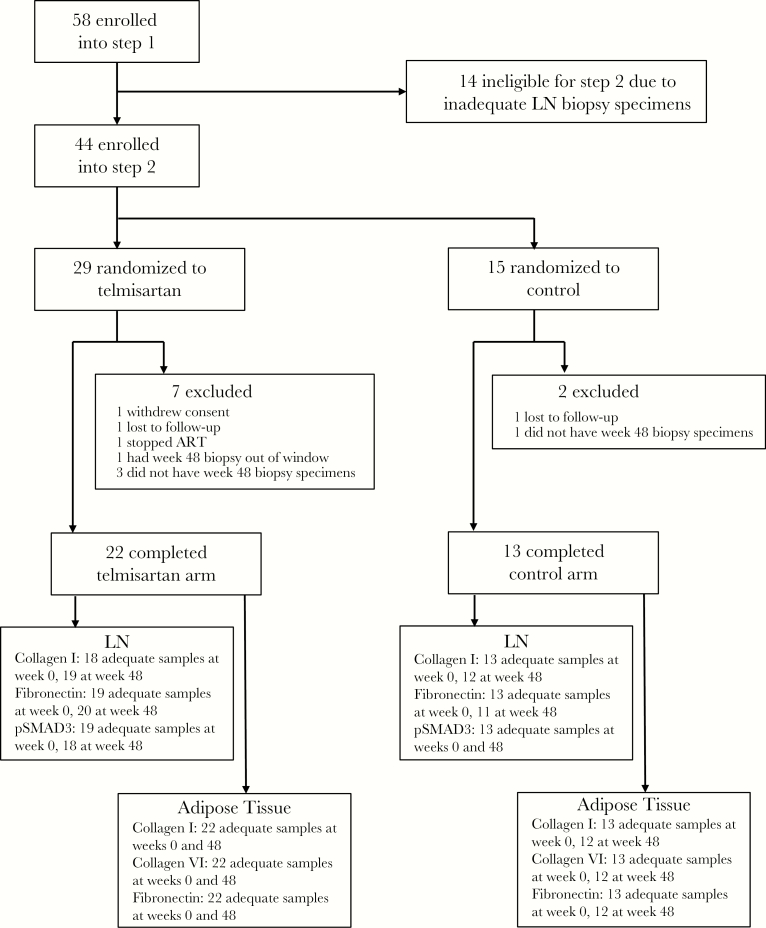
Figure 1.
Cohort diagram. Participants receiving suppressive antiretroviral therapy (ART) were enrolled into step 1 and underwent lymph node (LN) and adipose tissue biopsies. If adequate tissue specimens were obtained, they were enrolled into step 2 and randomized to receive telmisartan therapy or no additional drug. All participants were instructed to continue ART. Some samples were of insufficient quantity or quality for immunohistochemical analysis for the various markers. LN, lymph node; pSMAD3, phosphorylated SMAD3.
Table 1.
Baseline Characteristics, Overall and by Study Arm
| Characteristic | ART + Telmisartan (n = 29) |
ART Alone (n = 15) |
Overall (n = 44) |
|---|---|---|---|
| Sex | |||
| Male | 27 (93) | 14 (93) | 41 (93) |
| Female | 2 (7) | 1 (7) | 3 (7) |
| Age, y | 47 (41–51) | 50 (39–52) | 48 (41–52) |
| Race/ethnicity | |||
| White, non-Hispanic | 16 (55) | 6 (40) | 22 (50) |
| Black, non-Hispanic | 5 (17) | 8 (53) | 13 (30) |
| Hispanic (regardless of race) | 7 (24) | 1 (7) | 8 (18) |
| Body mass indexa | 25.4 (23.3–29.8) | 23.7 (21.4–26.6) | 25.0 (22.6–28.4) |
| Blood pressure, mm Hg | |||
| Systolic | 120 (115–133) | 116 (103–121) | 119 (112–125) |
| Diastolic | 75 (67–81) | 78 (67–79) | 75 (67–81) |
| Chronic hepatitis virus infection | |||
| Hepatitis B virus | 3 (10) | 0 (0) | 3 (7) |
| Hepatitis C virus | 2 (7) | 2 (13) | 4 (9) |
| Statin use | 4 (14) | 3 (20) | 7 (16) |
| CD4+ T-cell count, cells/mm3 | 604 (438–812) | 556 (444–906) | 588 (444–841) |
| HIV RNA | |||
| <40 copies/mL | 24 (83) | 12 (80) | 36 (82) |
| Detectable | 5 (17) | 3 (20) | 8 (18) |
| Overall, copies/mL, median (range) | 188 (48–899) | 114 (55–2340) | 151 (48–2340) |
| ART regimen | |||
| NRTI | 24 (83) | 15 (100) | 39 (89) |
| NNRTI | 9 (31) | 8 (53) | 17 (39) |
| PI | 17 (59) | 7 (47) | 24 (55) |
| INSTI | 14 (48) | 0 (0) | 14 (32) |
Data are no. (%) of participants or median value (interquartile range), unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus type 1; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitor.
aCalculated as the weight in kilograms divided by the height in meters squared.
Three participants (10%) in the telmisartan arm did not complete treatment and were excluded from the per-protocol analysis: 1 developed a grade 4 increase in transaminase levels unrelated to study treatment and stopped ART, 1 withdrew consent, and 1 was lost to follow-up. One participant with a week 48 biopsy performed out of window and 3 without week 48 biopsies were also excluded. In the control arm, one participant did not return to clinic, and another participant had no week 48 biopsy. Thus, 35 participants (22 in the telmisartan group and 13 in the control group) were included in the per-protocol analysis. Pill counts suggested excellent adherence, except 1 participant missed treatment on 2 days (during week 6) and 12 days (during weeks 14–16), 1 missed treatment on 5 days (during weeks 11–12), and 1 missed treatment during 2 weeks. Baseline characteristics of the per-protocol population were similar to those of the randomized study population (data not shown).
Lymph Node Fibrosis
The median baseline collagen I percentage deposition in inguinal lymph node specimens was 13.7% (interquartile range [IQR], 7.5%–21.4%) overall, with values of 14.4% (IQR, 6.0%–23.0%) in the telmisartan arm and 12.1% (IQR, 10.3%–18.2%) in the control arm (Figure 2A, 2B, and 3A). At week 48, the median collagen I percentage deposition was 11.5% (IQR, 5.8%–15.1%) overall, with values of 11.9% (IQR, 5.8%–16.0%) in the telmisartan arm and 10.1% (IQR, 4.5%–13.5%) in the control arm. The median change in the collagen I percentage deposition was −2.6 percentage points (IQR, −10.6–4.6 percentage points; P = .08) overall, with changes of −2.4 percentage points (IQR, −10.6–3.8 percentage points; P = .24) in the telmisartan arm and −6.1 percentage points (IQR, −11.7–5.8 percentage points; P = .23) in the control arm and no statistically significant difference between groups (θ = 0.50 [95% confidence interval {CI}, .26–.73]; P = .97). Thus, lymph node collagen I deposition tended to decrease over 48 weeks with continued ART alone, without reaching statistical significance and with no additional benefit from telmisartan therapy.
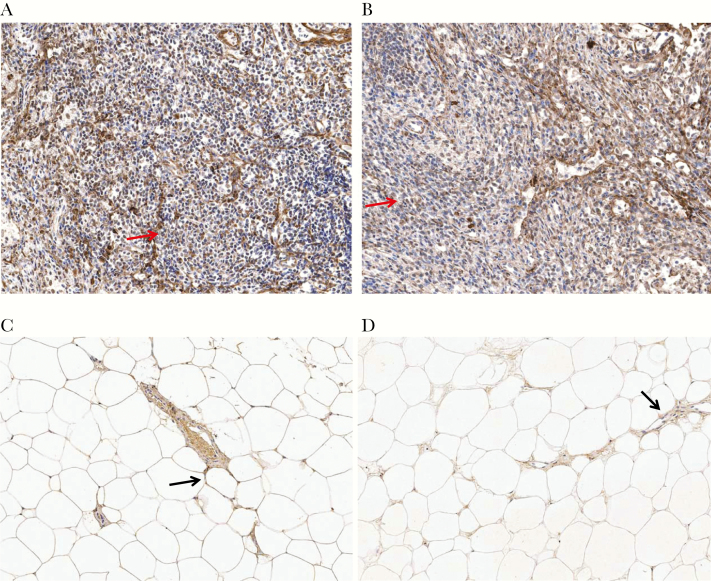
Figure 2.
Collagen I in lymph node (LN) and adipose tissue biopsy specimens. A, Substantial collagen I deposition (brown) weaving through the LN (arrow) with disruption of normal architecture. B, Low collagen I deposition in LN, with some preservation of LN architecture (arrow). C, Collagen I deposition in a vessel within adipose tissue and around adipocytes (arrow). D, Collagen I deposition in the interstitial spaces between adipocytes with inflammatory cell infiltrate (arrow).
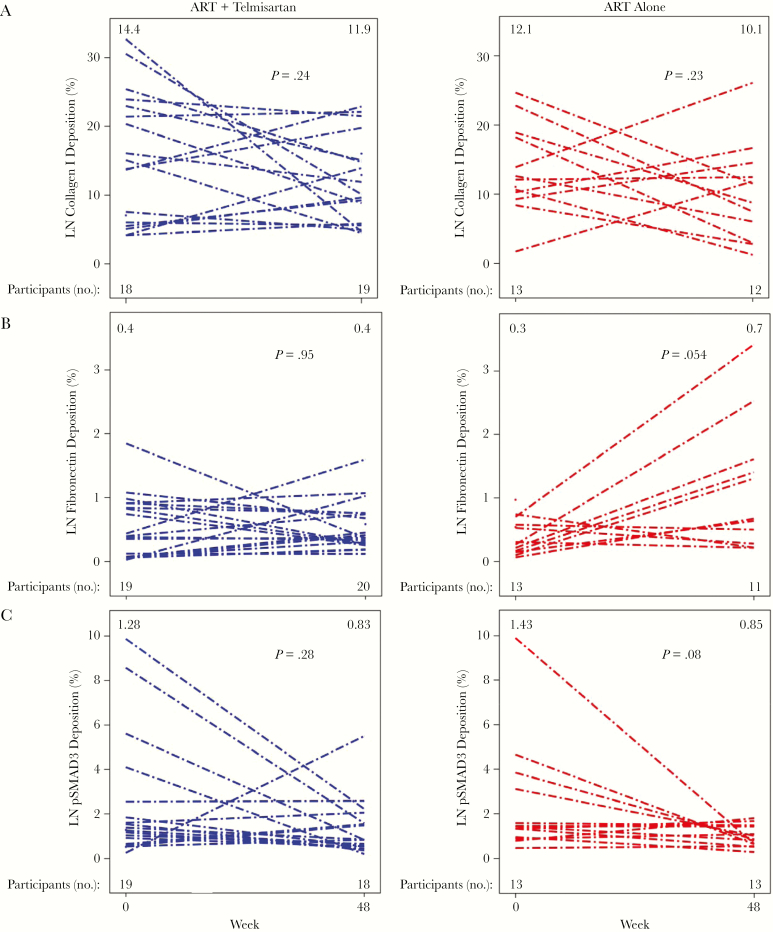
Figure 3.
Fibrosis in lymph node (LN) biopsy specimens. A, Collagen I deposition in LN specimens was dynamic, with most participants experiencing a decrease regardless of study arm. B, LN fibronectin deposition increased in the control arm but not in the telmisartan arm. C, Phosphorylated SMAD3 (pSMAD3), a marker of transforming growth factor β signaling, decreased with continued ART, with no additional decrease with telmisartan. No significant differences were seen in changes over time between telmisartan and control groups. Within-group changes over time were secondary end points. Each line represents a single participant. The median value for each tissue at each time point is indicated at the top of each figure. The number of participants with evaluable samples at each time point for each marker is indicated at the bottom of each figure. P values reflect within-arm differences between weeks 0 and 48 and were determined by the Wilcoxon signed rank test.
The median baseline fibronectin percentage deposition was 0.4% (IQR, 0.11%–0.8%) overall, with values of 0.4% (IQR, 0.1%–0.9%) in the telmisartan arm and 0.3% (IQR, 0.2%–0.6%) in the control arm (Figure 3B). At week 48, the fibronectin percentage deposition remained at 0.4% (IQR, 0.3%–0.8%) in the telmisartan arm and increased to 0.7% (IQR, 0.3%–1.6%) in the control arm, with an overall value of 0.5% (IQR, 0.3%–1.0%). The median change in fibronectin percentage deposition was 0.1 percentage points (IQR, −0.2–0.6 percentage points; P = .16) overall, with values of 0.0 percentage points (IQR, −0.4–0.4 percentage points; P = .95) in the telmisartan arm and 0.6 percentage points (IQR, −0.1–1.4 percentage points; P = .05) in the control arm and a between-arm difference (θ) of 0.72 (95% CI, .49–.94; P = .06). In sum, telmisartan therapy did not statistically significantly attenuate increases in fibronectin deposition.
The median baseline percentage deposition of pSMAD3, a surrogate marker of TGF-β signaling [29], was 1.4% (IQR, 0.9%–2.8%) overall. By week 48, this value changed by a median of −0.5 percentage points (IQR, −2.1–0.3 percentage points; P = .04) overall, by −0.47 percentage points (IQR, −1.33–0.46 percentage points; P = .28) in the telmisartan group, and by −0.62 percentage points (IQR, −2.10–0.08 percentage points; P = .08) in the control group (Figure 3C). Telmisartan did not attenuate pSMAD3 deposition more than ART alone (θ = 0.46 [95% CI, .25–.67]; P = .69), consistent with telmisartan’s lack of effect on collagen I deposition. However, decreases in pSMAD3 deposition correlated with decreases in collagen I deposition (r = 0.48; P = .01). Thus, lymph node TGF-β signaling decreased with continued ART, with no additional decrease with telmisartan therapy.
Adipose Tissue Fibrosis
The median baseline collagen I percentage deposition in subcutaneous lower abdominal adipose tissue was 1.9% (IQR, 0.4%–3.4%) overall, with a lower median value in the telmisartan group (1.3% [IQR, 0.3%–2.6%]) as compared to the control group (2.8% [IQR, 1.6%–3.7%]; between-group P = .06; Figure 2C, 2D, and 4A). At week 48, collagen I deposition changed by a median of −1.3 percentage points (IQR, −2.5–0 percentage points; P = .001) overall, with values of −1.1 percentage points (IQR, −2.1–0 percentage points; P = .02) in the telmisartan arm and −2.2 percentage points (IQR, −3.5–0 percentage points; P = .03) in the control arm and no significant between-arm difference (θ = 0.37 [95% CI, .14–.60]; P = .27). In sum, adipose tissue collagen I deposition significantly decreased with continued ART alone, with no additional benefit from telmisartan therapy.
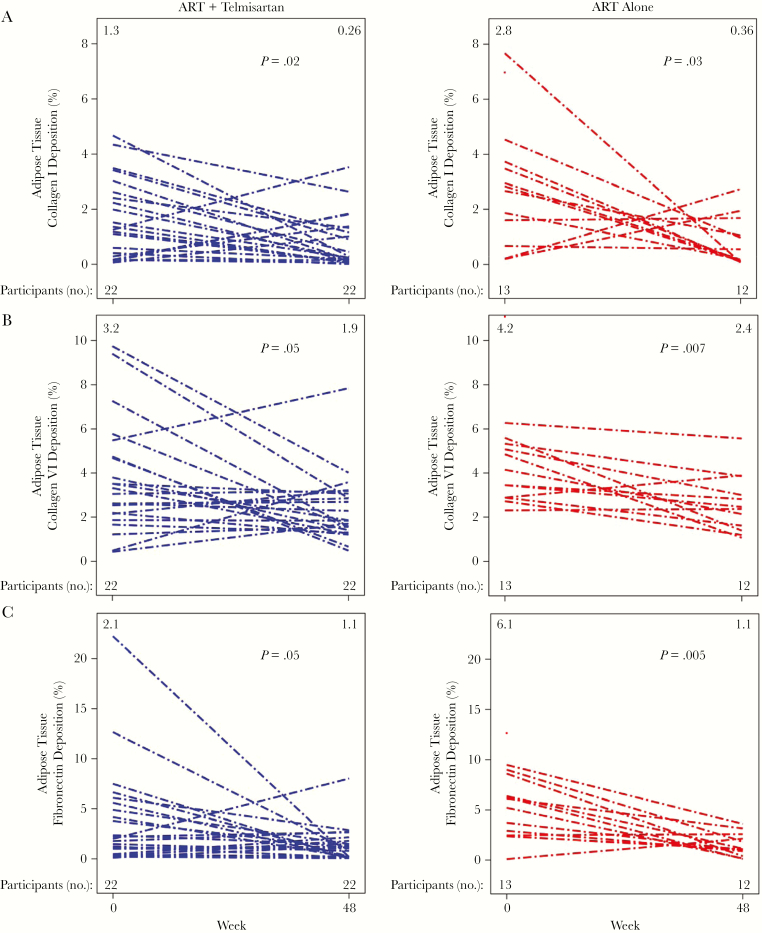
Figure 4.
Fibrosis in adipose tissue biopsy specimens. Collagen I (A), collagen VI (B), and fibronectin (C) deposition in adipose tissue decreased in both study arms, with no additional benefit of telmisartan therapy. No significant differences were seen in changes over time between telmisartan and control groups. Within-group changes over time were secondary end points. Each line represents a single participant. The median value for each tissue at each time point is indicated at the top of each figure. The number of participants with evaluable samples at each time point for each marker is indicated at the bottom of each figure. P values reflect within-arm differences between weeks 0 and 48 and were determined by the Wilcoxon signed rank test.
Collagen VI, a major component of fibrotic adipose tissue (primarily studied in obesity [30]), followed a similar pattern (Figure 4B). The median baseline collagen VI percentage deposition was 3.5% (IQR, 2.5%–5.3%) overall, with values of 3.2% (IQR, 2.2%–4.7%) in the telmisartan arm and 4.2% (IQR, 2.9%–5.3%) in the control arm (between-group P = .15). Over 48 weeks, the collagen VI percentage deposition changed significantly in both arms, with a median change of −1.0 percentage points (IQR, −2.4–0.1 percentage points; P = .001) overall, −0.4 percentage points (IQR, −4.0–0.3 percentage points; P = .05) in the telmisartan group, and −1.4 percentage points (IQR, −2.0 to −0.7 percentage points; P = .007) in the control group. Telmisartan therapy had no additional benefit as compared to ART alone (θ = 0.44 [95% CI, .24–.63]; P = .52).
The median baseline fibronectin percentage deposition was lower in the telmisartan arm as compared to the control arm, with values of 3.7% (IQR, 1.2%–6.4%) overall, 2.1% (IQR, 1.0%–5.6%) in the telmisartan arm, and 6.1% (IQR, 2.9%–8.6%) in the control arm (between-group P = .05; Figure 4C). The fibronectin percentage deposition significantly declined over 48 weeks, with a median change of −2.4 percentage points (IQR, −5.5–0.1 percentage points; P < .001) overall, −0.8 percentage points (IQR, −4.0–0.5 percentage points; P = .05) in the telmisartan group, and −4.0 percentage points (IQR, −6.0 to −1.6 percentage points; P = .005) in the control group. Telmisartan therapy had no apparent impact on fibronectin deposition in adipose tissue (θ = 0.36 [95% CI, .16–.56]; P = .17).
Secondary Analyses
Overall, telmisartan treatment did not affect collagen I deposition in lymph node or adipose tissues, after stratifying by ART class, statin use, or baseline CD4+ T-cell count. Participants with CD4+ T-cell counts below the median value (ie, 572 cells/mm3) had statistically significant changes in lymph node collagen I percentage deposition (median −8.6 percentage points [IQR, −11.5–1.7 percentage points]; P = .04), which were not observed among participants with higher CD4+ T-cell counts (0.1 percentage points [IQR, −5.6–6.4 percentage points]). Changes in adipose tissue collagen I percentage deposition were smaller for participants with excess adiposity (BMI, ≥30; waist circumference, ≥95 cm for men and ≥94 cm for women, and/or waist-to-hip ratio of ≥0.94 for men and 0.88 for women), with a median of −0.4 percentage points (IQR, −1.6–1.0 percentage points; P = .54) among participants with excess adiposity, compared with −1.8 percentage points (IQR, −2.9 to −1.1 percentage points; P < .001) among those without excess adiposity.
As PI therapy has been associated with adipose tissue inflammation, PPAR-γ suppression, and renin angiotensin system activation [31–34], we hypothesized that telmisartan therapy may affect participants receiving PIs differently. Indeed, the adipose tissue collagen I percentage deposition improved more after 48 weeks in PI-treated participants who received telmisartan therapy median (change, −1.7 percentage points [IQR, −2.2 to −1.1 percentage points]; P = .04) than in control participants taking PIs (change, −0.9 percentage points [IQR, −3.6–0.1 percentage points]; P = .31) and in telmisartan-treated participants receiving non–PI-based regimens (change, −0.1 percentage points [IQR, −0.4–1.0 percentage points]; P = .82); however, control participants not taking PIs had the greatest improvements (change, −2.7 percentage points [IQR, −3.4 to −1.7 percentage points]; P = .09). Excluding participants who started prohibited medications or were viremic at baseline did not change results for the primary end point (data not shown).
CD4+ and CD8+ T-cell counts did not change significantly during the 48-week study period. Telmisartan therapy did not significantly affect the CD4+ T-cell count over 48 weeks (median change, 9 cells/mm3 [IQR, −24–45 cells/mm3]; P = .70), compared with control (change, 97 cells/mm3 [IQR, 4–111 cells/mm3]; P = .22), with a between-group difference (θ) of 0.67 (95% CI, .50–.83; P = .06). The 48-week change in CD8+ T-cell count was, however, significantly smaller in the telmisartan arm (median 10 cells/mm3 [IQR, −72–101 cells/mm3]; P = .99), compared with the control arm (97 cells/mm3 [IQR, −24–169]; P = .11), with a between-group difference (θ) of 0.67 (95% CI, .51–.94; P = .04). The ratio of CD4+ to CD8+ T cells, which is predictive of non–AIDS-related morbidity and mortality [35], was similar in each arm at week 0 (0.84 in the telmisartan group and 0.89 in the control group) and did not change significantly in either arm (0.7% [IQR, −6.2%–9.3%] in the telmisartan group [P = .45] and 5.3% [IQR, −1.3%–13.4%] in the control group [P = .41]; θ = 0.53 [95% CI, .36–.71; P = .70]).
Metabolic parameters, including fasting glucose level, insulin level, homeostatic model assessment of insulin resistance value, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, triglycerides level, waist circumference, waist-to-hip ratio, or percentage of participants with metabolic syndrome, did not change significantly during the study, and telmisartan therapy had no significant effect on these parameters (data not shown). Telmisartan treatment significantly lowered blood pressure: the median change in systolic blood pressure was 3 mm Hg (IQR, −15–6 mm Hg; P = .79) overall, with values of −5 mm Hg (IQR, −20–5 mm Hg; P = .051) in the telmisartan arm and 5 mm Hg (IQR, 3–12 mm Hg ; P = .01) in the control arm (θ = 0.66 [95% CI, .52–.80]; P = .02). The median change in diastolic blood pressure was −2 mm Hg (IQR, −5–3 mm Hg; P = .40) overall, with values of −4 mm Hg (IQR, −5–0 mm Hg; P = .03) in the telmisartan arm and 1 mm Hg (IQR, −4–11 mm Hg; P = .28) in the control arm and no significant effect of telmisartan therapy (θ = 0.54 [95% CI, .39–.69]; P = .56).
Adverse Events
All randomized participants were included in the safety analysis. Telmisartan therapy was well tolerated. Five participants developed grade ≥3 adverse events, none of which were deemed related to study treatment (Table 2). No episodes of hypotension (symptomatic or asymptomatic) occurred.
Table 2.
Grade ≥3 Adverse Events (AEs), Overall and by Study Arm
| Grade |
ART + Telmisartan (n = 29) |
ART Alone (n = 15) |
Overall (n = 44) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 | Total | Grade 3 | Grade 4 | Total | |
| Overall | 1 (3) | 2 (7) | 3 (10) | 1 (7) | 1 (7) | 2 (13) | 2 (5) | 3 (7) | 5 (11) |
| Elevated liver enzyme levels | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Unstable angina | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| Acute diarrhea | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Disseminated herpes zoster | 1a | 0 | 1 | 0 | 0 | 0 | 1 a | 0 | 1 |
| Hydrocephaly | 0 | 1a | 1 | 0 | 0 | 0 | 0 | 1 a | 1 |
| Anal fistula | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
Data are no. (%) of participants.
Abbreviations: ART, antiretroviral therapy.
aSame participant. This individual was included in the total no. of participants with grade 4 events, rather than the total no. with grade 3 AEs.
DISCUSSION
Lymphoid tissue fibrosis occurs early in HIV infection, is associated with CD4+ T-cell depletion, and was previously thought to persist despite suppressive ART [36–38]. Adipose tissue fibrosis has been described primarily in obesity, where it can contribute to metabolic dysfunction, tissue hypoxia, and an ongoing, proinflammatory state [30, 39–41]. In HIV-infected persons, adipose tissue fibrosis has only been described in clinically significant lipoatrophy and dorsocervical fat accumulation [42, 43]. Thus, in the general population, tissue fibrosis is considered a response to a pathologic state or tissue dysfunction. Since chronic inflammation and immune activation persist despite suppressive ART, we hypothesized that lymph node and subcutaneous adipose tissue fibrosis are prevalent in HIV-infected adults receiving suppressive ART and that treatment with telmisartan, an ARB and PPAR-γ agonist with putative antifibrotic properties, would attenuate lymph node and adipose tissue fibrosis as compared to suppressive ART alone.
This randomized trial documented several important, novel findings. First, subcutaneous adipose tissue fibrosis exists in HIV-infected adults outside of obesity and clinically significant lipodystrophy, a finding that may help explain metabolic disturbances in HIV-infected adults. Additionally, (1) adipose tissue collagen I, collagen VI, and fibronectin deposition decreased with continued ART alone; (2) continued ART decreased lymph node collagen I deposition among participants with lower baseline CD4+ T-cell counts; and (3) telmisartan therapy had no additional impact on collagen I deposition in lymph nodes or adipose tissue or on fibronectin or collagen VI deposition in adipose tissue in HIV-infected adults, compared with continued ART alone.
Previous studies of lymphoid tissue fibrosis have demonstrated no effect of ART but have only evaluated up to 1 year of treatment [37, 44, 45]. Thus, fibrosis may be stable early after starting ART, but continued suppressive ART may eventually permit resumption of wound healing processes and modest fibrosis reversal. The stimulus for this transition to improved wound healing/fibrosis reversal is unknown, but possibilities include insufficient local viral stimulus, reduced microbial or other profibrotic stimuli, and immune system alterations. Importantly, the percentage improvement in tissue fibrosis needed to exert a clinical benefit is also unknown. Given our small sample size and heterogeneous population and results, additional studies are needed.
Telmisartan’s lack of additional benefit against fibrosis in this study may be multifactorial. The observed declines in collagen I, collagen VI, and/or fibronectin deposition with ART alone suggest that antifibrotic pathways were activated relative to those closer to ART initiation, consistent with suppression of TGF-β signaling. The observed decreases in pSMAD3 deposition reflect further suppression of TGF-β signaling at week 48 as compared to week 0, supporting the hypothesis of antifibrotic pathway activation with continued ART alone. Consequently, blocking TGF-β signaling with telmisartan therapy may not have had an additional impact. Alternatively, although TGF-β upregulation has been documented in fibrotic lymphoid tissue in a simian immunodeficiency virus model [5], whether TGF-β–independent pathways, such as interleukin 13 or interleukin 33 signaling, contribute is unknown.
Similarly, continued suppression of HIV replication may have resulted in minimal residual tissue inflammation, rendering telmisartan’s antiinflammatory properties inconsequential. Participants with lower CD4+ T-cell counts, who may have greater systemic immune activation [46], had the greatest declines in lymph node collagen I deposition, possibly because of the antiinflammatory properties of telmisartan. Similarly, participants receiving PIs may have had greater adipose tissue inflammation, making them more susceptible to telmisartan’s antiinflammatory benefits.
This study had several limitations. First, the study was designed to provide 90% power to detect a 4-percentage-point change in collagen I deposition, which required 39 participants. With 35 participants in the per-protocol analysis, the study was underpowered for all end points. Nonetheless, no trend toward a benefit of adding telmisartan to ART was observed in lymph node or adipose tissue. Second, both immunohistochemistry laboratories were blinded to group but not time point, so some bias could have been introduced. The lack of placebo and numerous comparisons made may have also introduced type 1 error. Third, participants had high median CD4+ T-cell counts, were overwhelmingly male, and were primarily nonobese, possibly limiting generalizability to other populations. Fourth, since the precise duration of ART was unknown, the effect of ART duration on fibrosis changes is unclear. Given differences in tissue penetration with INSTIs, particularly raltegravir [47], and potential differences in local or circulating inflammatory profiles [48–50], the effects of INSTI imbalance between arms (48% in the telmisartan arm and 0% in the control arm) cannot fully be elucidated in this pilot study. Whether telmisartan penetrated the fibrotic tissues sufficiently to effect a change is unknown, as tissue-level telmisartan concentrations are not available. Finally, the lack of statistical significance in some outcome measures and the number of inadequate and missed biopsy specimens also suggest that similar studies will need to enroll more participants.
Nonetheless, this study is the first to document the presence of abdominal adipose tissue fibrosis in HIV-infected persons outside of obesity or clinical lipodystrophy. This study was also the first clinical trial involving participants with treated HIV infection and longitudinal lymph node and adipose tissue biopsies, demonstrating the feasibility of such studies. It is the first study to document improvements in adipose tissue fibrosis and suggest possible improvements in lymph node fibrosis with continued ART alone, indicating that tissue-level inflammation may be minimized sufficiently to allow wound healing with chronic, sustained virologic suppression.
In summary, in this pilot study, continued suppressive ART decreased subcutaneous abdominal adipose tissue fibrosis and may decrease lymph node fibrosis. The addition of telmisartan, an ARB and PPAR-γ agonist, did not provide additional benefit. Analysis of tissue-level and systemic inflammatory and immunologic environments may help determine the specific mechanisms contributing to this change and/or identify future therapeutic opportunities.
Notes
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 13–16 February 2007. Abstract 251.
Acknowledgments. We thank the participants for volunteering for this study.
Disclaimer. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views or policies of the National Institutes of Health or the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants UM1 AI068634, UM1 AI068636, and UM1 AI106701; grant K23AI110532 to J. E. L.) and the National Cancer Institute (contract HHSN261200800001E to J. D. E.), National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
A5317 AIDS Clinical Trials Group Team:
Francesca Aweeka, Jenifer Baer, Alex Benns, Joan Dragavon, Christopher Hensel, Priscilla Hsue, Andy Kaytes, Heather Ribaudo, David Rusin, Katherine Shin, Antoine Simmons, and Xinyan Zhan
STUDY GROUP MEMBERS AND SITES
ACTG A5317 Team Members other than the coauthors are as follows: Francesca Aweeka, Jenifer Baer, Alex Benns, Joan Dragavon, Christopher Hensel, Priscilla Hsue, Andy Kaytes, Heather Ribaudo, David Rusin, Katherine Shin, Antoine Simmons, and Xinyan Zhang. Scanning and analyses were performed through the Translational Pathology Core Laboratory, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA. Enrolling research sites (and supporting grants) were as follows: UCLA CARE Center Clinical Research Site (CRS; UM1AI069424), University of Rochester Adult HIV Therapeutic Strategies Network (UM1AI069511), Cincinnati CRS (UM1AI069501), University of Colorado Hospital CRS (UM1AI069432), Vanderbilt Therapeutics CRS (UM1AI069439), Washington University Therapeutics (UM1AI069439), University of Washington AIDS Clinical Trials Unit (CTU; UM1AI069481), Case Western Reserve University CRS (UM1AI069501), Chapel Hill CRS (UM1AI069423), Houston AIDS Research Team (HART) CRS (UM1AI069503), Puerto Rico AIDS CTU (UM1AI069415), and Massachusetts General Hospital CRS (UM1AI069412).
References
- 1. Lohse N, Hansen AB, Pedersen G et al. . Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007; 146:87–95. [DOI] [PubMed] [Google Scholar]
- 2. Smith CJ, Ryom L, Weber R et al. . Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 3. Sandler NG, Wand H, Roque A et al. ; INSIGHT SMART Study Group. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boulware DR, Hullsiek KH, Puronen CE et al. ; INSIGHT Study Group. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estes JD, Wietgrefe S, Schacker T et al. . Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis 2007; 195:551–61. [DOI] [PubMed] [Google Scholar]
- 6. Schacker TW, Reilly C, Beilman GJ et al. . Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS 2005; 19:2169–71. [DOI] [PubMed] [Google Scholar]
- 7. Rothenberger MK, Keele BF, Wietgrefe SW et al. . Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 2015; 112:E1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodríguez A, Ezquerro S, Méndez-Giménez L, Becerril S, Frühbeck G. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab 2015; 309:E691–714. [DOI] [PubMed] [Google Scholar]
- 9. Longenecker CT, Jiang Y, Yun CH et al. . Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol 2013; 168:4039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brener M, Ketlogetswe K, Budoff M et al. . Epicardial fat is associated with duration of antiretroviral therapy and coronary atherosclerosis. AIDS 2014; 28:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lonardo A, Ballestri S, Guaraldi G et al. . Fatty liver is associated with an increased risk of diabetes and cardiovascular disease - Evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol 2016; 22:9674–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lake JE, Wohl D, Scherzer R et al. . Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care 2011; 23:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuller LH, Tracy R, Belloso W et al. ; INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spencer M, Yao-Borengasser A, Unal R et al. . Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab 2010; 299:E1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vila IK, Badin PM, Marques MA et al. . Immune cell toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep 2014; 7:1116–29. [DOI] [PubMed] [Google Scholar]
- 16. Knight SC. Specialized perinodal fat fuels and fashions immunity. Immunity 2008; 28:135–8. [DOI] [PubMed] [Google Scholar]
- 17. Tomasoni L, Sitia S, Borghi C et al. . Effects of treatment strategy on endothelial function. Autoimmun Rev 2010; 9:840–4. [DOI] [PubMed] [Google Scholar]
- 18. Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 2006; 20:953–70. [DOI] [PubMed] [Google Scholar]
- 19. Sata M, Fukuda D. Crucial role of renin-angiotensin system in the pathogenesis of atherosclerosis. J Med Invest 2010; 57:12–25. [DOI] [PubMed] [Google Scholar]
- 20. de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol 2009; 296:H550–8. [DOI] [PubMed] [Google Scholar]
- 21. Halici Z, Bilen H, Albayrak F et al. . Does telmisartan prevent hepatic fibrosis in rats with alloxan-induced diabetes?Eur J Pharmacol 2009; 614:146–52. [DOI] [PubMed] [Google Scholar]
- 22. Jin H, Yamamoto N, Uchida K, Terai S, Sakaida I. Telmisartan prevents hepatic fibrosis and enzyme-altered lesions in liver cirrhosis rat induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun 2007; 364:801–7. [DOI] [PubMed] [Google Scholar]
- 23. Kaschina E, Schrader F, Sommerfeld M et al. . Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J Hypertens 2008; 26:2361–73. [DOI] [PubMed] [Google Scholar]
- 24. Guan HS, Shangguan HJ, Shang Z, Yang L, Meng XM, Qiao SB. Endoplasmic reticulum stress caused by left ventricular hypertrophy in rats: effects of telmisartan. Am J Med Sci 2011; 342:318–23. [DOI] [PubMed] [Google Scholar]
- 25. Maejima Y, Okada H, Haraguchi G et al. . Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Lab Invest 2011; 91:932–44. [DOI] [PubMed] [Google Scholar]
- 26. Estes JD, Reilly C, Trubey CM et al. . Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ T-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis 2015; 211:744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burnier M. Telmisartan: a different angiotensin II receptor blocker protecting a different population?J Int Med Res 2009; 37:1662–79. [DOI] [PubMed] [Google Scholar]
- 28. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–45. [PubMed] [Google Scholar]
- 29. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012; 18:1028–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Divoux A, Tordjman J, Lacasa D et al. . Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010; 59:2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mencarelli A, Francisci D, Renga B et al. . Ritonavir-induced lipoatrophy and dyslipidaemia is reversed by the anti-inflammatory drug leflunomide in a PPAR-γ-dependent manner. Antivir Ther 2012; 17:669–78. [DOI] [PubMed] [Google Scholar]
- 32. Boccara F, Auclair M, Cohen A et al. . HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther 2010; 15:363–75. [DOI] [PubMed] [Google Scholar]
- 33. Loonam CR, O’Dell SD, Sharp PA, Mullen A. Microarray analysis reveals altered lipid and glucose metabolism genes in differentiated, ritonavir-treated 3T3-L1 adipocytes. Curr HIV Res 2016; 14:37–46. [DOI] [PubMed] [Google Scholar]
- 34. Erlandson KM, Lake JE. Fat matters: understanding the role of adipose tissue in health in HIV infection. Curr HIV/AIDS Rep 2016; 13:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy JP. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015; 18:20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nies-Kraske E, Schacker TW, Condoluci D et al. . Evaluation of the pathogenesis of decreasing CD4(+) T cell counts in human immunodeficiency virus type 1-infected patients receiving successfully suppressive antiretroviral therapy. J Infect Dis 2009; 199:1648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez JL, Hunt PW, Reilly CS et al. . Lymphoid fibrosis occurs in long-term nonprogressors and persists with antiretroviral therapy but may be reversible with curative interventions. J Infect Dis 2015; 211:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng M, Southern PJ, Reilly CS. et al. Lymphoid Tissue Damage in HIV-1 Infection Depletes Naïve T Cells and Limits T Cell Reconstitution after Antiretroviral Therapy. PLoS Pathog 2012; 8(1):e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halberg N, Khan T, Trujillo ME et al. . Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 2009; 29:4467–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henegar C, Tordjman J, Achard V et al. . Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 2008; 9:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spencer M, Unal R, Zhu B et al. . Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab 2011; 96:E1990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jan V, Cervera P, Maachi M et al. . Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther 2004; 9:555–64. [PubMed] [Google Scholar]
- 43. Béréziat V, Cervera P, Le Dour C et al. ; Lipodystrophy Study Group LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue. Am J Pathol 2011; 179:2443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol 2008; 20:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torres B, Rallón NI, Loncá M et al. . Immunological function restoration with lopinavir/ritonavir versus efavirenz containing regimens in HIV-infected patients: a randomized clinical trial. AIDS Res Hum Retroviruses 2014; 30:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lederman MM, Calabrese L, Funderburg NT et al. . Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patterson KB, Prince HA, Stevens T et al. . Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS 2013; 27:1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lake JE, McComsey GA, Hulgan T et al. . Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the Women, Integrase and Fat Accumulation Trial. HIV Med 2014; 15:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Afonso P, Auclair M, Caron-Debarle M, Capeau J. Impact of CCR5, integrase and protease inhibitors on human endothelial cell function, stress, inflammation and senescence. Antivir Ther 2017; 22:645–657. [DOI] [PubMed] [Google Scholar]
- 50. Villanueva-Millan MJ, Perez-Matute P, Recio-Fernandez E, Lezana Rosales JM, Oteo JA. Differential effects of antiretrovirals on microbial translocation and gut microbiota composition of HIV-infected patients. J Int AIDS Soc 2017; 20:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]