Abstract
Objective
This study was aimed to compare survival outcomes in high-risk prostate cancer (PCa) patients receiving external beam radiotherapy (EBRT) or radical prostatectomy (RP).
Materials and methods
The Surveillance, Epidemiology, and End Results (SEER) database was used to identify PCa patients with high-risk features who received RP alone or EBRT alone from 2004 to 2008. Propensity-score matching (PSM) was performed. Kaplan–Meier survival analysis was used to compare cancer-specific survival (CSS) and overall survival (OS). Multivariate Cox regression analysis was used to identify independent prognostic factors.
Results
A total of 24,293 patients were identified, 14,460 patients receiving RP and 9833 patients receiving EBRT. Through PSM, 3828 patients were identified in each group. The mean CSS was 128.6 and 126.7 months for RP and EBRT groups, respectively (P<0.001). The subgroup analyses showed that CSS of the RP group was better than that of the EBRT group for patients aged <65 years (P<0.001), White race (P<0.001), and married status (P<0.001). However, there was no significant difference in CSS for patients aged ≥65 years, Black race, other race, and unmarried status. Similar trends were observed for OS. Multivariate analysis showed that EBRT treatment modality, T3–T4 stage, Gleason score 8–10, and prostate-specific antigen >20 ng/mL were significant risk factors for both CSS and OS.
Conclusion
This study suggested that survival outcomes might be better with RP than EBRT in high-risk PCa patients aged <65 years; however, RP and EBRT provided equivalent survival outcomes in older patients, which argues for primary radiotherapy in this older cohort.
Keywords: prostate cancer, SEER, radical prostatectomy, EBRT
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer and the third leading cause of cancer-related deaths in men in the USA.1 More than 90% of PCa is clinically localized during the prostate-specific antigen (PSA) era.2 High-risk factors include T3–T4 stage, Gleason score (GS) 8–10, or PSA >20 ng/mL.3 According to European Association of Urology guidelines, radical prostatectomy (RP) is the preferred option. However, in the National Comprehensive Cancer Network (NCCN) guidelines, external beam radiotherapy (EBRT) in combination with long-term androgen-deprivation therapy (ADT) is preferred (category 1 recommended) and EBRT with brachytherapy with or without ADT or RP with pelvic lymph node dissection is used in selected patients.4 A recent randomized trial showed the comparative effectiveness of active monitoring, RP, and EBRT for almost all low-risk to favorably intermediate-risk clinically localized PCa.5 However, there is no randomized controlled trial examining the comparative effectiveness of different therapeutic modalities for high-risk clinically localized PCa to date.
Several retrospective studies have investigated survival outcomes after RP or EBRT for high-risk PCa, with mixed findings.6,7 For example, RP improved overall survival (OS) more than EBRT,6 while survival outcomes associated with EBRT with ADT were not inferior to RP in other studies.8 Of note, some of the above studies recruited patients treated with conventional radiotherapy (RT) and dose regime, whereas dose-escalation studies using advanced radiation oncology technologies such as intensity-modulated radiotherapy and image-guided radiotherapy have been utilized since early 2000s.9 Therefore, it remains unclear whether or not therapeutic effects of modern EBRT with high dose and RP are equivalent or different depending on individual risk factor(s). In this study, we examined survival outcomes including cancer-specific survival (CSS) and OS of high-risk PCa patients receiving RP or EBRT as the initial therapy, from contemporary Surveillance, Epidemiology, and End Results (SEER) database (2004–2008). Moreover, we performed propensity-score matching (PSM) to select similar numbers of patients with balanced risk factors between the two groups, in order to fairly compare survival outcomes between the groups.
Materials and methods
Data source
SEER database of the National Cancer Institute reports cancer incidence and survival data of 18 regional or statewide cancer registries in the USA. The SEER catchment area includes ~28% of the USA. All of the high-risk PCa cases were extracted from the SEER database using the SEER Stat 8.3.4 software (National Institutes of Health, Bethesda, MD, USA). The SEER database includes de-identified patients information. This study was exempt from review by our institutional review board.
Study population
Our cohort included PCa patients registered in the SEER database from 2004 to 2008, in which GS information was available in SEER. PSA information was available in SEER in 2017 release,10 and high-risk factors were defined as T3–T4, GS 8–10, or PSA >20 ng/mL.3 TNM stage was classified according to the American Joint Committee on Cancer, Cancer Staging Manual (6th ed., 2002). Other inclusion criteria were as follows: 1) N0 and M0 stage, 2) adenocarcinoma of the prostate (ICD-O-3 Hist/Behav code=8140/3), 3) PCa as the first and only primary cancer, 4) known survival time and cause of death, and 5) RP or EBRT as the primary treatment. Patients who received neoadjuvant and/or adjuvant radiation were excluded.
Outcomes
Objectives of this study are to compare CSS, which was identified from the date of diagnosis to the date when patients died from PCa, and OS, which was identified from the date of diagnosis to the date when patients died of any cause. The last date of follow-up was December 31, 2014.
Statistical analyses
The differences in the demographic and clinicopathological features of patients who received RP or EBRT were assessed using the chi-square (χ2) test. Continuous variables were compared using the Student’s t-test. Univariate comparisons of OS and CSS were assessed using Kaplan–Meier method with log-rank test. Multivariate analyses were performed using Cox regression model. PSM method11 was used to balance observed covariates between RP and EBRT. Propensity scores were calculated according to the range of each characteristic including age, marital status, race, year of diagnosis, T stage, GS, PSA level, and SEER region. The matched ratio was 1:1, and the matching was conducted based on nearest-neighbor matching principle. The matching approach was without replacements. All statistical analyses were performed using SPSS ver. 22.0 (IBM Corporation, Armonk, NY, USA). A value of P<0.05 was considered as statistically significant.
Results
Patient characteristics
A total of 24,293 patients were identified, 14,460 patients receiving RP and 9833 patients receiving EBRT. The baseline characteristics of patients are shown in Table 1. There were statistically significant differences in all baseline factors between the RP and EBRT groups in the overall cohort. Notably, the median age of EBRT group was 71 years, whereas the median age of RP group was 62 years. PSM was conducted between the two groups. In the PSM cohort, as shown in Table 1, 3828 patients were identified in each RP and EBRT group. There was no significant difference between groups regarding all patient characteristics including age, marital status, race, T stage, GS, PSA, and year of diagnosis. The following analyses were conducted in the PSM cohorts from each group.
Table 1.
Patient characteristics
| Variables | Overall cohort
|
Propensity-matched cohort
|
||||||
|---|---|---|---|---|---|---|---|---|
| Total | RP | EBRT | P-value | Total | RP | EBRT | P-value | |
| Number of patients (%) | 24,293 (100) | 14,460 (59.5) | 9833 (40.5) | 7656 (100) | 3828 (50) | 3828 (50) | ||
| Age (years) | <0.001 | 0.817 | ||||||
| Median, IQR | 65 (59–71) | 62 (57–67) | 71 (64–76) | 66 (61–70) | 66 (61–70) | 66 (61–70) | ||
| Age (years), n (%) | <0.001 | 0.926 | ||||||
| <65 | 11,658 (48) | 9151 (63.3) | 2507 (25.5) | 3162 (41.3) | 1583 (41.4) | 1579 (41.2) | ||
| ≥65 | 12,635 (52) | 5309 (36.7) | 7326 (74.5) | 4494 (58.7) | 2245 (58.6) | 2249 (58.8) | ||
| Marital status, n (%) | <0.001 | 0.866 | ||||||
| Married | 17,751 (73.1) | 11,122 (76.9) | 6629 (67.4) | 5949 (77.7) | 2965 (77.5) | 2984 (78) | ||
| Unmarried | 5250 (21.6) | 2676 (18.5) | 2574 (26.2) | 1503 (19.6) | 759 (19.8) | 744 (19.4) | ||
| Unknown | 1292 (5.3) | 662 (4.6) | 630 (6.4) | 204 (2.7) | 104 (2.7) | 100 (2.6) | ||
| Race, n (%) | <0.001 | 0.429 | ||||||
| White | 19,057 (78.4) | 11,805 (81.6) | 7252 (73.8) | 6396 (83.5) | 3193 (83.4) | 3203 (83.7) | ||
| Black | 3487 (14.4) | 1773 (12.3) | 1714 (17.4) | 864 (11.3) | 422 (11) | 442 (11.5) | ||
| Others | 1517 (6.2) | 770 (5.3) | 747 (7.6) | 365 (4.8) | 196 (5.1) | 169 (4.4) | ||
| Unknown | 232 (1) | 112 (0.8) | 120 (1.2) | 17 (0.4) | 14 (0.4) | 31 (0.4) | ||
| T stage, n (%) | 0.027 | 0.066 | ||||||
| T1–T2 | 12,350 (50.8) | 7273 (50.3) | 5077 (51.6) | 3850 (50.3) | 1912 (49.9) | 1938 (50.6) | ||
| T3–T4 | 11,908 (49) | 7171 (49.6) | 4737 (48.2) | 3801 (49.6) | 1916 (50.1) | 1885 (49.2) | ||
| Unknown | 35 (0.1) | 16 (0.1) | 19 (0.2) | 5 (0.1) | 0 (0) | 5 (0.1) | ||
| Gleason score, n (%) | 0.462 | 0.134 | ||||||
| 2–7 | 12,085 (49.7) | 7169 (49.6) | 4916 (50) | 3997 (52.2) | 2037 (53.2) | 1960 (51.2) | ||
| 8–10 | 11,918 (49.1) | 7109 (49.2) | 4809 (48.9) | 3622 (47.3) | 1770 (46.2) | 1852 (48.4) | ||
| Unknown | 290 (1.2) | 183 (1.3) | 108 (1.1) | 37 (0.5) | 21 (0.5) | 16 (0.4) | ||
| PSA (ng/mL), n (%) | 0.089 | 0.934 | ||||||
| ≤20 | 16,852 (69.4) | 10,090 (69.8) | 6762 (68.8) | 5725 (73.8) | 2866 (74.9) | 2859 (74.7) | ||
| >20 | 5921 (24.4) | 3453 (23.9) | 2468 (25.1) | 1641 (21.4) | 815 (21.3) | 826 (21.6) | ||
| Unknown | 1520 (6.3) | 917 (6.3) | 603 (6.1) | 290 (3.8) | 147 (3.8) | 143 (3.7) | ||
| Region, n (%) | <0.001 | 0.852 | ||||||
| East | 9038 (37.2) | 5279 (36.5) | 3759 (38.2) | 2794 (36.5) | 1401 (36.6) | 1393 (36.4) | ||
| Northern Plains | 2588 (10.7) | 1533 (10.6) | 1055 (10.7) | 530 (6.9) | 263 (6.9) | 267 (7) | ||
| Pacific Coast | 11,535 (47.5) | 6844 (47.3) | 4691 (47.7) | 4155 (54.3) | 2070 (54.1) | 2085 (54.5) | ||
| Southwest | 1120 (4.6) | 797 (5.5) | 323 (3.3) | 177 (2.3) | 94 (2.5) | 83 (2.2) | ||
| Alaska | 12 (0) | 7 (0) | 5 (0.1) | 0 (0) | 0 (0) | 0 (0) | ||
| Year of diagnosis, n (%) | <0.001 | 0.643 | ||||||
| 2004 | 4290 (17.7) | 2452 (17) | 1838 (18.7) | 1350 (17.6) | 660 (17.2) | 690 (18) | ||
| 2005 | 4024 (16.6) | 2357 (16.3) | 1667 (17) | 1241 (16.2) | 616 (16.1) | 625 (16.3) | ||
| 2006 | 4943 (20.3) | 2861 (19.8) | 2082 (21.2) | 1560 (20.4) | 769 (20.1) | 791 (20.7) | ||
| 2007 | 5506 (22.7) | 3393 (23.5) | 2113 (21.5) | 1623 (21.2) | 835 (21.8) | 788 (20.6) | ||
| 2008 | 5530 (22.8) | 3397 (23.5) | 2133 (21.7) | 1882 (24.6) | 948 (24.8) | 934 (24.4) | ||
Abbreviations: IQR, interquartile range; EBRT, external beam radiotherapy; PSA, prostate-specific antigen; RP, radical prostatectomy.
CSS and OS in these two PSM cohorts
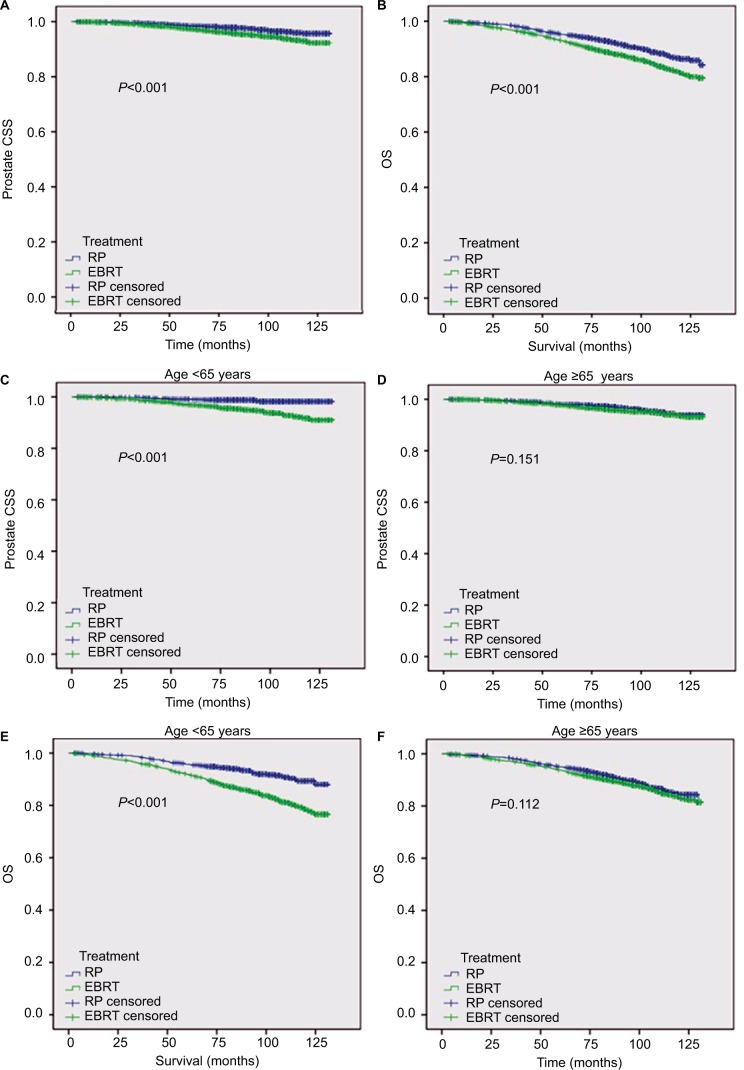
There was no substantial difference in patent characteristics between the two PSM cohorts. The median age was 66 years with interquartile range =61–70 years for both groups. For CSS, the mean survival time (MST) was 128.6 months with 95% CI=128.2–129.1 for RP group and was 126.7 months with 95% CI=126.1–127.3 for EBRT group (P<0.001; Figure 1). The subgroup analyses showed that CSS of the RP group was better than that of the EBRT group in patients with <65 years (P<0.001), White race (P<0.001), and married status (P<0.001). However, there was no significant difference in patients with ≥65 years (P=0.151), Black race (P=0.418), other race (P=0.874), and unmarried status (P=0.146). For OS, the MST and 95% CI were 123.3 months and 122.5–124.1 for RP vs 119.6 months and 118.7–120.5 for EBRT group (P<0.001). The subgroup analyses revealed that OS was better in the RP group than that in the EBRT group in patients with <65 years (P<0.001), White race (P<0.001), and married status (P<0.001). Similarly, there was no significant difference in OS between the groups in patients with age ≥65 years (P=0.112), Black race (P=0.936), other race (P=0.499), and unmarried status (P=0.257).
Figure 1.
Survival analysis of matched cohorts.
Notes: CSS (A) and OS (B) in all patients; CSS in patients aged <65 years (C) vs patients aged ≥65 years (D); and OS in patients aged <65 years (E) vs patients aged ≥65 years (F).
Abbreviations: CSS, cancer-specific survival; EBRT, external beam radiotherapy; OS, overall survival; RP, radical prostatectomy.
Multivariate Cox regression analysis
To identify independent prognostic factors for high-risk patients, multivariate Cox regression analysis was performed in the matched population. Basic clinicopathological factors were evaluated. The multivariate analysis showed that EBRT treatment modality, T3–T4 stage, GS 8–10, and PSA >20 ng/mL were significant risk factors for both CSS and OS (Table 2). Moreover, unmarried status is a prognostic factor for poor CSS but not for poor OS. Patients being diagnosed in recent years usually showed fine survival outcomes. At last, other clinical factors including age, race, and region had no significant impact on CSS and OS (Table 2).
Table 2.
Multivariate Cox regression analysis of prognostic factors for CSS and OS in the matched population
| Variables | CSS
|
OS
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Treatment | ||||||
| RP | 1 | Reference | 1 | Reference | ||
| EBRT | 1.811 | 1.425–2.301 | <0.001 | 1.482 | 1.295–1.696 | <0.001 |
| Age (years) | ||||||
| <65 | 1 | Reference | 1 | Reference | ||
| ≥65 | 1.048 | 0.832–1.32 | 0.691 | 0.946 | 0.829–1.08 | 0.413 |
| Marital status | ||||||
| Married | 1 | Reference | 1 | Reference | ||
| Unmarried | 1.322 | 1.001–1.746 | 0.049 | 1.103 | 0.935–1.302 | 0.244 |
| Race | ||||||
| White | 1 | Reference | 1 | Reference | ||
| Black | 0.959 | 0.671–1.372 | 0.819 | 1.112 | 0.913–1.355 | 0.291 |
| Others | 1.383 | 0.857–1.234 | 0.184 | 1.251 | 0.936–1.671 | 0.13 |
| T stage | 1.992 | 0.492–8.057 | 0.334 | 2.219 | 1.049–4.693 | 0.037 |
| T1–T2 | 1 | Reference | 1 | Reference | ||
| T3–T4 | 1.955 | 1.543–2.477 | <0.001 | 1.026 | 0.88–1.196 | 0.741 |
| Gleason score | ||||||
| 2–7 | 1 | Reference | 1 | Reference | ||
| 8–10 | 5.504 | 4.197–7.217 | <0.001 | 2.869 | 2.455–3.352 | <0.001 |
| PSA (ng/mL) | ||||||
| ≤20 | 1 | Reference | 1 | Reference | ||
| >20 | 3.226 | 2.521–4.129 | <0.001 | 2.71 | 2.328–3.154 | <0.001 |
| Region | ||||||
| East | 1 | Reference | 1 | Reference | ||
| Northern plains | 0.79 | 0.474–1.315 | 0.364 | 1.15 | 0.891–1.484 | 0.282 |
| Pacific coast | 0.847 | 0.666–1.077 | 0.174 | 0.97 | 0.841–1.119 | 0.676 |
| Southwest | 1.388 | 0.724–2.66 | 0.323 | 1.361 | 0.91–2.035 | 0.133 |
| Year of diagnosis | ||||||
| 2004 | 1 | Reference | 1 | Reference | ||
| 2005 | 1.069 | 0.776–1.473 | 0.681 | 0.921 | 0.76–1.115 | 0.399 |
| 2006 | 0.81 | 0.577–1.138 | 0.225 | 0.726 | 0.596–0.886 | 0.002 |
| 2007 | 0.756 | 0.529–1.078 | 0.122 | 0.73 | 0.594–0.896 | 0.003 |
| 2008 | 0.611 | 0.425–0.879 | 0.008 | 0.72 | 0.589–0.879 | 0.001 |
Abbreviations: CSS, cancer-specific survival; EBRT, external beam radiotherapy; HR, hazard ratio; OS, overall survival; PSA, prostate-specific antigen; RP, radical prostatectomy.
Discussion
Selection of RP or EBRT as an initial therapy for clinically high-risk PCa patients is often a clinical challenge. Typically, young and healthy patients are often selected by urologists for RP, while elderly patients or patients with poor performance status and/or comorbidities are often recommended to receive no treatment or EBRT. However, there is no detailed consensus on the recommendation of RP or EBRT, simply because of no randomized controlled trials to examine survival outcomes between RP and EBRT in this high-risk cohort and mixed findings from previous studies as discussed earlier.6–8 Results from two meta-analyses indeed supported RP over RT in high-risk PCa in aspects of OS, PCa-specific mortality (PCSM), and non-PCSM.12,13 However, RP and RT cohorts were not matched by clinical risk factors and some of these RT studies were before 2000s (modern RT era). For example, there was a substantial gap in age between EBRT and RP groups. The median age of EBRT group is usually 5–10 years older than that of the RP group. In this study, we balanced important clinical risk factors (age, marital status, race, year of diagnosis, T stage, GS, PSA level, and SEER region) in clinically localized high-risk PCa patients derived from the SEER data in the 2004–2008 and then compared survival outcomes between these RP vs EBRT in PSM cohorts. Results of this study suggest that young patients (<65 years) might benefit from RP while old patients (≥65 years) might not need RP since EBRT is likely to produce similar survival outcomes. Results of this study have provided important information to clinicians and patients in the management of clinical high-risk PCa.
Pitfalls of this study include retrospective analysis of the SEER data in which many other important clinical factors were not available for this analysis, including comorbidities, performance status, other pathological features such as residual PSA and margin status, and use of ADT (long-term currently recommended). For example, in addition to survival outcomes, toxicity profiles and quality of life associated with RP vs EBRT are completely different between RP and EBRT14 and should also be considered when RP or EBRT is recommended. Typically, urinary incontinence and impotence frequently occur after RP, and older patients are often difficult to recover from treatment-related morbidities such as urinary incontinence, while urinary incontinence are rarely observed after EBRT. Therefore, EBRT is often argued as a primary treatment option for older patients with high-risk PCa when EBRT is proved to be not inferior to RP in survival outcomes.
In addition, radiation dose and dose fractionation regimen are important information but were not available in this SEER cohort. This is because high-dose radiotherapy has been clearly associated with clinical outcomes when compared with conventional radiotherapy.15–18 Results of these dose escalation studies have led to the NCCN recommendation of high-dose EBRT (79.2–81 Gy) or the combination of EBRT and brachytherapy. Therefore, it is important to know if young patients (<65 years) received high-dose radiotherapy and inferior survival outcomes in the young age EBRT group are not secondary to conventional dose RT. Results of this study suggest a need of a randomized controlled trial for the comparison of these two local treatment modalities for this young cohort.
Another important pitfall of this study is exclusion of neoadjuvant or adjuvant RT in this study. For the clean comparison of RP and EBRT for survival outcomes, we have to exclude patients with neoadjuvant or adjuvant RT. However, adjuvant RT was indicated in 2000s as a treatment option for patients with poor risk factors including residual PSA, positive margin, high GS, and T3–T4.19–21 Therefore, improved survival outcomes in the RP cohort in this study might come at least in part from adjuvant RT.
Conclusion
Results of this population-based propensity score matched study indicated that RP might provide favorable survival outcomes compared to EBRT in high-risk PCa for young patients (<65 years), while survival outcomes were comparable between EBRT and RP in older patients (≥65 years), which argues for definitive RT as a primary modality for this older cohort. This information is important in the clinical management of high-risk PCa, and a well-controlled randomized trial is required to assess therapeutic effectiveness of RP vs modern high-dose RT with long-term ADT in the treatment of young patients with high-risk factors.
Acknowledgments
This study was supported by a grant from the Clinical Features Research of Capital (no Z141107002514160).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69(6):1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016 featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2016;14(1):19–30. [Google Scholar]
- 5.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 6.Sooriakumaran P, Nyberg T, Akre O, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ. 2014;348:g1502. doi: 10.1136/bmj.g1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto S, Kawakami S, Yonese J, et al. Long-term oncological outcome in men with T3 prostate cancer: radical prostatectomy versus external-beam radiation therapy at a single institution. Int J Clin Oncol. 2014;19(6):1085–1091. doi: 10.1007/s10147-013-0654-2. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Kitamura Y, Komatsubara S, Matsumoto Y, Sugita T, Hara N. Outcomes of locally advanced prostate cancer: a single institution study of 209 patients in Japan. Asian J Androl. 2006;8(5):555–561. doi: 10.1111/j.1745-7262.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 9.Thariat J, Hannoun-Levi JM, Myint AS, Vuong T, Gerard JP. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol. 2013;10(1):52–60. doi: 10.1038/nrclinonc.2012.203. [DOI] [PubMed] [Google Scholar]
- 10.Database S [webpage on the Internet] PSA Values and SEER Data. 2017. [Accessed April 14, 2017]. Available from: https://seer.cancer.gov/data/psa-values.html.
- 11.D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Petrelli F, Vavassori I, Coinu A, Borgonovo K, Sarti E, Barni S. Radical prostatectomy or radiotherapy in high-risk prostate cancer: a systematic review and metaanalysis. Clin Genitourin Cancer. 2014;12(4):215–224. doi: 10.1016/j.clgc.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Wallis CJD, Saskin R, Choo R, et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(1):21–30. doi: 10.1016/j.eururo.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 16.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 17.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15(4):464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 18.Kalbasi A, Li J, Berman A, et al. Dose-escalated irradiation and overall survival in men with nonmetastatic prostate cancer. JAMA Oncol. 2015;1(7):897–906. doi: 10.1001/jamaoncol.2015.2316. [DOI] [PubMed] [Google Scholar]
- 19.Van der Kwast TH, Bolla M, Van Poppel H, et al. EORTC 22911. Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol. 2007;25(27):4178–4186. doi: 10.1200/JCO.2006.10.4067. [DOI] [PubMed] [Google Scholar]
- 20.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]