Abstract
Background
There is growing concern about overtreatment of breast cancer as outcomes have improved over time. However, little is known about how chemotherapy use and oncologists’ recommendations have changed in recent years.
Methods
We surveyed 5080 women (70% response rate) diagnosed with breast cancer between 2013 and 2015 and accrued through two Surveillance, Epidemiology, and End Results registries (Georgia and Los Angeles) about chemotherapy receipt and their oncologists’ chemotherapy recommendations. We surveyed 504 attending oncologists (60.3% response rate ) about chemotherapy recommendations in node-negative and node-positive case scenarios. We conducted descriptive statistics of chemotherapy use and patients’ report of oncologists’ recommendations and used a generalized linear mixed model of chemotherapy use according to time and clinical factors. All statistical tests were two-sided.
Results
The analytic sample was 2926 patients with stage I–II, estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer. From 2013 to 2015, keeping other factors constant, chemotherapy use was estimated to decline from 34.5% (95% confidence interval [CI] = 30.8% to 38.3%) to 21.3% (95% CI = 19.0% to 23.7%, P < .001). Estimated decline in chemotherapy use was from 26.6% (95% CI = 23.0% to 30.7%) to 14.1% (95% CI = 12.0% to 16.3%) for node-negative/micrometastasis patients and from 81.1% (95% CI = 76.6% to 85.0%) to 64.2% (95% CI = 58.6% to 69.6%) for node-positive patients. Use of the 21-gene recurrence score (RS) did not change among node-negative/micrometastasis patients, and increasing RS use in node-positive patients accounted for one-third of the chemotherapy decline. Patients’ report of oncologists’ recommendations for chemotherapy declined from 44.9% (95% CI = 40.2% to 49.7%) to 31.6% (95% CI = 25.9% to 37.9%), controlling for other factors. Oncologists were much more likely to order RS if patient preferences were discordant with their recommendations (67.4%, 95% CI = 61.7% to 73.0%, vs 17.5%, 95% CI = 13.1% to 22.0%, concordant), and they adjusted recommendations based on patient preferences and RS results.
Conclusions
For both node-negative/micrometastasis and node-positive patients, chemotherapy receipt and oncologists’ recommendations for chemotherapy declined markedly over time, without substantial change in practice guidelines. Results of ongoing trials will be essential to confirm the quality of this approach to breast cancer care.
Medical oncologists are leading efforts to reduce the burden of treatment for patients diagnosed with curable breast cancer. Recent advances in test algorithms enable increasingly precise estimates of the benefit of adjuvant chemotherapy for individual patients (1–3). The growing concern about overtreatment is particularly acute for patients with early-stage disease, for some of whom the benefit of chemotherapy approaches nil in the face of substantial harms. The criteria for chemotherapy decision-making are evolving from being based primarily on anatomy to biology (2–5). Prior studies have shown decreasing use of adjuvant chemotherapy concomitant with increasing use of tumor genomic profiling in patients diagnosed from 2006 to 2013 (6–9). Despite this progress, questions remain about the causes of this downward trend in chemotherapy receipt and whether it persists. Prior studies have been limited by a lack of granular information from patients’ and oncologists’ reports of the treatment decision-making context, and by a lack of direct assessment of the impact of clinical factors including changes in the use of 21-gene recurrence score (RS) testing on treatment trends (10–12).
Treatment decisions are influenced by factors other than clinical information, including patients’ fear of recurrence and physicians’ reluctance to miss any potential opportunity to improve survival (13). Yet little is known about the perspectives of patients and their attending oncologists during the most recent period of changing views about adjuvant therapy. Understanding the shifting patterns of systemic therapy decisions is essential to initiatives that aim to advance the individualization and quality of cancer care. We therefore examined trends in chemotherapy receipt and oncologists’ recommendations in a large, contemporary, diverse, population-based sample of newly diagnosed breast cancer patients, along with their oncologists’ perspectives on chemotherapy decision-making.
Methods
Study Sample
After approval by institutional review boards (including a waiver of signed informed consent), the iCanCare study selected 7303 women age 20 to 79 years who had been diagnosed with stage 0–II breast cancer and reported to the Surveillance, Epidemiology and End Results (SEER) registries of Los Angeles County and Georgia. Patients diagnosed in the first quarter of 2013 through the second quarter of 2015 were sent surveys approximately three months after surgery on a monthly basis; 5080 women completed the survey (response rate = 70%) (Supplementary Figure 1, available online). Patients with distant metastasis and/or tumors larger than 5 cm were excluded. We oversampled Asian, black, and Hispanic women in Los Angeles, as previously reported (14). For the presented analysis, patients with stage 0 disease were excluded because they were not eligible for treatment with chemotherapy, while all other iCanCare participants were included.
Data Collection
We developed survey content using a conceptual framework, the published literature, and our previous work. Content validity was evaluated through systematic review by design experts, cognitive pretesting with patients, and pilot research in representative populations (15,16).
We used a modified Dillman method (17), including a $20 cash incentive and reminders to patients who did not respond. All survey materials were in English, with Spanish-translated materials added for women whose surnames suggested Hispanic ethnicity. We merged survey responses with SEER data. Patients reported their oncologists’ names and their specialties, the address was verified by the SEER registries, and these doctors were surveyed (using a similar Dillman approach) in 2015.
Measures
SEER registries provided diagnosis date, cancer stage, grade (1–3), tumor size, and estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2)/neu status. RS testing and results (low, intermediate, high) were provided by the testing laboratory, Genomic Health, Inc., to SEER registries and merged with survey and SEER data, as previously reported (10). Patients were eligible for this study if they had invasive disease (stage I–II) that was ER-positive and HER2-negative. Nodal status grouping followed National Comprehensive Cancer Network (NCCN) guidelines for adjuvant systemic therapy (5): node-positive (American Joint Committee on Cancer [AJCC] Anatomic Staging categories N1 or N2) vs node-negative or only micrometastasis smaller than 2 mm in size (AJCC categories N0 and N1mi, defined hereafter as “node-negative/micrometastasis”).
Patients provided information about age at diagnosis, race/ethnicity, chemotherapy receipt, whether they ever met with an oncologist, and whether the oncologist recommended chemotherapy on a five-point scale (strongly in favor of chemotherapy, somewhat in favor, left it up to me, somewhat against chemotherapy, strongly against; the first two responses were defined as recommendation for and the last two as recommendation against chemotherapy).
Oncologists were asked whether they would recommend chemotherapy and/or order a tumor genomic test in response to two clinical scenarios in hormone receptor–positive, HER2-negative disease. The first scenario was a patient with more favorable disease: age 60 years, postmenopausal, 0.7 cm tumor, node-negative. The second scenario was a patient with less favorable disease: age 48 years, premenopausal, 2.2 cm tumor, two involved lymph nodes. Oncologists were asked after each scenario 1) whether they would recommend adjuvant chemotherapy (yes/no) and 2) whether they would order a tumor genomic test that estimates the probability of distant recurrence and benefit of chemotherapy (eg, RS) before making a chemotherapy decision (yes/no). Oncologists were then given additional information for each scenario followed by the same two questions, intended to elicit the extent to which their recommendations could be influenced by patient preferences. For the node-negative scenario, the patient expressed an initial desire to receive chemotherapy, and for the node-positive scenario the patient expressed an initial desire to avoid chemotherapy. For each scenario, oncologists were asked whether they would recommend chemotherapy if a genomic test yielded a score that ran counter to the patient’s nodal status (RS = 34, in the high-risk category if node-negative; RS = 16, in the low risk-category if node-positive) (1,3). The oncologist questionnaire is included as Supplementary Material (available online), and the patient questionnaire has been previously published (18).
Statistical Methods
We conducted descriptive statistics of the patterns and timing of chemotherapy use and patients’ report of oncologists’ recommendations. We used a generalized linear mixed model with logit link to model chemotherapy receipt (yes/no) as a function of time and clinical factors, considering a potential correlation in chemotherapy receipt among patients seeing the same oncologist. Modeled factors consisted of age, study site, RS testing, tumor grade, tumor size, nodal status, and time between diagnosis and survey completion and site. We tested nodal status and other clinical factors for interactions with diagnosis date. We used this model to estimate the marginal predicted probabilities for the overall sample and prespecified subsamples averaged over all clinical and demographic factors (19). We then estimated marginal probabilities of chemotherapy receipt at each quarter under two different scenarios: one assuming constant RS testing across time and the other modeling changes in RS testing rate observed in the node-positive subsample. For each scenario, we estimated the rate of change in chemotherapy use and compared these rates in order to estimate the impact of changes in RS testing frequency. We used the bootstrap method to estimate variance of these estimates (Supplementary Figure 2, available online). We used estimates from a generalized ordinal mixed model that included the same predictors as in the model for chemotherapy use to estimate marginal trends in patients’ reports of oncologists’ recommendations. All models incorporated patient survey and nonresponse weights so that statistical inference was representative of our target population. Oncologist survey descriptive results incorporated nonresponse weights based on both physician and average patient characteristics. Analyses using multiply imputed data (not shown) were consistent with reported results. We used Proc NMIXED and proc SURVEYFREQ (SAS version 9.4).
We used the Rao-Scott chi-square test to assess the statistical signficiance of differences in proportions and likelihood ratio tests to compare the goodness of fit of different models. P values of less than .05 were considered statistically significant. All statistical tests were two-sided.
Results
Patient Characteristics
A total of 2926 women met inclusion criteria (Supplementary Figure 1, available online), of whom 2844 had data available for all covariates and comprised the analytic sample for chemotherapy receipt. The analysis of trends in patient report of oncologist recommendations was restricted to patients who reported seeing an oncologist (2393) and had complete information for all covariates (2347). Mean patient age was 62 years, 56.2% white, 15.9% black, 17.4% Hispanic, and 7.9% Asian (Table 1). There were no statistically significant changes in patient characteristics over time.
Table 1.
Patient demographic and clinical characteristics (n = 2926)
| Characteristic | No. (%) |
|---|---|
| Age at time of survey, y | |
| ≥50 | 2493 (85.2) |
| <50 | 433 (14.8) |
| Mean age (SD)*, y | 61.8 (10.2) |
| Mean time since diagnosis (SD)†, y | 7.4 (2.8) |
| Study site | |
| Georgia | 1580 (54.0) |
| Los Angeles County | 1346 (46.0) |
| Race/ethnicity | |
| White | 1644 (56.2) |
| Black | 466 (15.9) |
| Hispanic | 508 (17.4) |
| Asian | 230 (7.9) |
| Other/unknown/missing | 78 (2.7) |
| Any comorbidities | |
| No | 1992 (68.1) |
| Yes | 894 (30.6) |
| Missing | 40 (1.4) |
| Tumor grade | |
| 1 | 1080 (36.9) |
| 2 | 1407 (48.1) |
| 3 | 416 (14.2) |
| Missing | 23 (0.8) |
| Cancer stage | |
| I | 2039 (69.7) |
| II | 889 (30.3) |
| Tumor size, mm | |
| ≤10 | 936 (32.0) |
| >10, ≤20 | 1320 (45.1) |
| >20, ≤50 | 670 (22.9) |
| Lymph node involvement (AJCC 7 staging) | |
| Node-negative (N0) | 2357 (80.6) |
| Micrometastases (N1mi)‡ | 149 (5.1) |
| Node-positive (N1) | 420 (14.4) |
| 21-gene recurrence score testing | |
| No test | 1510 (50.7) |
| Low score | 904 (30.9) |
| Intermediate score | 410 (14.0) |
| High score | 102 (3.5) |
| Patient report of medical oncologist recommendation | |
| Recommended in favor of chemotherapy | 1172 (40.0) |
| Left it up to me | 306 (10.5) |
| Recommended against chemotherapy | 915 (31.3) |
| Did not indicate visit with oncologist | 533 (18.2) |
| Received chemotherapy | |
| No | 2102 (71.8) |
| Yes | 766 (26.2) |
| Missing | 58 (2.0) |
n = 2926. AJCC = American Joint Committee on Cancer staging.
n = 2924.
N1mi is grouped with N0 for analyses, reflecting treatment algorithms in guidelines of the National Comprehensive Cancer Network.
Chemotherapy Receipt Trends
Chemotherapy receipt was associated with younger age (per 10 years: odds ratio [OR] = 0.44, 95% confidence interval, 95% CI = 0.38 to 0.51), high RS (vs not tested: OR = 14.14, 95% CI = 6.08 to 32.88), nodal involvement (OR = 13.46, 95% CI = 9.39 to 19.28 for node-positive vs node-negative/micrometastasis), and grade (grade 3 vs grade 1: OR = 10.45, 95% CI = 6.90 to 15.83), but not with study site (OR = 1.11, 95% CI = 0.81 to 1.53) (Supplementary Figure 2, available online).
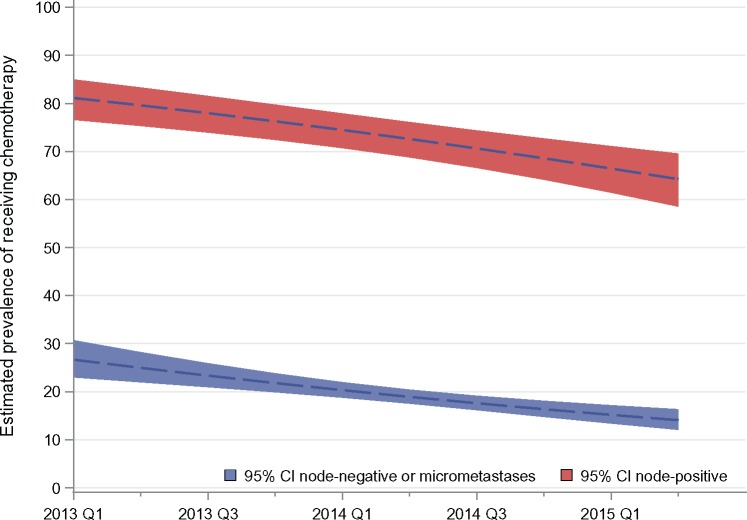
From 2013 to 2015, odds of receiving chemotherapy declined by 14.3% per quarter, controlling for testing and all other patient covariates (OR = 0.86, 95% CI = 0.80 to 0.91) (Supplementary Figure 2, available online). Receipt of chemotherapy declined from 34.5% (95% CI = 30.8% to 38.3%) to 21.3% (95% CI = 19.0% to 23.7%) based on estimates from Supplementary Figure 2 (available online) applied to the sample, while holding clinical and testing characteristics constant across the time. Figure 1 shows chemotherapy estimated trends by nodal status, controlling for change in all clinical variables including RS testing. In the node-positive group, chemotherapy declined from 81.1% (95% CI = 76.6% to 85.0%) to 64.2% (95% CI = 58.6% to 69.6%), and in the node-negative/micrometastatic group, it declined from 26.6% (95% CI = 23.0% to 30.7%) to 14.1% (95% CI = 12.0% to 16.3%).
Figure 1.
Trends in the marginal probability of a patient reporting chemotherapy receipt over time (by calendar year and quarter) by axillary lymph node involvement (node-positive, American Joint Committee on Cancer [AJCC] stage N1; node-negative/micrometastasis, AJCC stages N0 and N1mi), averaging over the demographic and clinical characteristics included in the model, as specified in the “Methods,” including the receipt of the 21-gene recurrence score and its interaction with lymph node status, in a sample of 2926 patients with estrogen receptor–positive, HER2-negative stage I–II breast cancer. The dotted lines represent the trends concurrent with the observed rate of testing, and the shaded areas represent the 95% confidence intervals. CI = confidence interval.
Trends in RS Receipt and Effect on Chemotherapy Use
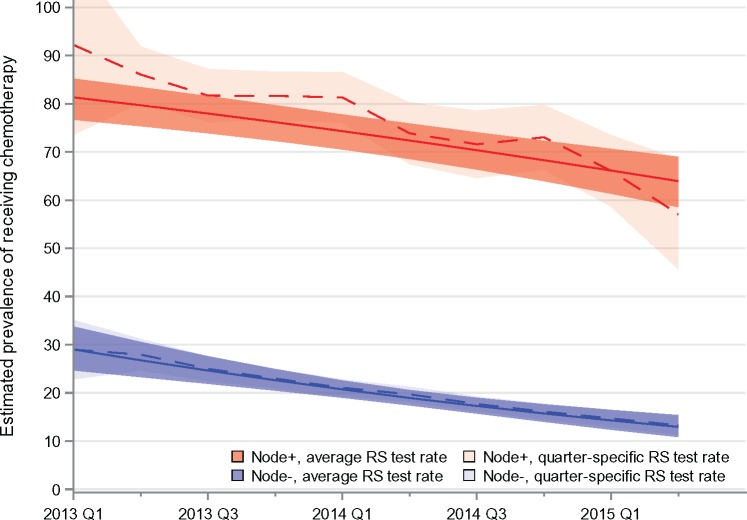
Half of the patients with node-negative/micrometastatic disease received RS, with a slow increase over time from 52.1% (95% CI = 43.8% to 60.3%) in the first half of 2013 to 54.1% (95% CI = 48.4% to 59.8.1%) in the first half of 2015 (Supplementary Figure 3, available online). By contrast, during the same time period, RS testing in patients with node-positive disease increased from 26.1% (95% CI = 11.3% to 40.9%) to 42.7% (95% CI = 28.3% to 57.1%) (Supplementary Figure 3, available online). Figure 2 shows trends in chemotherapy receipt by nodal status, estimated in two different ways in order to isolate the effect of changes in RS test rates on treatment: the solid lines represent results when RS use and all other covariates are held constant over the entire time period; the dashed lines show estimated chemotherapy rates, allowing the rate of RS testing to vary as observed over time. For the node-positive group, the average decline per quarter in these two scenarios is –2.0% per quarter (95% CI = –2.6% to –1.4%) when testing is held constant and –3.0% (95% CI = –4.0% to –1.9%) when testing rates are allowed to vary as observed (Figure 2).The difference in rates is –1% per quarter (95% CI = –1.7% to –0.3%), suggesting that the changing rates of testing are responsible for about one-third of the decline estimated in a scenario where testing rates are allowed to vary as observed. In the node-negative/micrometastatic group, there was no statistically significant change (Ptrend = .66) in RS testing (Supplementary Figure 3, available online), and thus no difference was observed between the solid and dashed lines (Figure 2).
Figure 2.
Comparison of the effect of standardized vs changing rates of use of the 21-gene recurrence score (RS) on receipt of chemotherapy over time, stratified by axillary lymph node status (node-positive, American Joint Committee on Cancer [AJCC] stage N1; node-negative/micrometastasis, AJCC stages N0 and N1mi), in a sample of 2926 patients with estrogen receptor–positive, HER2-negative stage I–II breast cancer. Solid lines show estimated trends in chemotherapy use had there been no change in rates of RS use over time; dashed lines show the observed trends in chemotherapy use. RS = recurrence score.
Patients’ Reports of Oncologists’ Recommendations
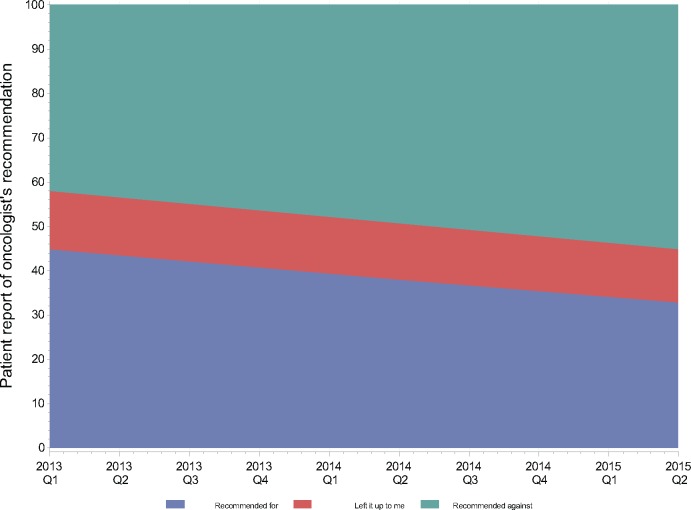
Among those who reported an oncologist recommendation about chemotherapy (n = 2393, 84.1%), 1.4% of patients received chemotherapy when oncologists recommended against it, vs 17.0% and 74.9% when oncologists “left it up to me” or recommended chemotherapy, respectively (chi-square test, P < .001). There was a marked decline over time in the odds of patients’ reporting that their oncologists recommended chemotherapy (OR = 0.92, 95% CI = 0.88 to 0.96), holding other characteristics constant, representing a decline of 8.2% per quarter (95% CI = 3.9% to 13.3%). Figure 3 shows that patient report of having received a recommendation for chemotherapy declined from 44.9% (95% CI = 40.2% to 49.7%) to 31.6 % (95% CI = 25.9% to 37.9%), controlling for other factors, with a corresponding increase in reports of receiving a recommendation against chemotherapy. The trend in reported oncologist recommendations about chemotherapy did not differ by node status (likelihood ratio test, P = .85).
Figure 3.
Trends in patients’ reports of medical oncologists’ recommendations about chemotherapy over time (by calendar year and quarter).
Oncologists’ Perspectives on Chemotherapy Use in Case Scenarios
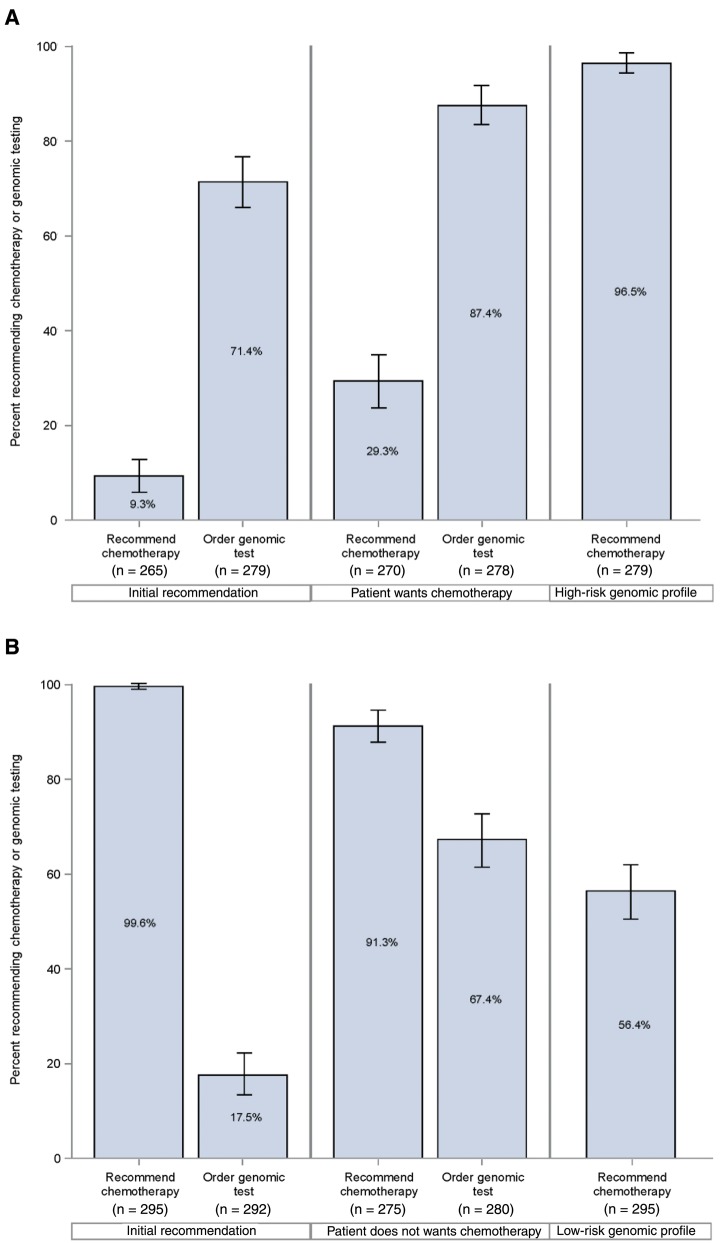
We identified 504 oncologists and 304 completed surveys (response rate = 60.3%). The yearly volume of new breast cancer patients was 21.2% (1–20 patients), 35.8% (21–50), and 34.9% (≥51). For the more favorable prognosis scenario, few oncologists would recommend chemotherapy (9.3%, 95% CI = 5.8% to 12.9%), while more than two-thirds (71.4%, 95% CI = 66.0% to 76.8% ) would order a genomic test before making a decision (Figure 4A). When presented with additional information that the patient initially desired to have chemotherapy, oncologists were more likely both to recommend chemotherapy (from 9.3%, 95% CI = 5.8% to 12.9%, to 29.3%, 95% CI = 23.7% to 34.9%, chi-square test, P < .001) and to order a genomic test before making a decision (from 71.4%, 95% CI = 66.0% to 76.8% to 87.4%, 95% CI = 83.3% to 91.4% , P < .001). When asked how their recommendation would change if a genomic profiling test (RS) predicted a high risk of distant recurrence (RS = 34), virtually all oncologists (96.5%, 95% CI = 94.4% to 98.6%) would recommend chemotherapy.
Figure 4.
Medical oncologists’ perspectives on recommending chemotherapy and ordering genomic testing in response to patient preference and genomic results for (A) node-negative and (B) node-positive disease.
For the less favorable prognosis scenario, virtually all (99.6%, 95% CI = 98.9% to 100.0%) oncologists would recommend chemotherapy, and few (17.5%, 95% CI = 13.1% to 22.0%) would order a tumor genomic test before making a decision (Figure 4B). When presented with information that the patient initially desired to avoid chemotherapy, oncologists were somewhat less likely to recommend chemotherapy (from 99.6%, 95% CI = 98.9% to 100.0%, to 91.3%, 95% CI = 87.9% to 94.7%, chi-square test, P < .001) but much more likely to order a genomic test (from 17.5%, 95% CI = 13.1% to 22.0%, to 67.4%, 95% CI = 61.7% to 73.0%, P < .001). When presented with an RS of 16 (low risk), oncologists were much less likely to recommend chemotherapy (from 91.3%, 95% CI = 87.9% to 94.7% to 56.4%, 95% CI = 50.7% to 62.2%, P < .001).
Discussion
In this large, diverse, contemporary population-based sample of newly diagnosed, early-stage breast cancer, we observed a marked decline in chemotherapy receipt from 2013 through 2015. The chemotherapy decline was independent of changes in clinical factors including RS use. RS testing rates did not change at all in node-negative/metastatic patients (the majority of patients) over time. RS testing did increase in the node-positive patients, but we estimated that RS testing accounted for only about one-third of the downward chemotherapy trend in node-positive patients before controlling for changes in testing rates.
Patients’ reports of their oncologists’ recommendations for chemotherapy declined at a rate commensurate with changes in chemotherapy receipt. Oncologists’ perspectives on chemotherapy recommendation, elicited independently at one point in time near the end of the study period, generally adhered to practice guidelines, yet also reflected willingness to be responsive to patient desires. For a patient with less favorable prognosis node-positive disease, the patient’s desire to avoid chemotherapy was associated with a marked increase in oncologists’ inclination to order genomic testing before making a recommendation, suggesting a possible mechanism for the 20% increase we observed in RS use among node-positive patients over time.
Several studies have examined trends in RS and chemotherapy use (6–9,20–22). These studies documented a decline in chemotherapy over the last decade with increasing RS testing. We and others have modeled the impact of RS use on chemotherapy decision-making and identified scenarios in which RS tends to encourage vs discourage chemotherapy (8,21,23). This study builds on earlier work by demonstrating an ongoing marked drop in chemotherapy use that is independent of RS testing trends. While it is possible that use of other genomic assays (eg, 70-gene signature) (24) increased during the study period, our survey respondents did not report frequent use of other assays, and guidelines stipulate RS as the only assay validated to predict chemotherapy response. Oncologists appeared to be a driving force in the observed chemotherapy trends, as there were powerful concomitant trends in patients’ reports of oncologists’ recommendations. During the study period, there was no major change in the most prominent US guidelines, those of the NCCN (which continue to recommend chemotherapy for node-positive disease) (5,25), although European guideline organizations did comment on genomic assays for node-positive patients in 2015 (26,27). The decline in chemotherapy use despite an incomplete evidence base suggests a broad change in culture, with oncologists moving away from chemotherapy for patients with hormone-responsive early breast cancer.
We speculate that this powerful trend is due to a shift in focus toward tumor biology and away from anatomy as we strive to identify individual patients whom chemotherapy is likely to harm more than it helps. Much of the decline in chemotherapy use may result from an evolution in oncologists’ attitudes about the management of “close calls” (cases in which there is uncertainty as to whether chemotherapy is warranted) because of a growing literature that supports omitting chemotherapy among patients with favorable tumor features (3,22,24). The evidence for such a paradigm shift lies in our finding that, when faced with the same clinical information, oncologists interpreted it differently in later than in earlier years of this study. Concurrently, patients may be increasingly concerned about the well-known and publicized toxic side effects of chemotherapy (28), particularly if oncologists express less enthusiasm about its benefits. This growing sea change in decision-making about chemotherapy is important for both oncologists and patients to recognize. In this context, evidence published after this study may rapidly accelerate the trend to reduce chemotherapy among node-positive patients (3,29).
Oncologists offered perspectives on chemotherapy use that generally followed standard algorithms; however, their recommendations were also sensitive to patient preferences. Our results suggest that oncologists use tumor genomic profiling to adjudicate mismatch between patient preference and practice guidelines. For example, oncologists initially indicated low use (17.5%) of a genomic profiling test in the node-positive case scenario, but when confronted by a discordant patient preference, oncologists’ inclination to order a genomic test surged to 67.4%. Notably, patient preferences that were discordant with guidelines (and most oncologists’ initial recommendations) shifted oncologists’ stance on chemotherapy by 10 to 20 percentage points. Yet when the combination of patient preferences and genomic results were both discordant with oncologists’ initial recommendation, oncologists’ stance shifted far more substantially. These results offer a window into the clinical encounter and demonstrate the process by which oncologists integrate practice guidelines, patient preferences, and genomic profiling to individualize breast cancer care.
Aspects of the study merit comment. This study’s strengths include a large, contemporary, diverse population-based patient sample, highly complete ascertainment of RS directly from the testing laboratory, detailed clinical information integrated with survey data from both patients and their attending oncologists, and high response rates. Limitations include the use of patient report, an indirect and imperfect measure of the strength of oncologists’ chemotherapy recommendations, a relatively small number of node-positive cases, absence of detailed information on lymph node number and size, extracapsular extension and specific chemotherapy agents, a relatively brief study period, and geographic restriction to two US regions. Oncologists were surveyed only once, limiting our ability to study treatment decision-making from their perspective over time.
The trends we document are remarkable for their steepness of decline, independent of clinical factors and despite no major change in the evidence base over the study period. This result represents an evolution of clinical oncology culture driven by oncologists’ appropriate concerns about overtreatment in this generally favorable-prognosis population. However, a lingering concern is that oncologists may be overshooting the mark in patients with less favorable prognosis, especially if pending trials do not support that genomic testing algorithms are equally predictive in node-positive vs node-negative disease. Thus, the results of pending clinical trials will be essential to confirm the rationale of this approach to treatment.
Funding
Research reported in this publication was supported by the National Cancer Institute (NCI) of the National Institutes of Health under award number P01 CA163233 to the University of Michigan. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the NCI’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California (USC), and contract HHSN261201000034C awarded to the Public Health Institute. The collection of cancer incidence data in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI, and cooperative agreement 5NU58DP003875-04-00 from the CDC.
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the NCI, or the CDC or their contractors and subcontractors is not intended and should not be inferred.
Supplementary Material
References
- 1. Paik S, Shak S, Tang G et al. , A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826.http://dx.doi.org/10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 2. Paik S, Tang G, Shak S et al. , Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734.http://dx.doi.org/10.1200/JCO.2005.04.7985 [DOI] [PubMed] [Google Scholar]
- 3. Sparano JA, Gray RJ, Makower DF et al. , Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–2014.http://dx.doi.org/10.1056/NEJMoa1510764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perou CM, Sorlie T, Eisen MB et al. , Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752.http://dx.doi.org/10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 5. Gradishar WJ, Anderson BO, Balassanian R et al. , Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(3):324–354.http://dx.doi.org/10.6004/jnccn.2016.0037 [DOI] [PubMed] [Google Scholar]
- 6. Parsons BM, Landercasper J, Smith AL et al. , 21-Gene recurrence score decreases receipt of chemotherapy in ER+ early-stage breast cancer: An analysis of the NCDB 2010–2013. Breast Cancer Res Treat. 2016;159(2):315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Potosky AL, O'Neill SC, Isaacs C et al. , Population-based study of the effect of gene expression profiling on adjuvant chemotherapy use in breast cancer patients under the age of 65 years. Cancer. 2015;121(22):4062–4070.http://dx.doi.org/10.1002/cncr.29621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hassett MJ, Silver SM, Hughes ME et al. , Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30(18):2218–2226.http://dx.doi.org/10.1200/JCO.2011.38.5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassett MJ, Hughes ME, Niland JC et al. , Chemotherapy use for hormone receptor-positive, lymph node-negative breast cancer. J Clin Oncol. 2008;26(34):5553–5560.http://dx.doi.org/10.1200/JCO.2008.17.9705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friese CR, Li Y, Bondarenko I et al. , Chemotherapy decisions and patient experience with the recurrence score assay for early-stage breast cancer. Cancer. 2017;123(1):43–51.http://dx.doi.org/10.1002/cncr.30324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dinan MA, Mi X, Reed SD et al. , Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the Medicare population. JAMA Oncol. 2015;1(2):158–166.http://dx.doi.org/10.1001/jamaoncol.2015.43 [DOI] [PubMed] [Google Scholar]
- 12. Afghahi A, Mathur M, Thompson CA et al. , Use of gene expression profiling and chemotherapy in early-stage breast cancer: A study of linked electronic medical records, cancer registry data, and genomic data across two health care systems. J Oncol Pract. 2016;12(6):e697–e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurian AW, Friese CR, Bondarenko I et al. , Second opinions from medical oncologists for early-stage breast cancer: Prevalence, correlates, and consequences. JAMA Oncol. 2017;3(3):391–397.http://dx.doi.org/10.1001/jamaoncol.2016.5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamilton AS, Hofer TP, Hawley ST et al. , Latinas and breast cancer outcomes: Population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2022–2029.http://dx.doi.org/10.1158/1055-9965.EPI-09-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawley ST, Jagsi R, Morrow M et al. , Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. 2014;149(6):582–589.http://dx.doi.org/10.1001/jamasurg.2013.5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jagsi R, Griffith KA, Kurian AW et al. , Concerns about cancer risk and experiences with genetic testing in a diverse population of patients with breast cancer. J Clin Oncol. 2015;33(14):1584–1591.http://dx.doi.org/10.1200/JCO.2014.58.5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dillman DA SJ, Christian LM.. Internet, Mail, and Mixed-Mode Surveys: The Tailored Design Method. 3rd ed. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 18. Kurian AW, Griffith KA, Hamilton AS et al. , Genetic testing and counseling among patients with newly diagnosed breast cancer. JAMA. 2017;317(5):531–534.http://dx.doi.org/10.1001/jama.2016.16918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou GY. Assessment of risks by predicting counterfactuals. Stat Med. 2009;28(30):3761–3781.http://dx.doi.org/10.1002/sim.3751 [DOI] [PubMed] [Google Scholar]
- 20. Dinan MA, Mi X, Reed SD et al. , Association between use of the 21-gene recurrence score assay and receipt of chemotherapy among medicare beneficiaries with early-stage breast cancer, 2005-2009. JAMA Oncol. 2015;1(8):1098–1109.http://dx.doi.org/10.1001/jamaoncol.2015.2722 [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Kurian AW, Bondarenko I et al. , The influence of 21-gene recurrence score assay on chemotherapy use in a population-based sample of breast cancer patients. Breast Cancer Res Treat. 2017;161(3):587–595.http://dx.doi.org/10.1007/s10549-016-4086-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albain KS, Barlow WE, Shak S et al. , Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65.http://dx.doi.org/10.1016/S1470-2045(09)70314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HS, Umbricht CB, Illei PB et al. , Optimizing the use of gene expression profiling in early-stage breast cancer. J Clin Oncol. 2016;34(36):4390–4397.http://dx.doi.org/10.1200/JCO.2016.67.7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardoso F, van't Veer LJ, Bogaerts J et al. , 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375(8):717–729.http://dx.doi.org/10.1056/NEJMoa1602253 [DOI] [PubMed] [Google Scholar]
- 25. Gradishar WJ, Anderson BO, Balassanian R et al. , NCCN guidelines insights: Breast cancer, version 1.2017. J Natl Compr Canc Netw. 2017;15(4):433–451. [DOI] [PubMed] [Google Scholar]
- 26. Gnant M, Thomssen C, Harbeck N.. St. Gallen/Vienna 2015: A brief summary of the consensus discussion. Breast Care (Basel). 2015;10(2):124–130.http://dx.doi.org/10.1159/000430488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senkus E, Kyriakides S, Ohno S et al. , Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v8–v30. [DOI] [PubMed] [Google Scholar]
- 28. Friese CR, Harrison JM, Janz NK et al. , Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramsey SD, Barlow WE, Gonzalez-Angulo AM et al. , Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34(1):1–9.http://dx.doi.org/10.1016/j.cct.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.