Abstract
Aims
To evaluate the long‐term safety and efficacy of tofogliflozin as an add‐on treatment to insulin over 52 weeks.
Materials and methods
This 52‐week, multicentre, Phase 4 study consisted of a 16‐week, randomized, double‐blind, placebo‐controlled phase and a 36‐week open label extension phase (NCT02201004). Japanese patients with type 2 diabetes mellitus, aged 20 to 75 years, with suboptimal glycaemic control (7.5%‐10.5%) receiving insulin monotherapy (basal‐bolus, bolus, premix [low and high] and basal) or receiving combination therapy with basal insulin and dipeptidyl peptidase‐4 inhibitor were eligible for participation. Patients who received tofogliflozin throughout the study (52 weeks) were referred to as the ‘tofo‐tofo group’ and patients who received placebo and tofogliflozin (36 weeks) were referred to as the ‘pla‐tofo group’.
Results
A total of 210 patients received treatment per randomization. Hypoglycaemia was the most common treatment‐emergent adverse event (AE) (42.9% in the tofo‐tofo group and 29.4% in the pla‐tofo group). Patients reported genital infection, urinary tract infection, excessive urination and AEs related to volume depletion (2.1%, 2.1%, 7.1% and 10.0% of patients in the tofo‐tofo group, and 0%, 1.5%, 2.9% and 7.4% of patients in the pla‐tofo group, respectively). Mean HbA1c and body weight at baseline (mean changes ± standard error from baseline to Week 52) in the tofo‐tofo and pla‐tofo groups were 8.53% (−0.76% ± 0.077) and 8.40% (−0.73% ± 0.102); 68.84 kg (−1.52 kg ± 0.207) and 72.24 kg (−2.13 kg ± 0.313), respectively.
Conclusions
This study demonstrates the safety and efficacy of tofogliflozin as add‐on to insulin therapy in type 2 diabetes mellitus patients, offering a new therapeutic solution to diabetes management.
Keywords: basal insulin, randomized trial, SGLT2 inhibitor, type 2 diabetes, weight control
1. INTRODUCTION
Tofogliflozin is a class of sodium glucose cotransporter 2 (SGLT2) inhibitors, approved in Japan in 2014 for treatment of type 2 diabetes mellitus (T2DM).1, 2 When used as either monotherapy or dual therapy with other oral anti‐diabetic drugs (OADs), tofogliflozin significantly reduced HbA1c, fasting plasma glucose (FPG), body weight and systolic/diastolic blood pressure.3, 4 Tofogliflozin was well tolerated, with a low risk of hypoglycaemia because of its insulin‐independent mechanism, as consistent with other SGLT2 inhibitors. However, the increased frequency of urinary tract and genital infections that are typical of the SGLT2 inhibitor class was also reported.
Combination therapy with insulin and OADs such as metformin and dipeptidyl peptidase‐4 (DPP‐4) inhibitors has been reported to be effective in terms of improving glycaemic control.5, 6 According to American Diabetes Association guidelines, use of an adjunctive SGLT2 inhibitor along with insulin may be helpful in ameliorating glycaemic control, as well as reducing the amount of insulin needed in patients with suboptimal glycaemic control, especially in those requiring increased insulin doses.7
To date, information regarding the long‐term safety and efficacy of combination therapy with insulin and tofogliflozin for T2DM patients is limited. Thus, we conducted a 52‐week, multicentre, Phase 4 study that integrated a 16‐week, randomized, double‐blind, placebo‐controlled phase and a subsequent 36‐week, open‐label extension phase to assess the safety and efficacy of tofogliflozin added to insulin in patients with T2DM (J‐STEP/INS).8 The previous report showed that combination therapy of insulin and tofogliflozin demonstrated significantly greater improvement in HbA1c (−0.59%, P < .0001), FPG (−27.2 mg/dL, P < .0001) and post‐prandial plasma glucose (PPG) (−65.0 mg/dL, P < .0001) at Week 16 compared to placebo. Diastolic blood pressure (−1.8 mmHg, P = .0218) and body weight (−1.34 kg, P < .0001) were also decreased in the tofogliflozin group, with statistical significances over placebo. With detailed information on results of the double‐blind phase reported elsewhere,8 this report further evaluates the long‐term safety and efficacy of tofogliflozin as add‐on treatment to insulin during 52 weeks.
2. MATERIALS AND METHODS
2.1. Study design
This was a 52‐week, multicentre, Phase 4 study that comprised 2 phases: 1) a 16‐week, randomized, double‐blind, placebo‐controlled phase, referred to as the double‐blind phase, and 2) a subsequent 36‐week open label extension phase, referred to as the extension phase, followed by 3‐day follow‐ups conducted from June 2014 to January 2016 (NCT02201004) (Figure S1).
2.2. Patients
Patients diagnosed with T2DM were recruited from diabetic clinics in Japan. Patients aged 20 to 75 years with suboptimal glycaemic control (7.5%‐10.5%) receiving insulin monotherapy (basal‐bolus, bolus, premix [low and high] and basal) or combination therapy with basal insulin and a DPP‐4 inhibitor were eligible for participation. Patients had been treated with stable insulin doses (± 20%) for ≥3 months prior to screening. No change in DPP‐4 inhibitor dose was permitted during the 3 months before screening.
Exclusion criteria included type 1 diabetes mellitus, unstable proliferative diabetic retinopathy or any other rapidly progressive diabetic retinopathy or macular edema likely to require treatment during the study period, history of metabolic acidosis ≤1 year prior to the screening visit, history of severe uncontrolled glycaemia, pregnancy or lactation, or the judgement by the attending investigators that participation was inappropriate based on any medical, psychological, social or geographical reasons. Further details are described elsewhere.8
2.3. Procedures
After a 2‐week screening period, we randomly assigned patients in a 2:1 ratio to tofogliflozin or placebo using the minimization method. Patients received 20 mg of tofogliflozin or placebo once daily, before or after breakfast, during the double‐blind phase. During the extension phase, all patients received 20 mg of tofogliflozin once daily. Patients who received tofogliflozin during both the double‐blind and extension phases were referred to as the ‘tofo‐tofo group’ and patients who received placebo during the double‐blind phase and tofogliflozin during extension phase were referred to as the ‘pla‐tofo group’. During the double‐blind phase, changing the insulin dose was prohibited except when hypoglycaemia developed or hypoglycaemia prevention was required. The insulin dose changed if the FPG was ≥240 mg/dL and did not decrease to below 240 mg/dL with appropriate treatment (defined as rescue therapy). During the extension phase, changing the insulin dose was permitted according to the discretion of the investigator. Background therapy, including time of injection and diet and exercise routine, was maintained without change. Other hypoglycaemic drugs, systemic glucocorticoids for more than 10 days and probenecid were prohibited during the study. The randomization code was broken after all data of the double‐blind phase were fixed.
Written informed consent was obtained from all patients. The study protocol was conducted in accordance with the Japanese Pharmaceutical Affairs Act (Articles 14.4.4, 14.4.7), Good Clinical Practice Standards for the Implementation of Clinical Trials (Ordinance of the Pharmaceutical Affairs Bureau, Ministry of Health and Welfare No. 28 of March 1997), Good Post‐Marketing Study Practice (Ordinance of the Japanese Pharmaceutical Affairs Bureau, Ministry of Health, Labour and Welfare No. 171 of December 2004) and related notices. Local institutional review boards approved the study protocol.
2.4. Study endpoints
Assessment of long‐term safety was based on adverse events (AEs), vital signs, electrocardiogram and clinical labouratory tests. All AEs were classified and coded according to the terms of the Medical Dictionary for Regulatory Activities (MedDRA). Treatment‐emergent adverse events (TEAEs) during the on‐treatment period were defined as AEs that developed, worsened or became serious during the period from first administration of tofogliflozin or placebo up to 7 days after last administration of tofogliflozin or placebo. AEs were assessed by patient diary entries, reviewed at each study visit, by telephone interviews at Weeks 20, 28, 36, 44, 48 and by follow‐up after Week 52. Hypoglycaemia was classified as severe, documented symptomatic, asymptomatic, probable symptomatic, nocturnal or daytime, of which definitions are available in a previous report.8 Urinary tract or genital infections were recorded, based on diagnosis by the investigator. Vital signs were collected at each study visit. Clinical labouratory tests were performed at randomization and at Weeks 16, 32, 40 and 52.
Long‐term efficacy was assessed by change in HbA1c, body weight, FPG, systolic and diastolic blood pressure (sitting position), uric acid, lipid parameter (total cholesterol, high‐density lipoprotein [HDL] cholesterol, low‐density lipoprotein [LDL] cholesterol and triglycerides) and insulin dose from baseline to Week 52.
2.5. Statistical analysis
All long‐term safety and efficacy analyses were conducted descriptively. Safety analyses were performed on the intention‐to‐treat (ITT) population, defined as all patients randomized and exposed to at least 1 dose of the study drug. Safety analyses for the 52‐week on‐treatment period were performed in patients randomized to the tofo‐tofo group. Safety analyses during the extension phase in the pla‐tofo group were performed on the ITT population for the open‐label period (opITT), defined as patients randomized to the pla‐tofo group who completed the double‐blind phase and were exposed to at least 1 dose of tofogliflozin. The percentage of patients who developed hypoglycaemia, urinary tract infection, genital infection, excessive urination, pollakiuria or volume depletion was summarized according to treatment groups. Incidence rates of patients who experienced hypoglycaemia per 100 person‐years during 52 weeks for the tofo‐tofo group and during the extension phase for the pla‐tofo group were also calculated. AEs were summarized as TEAEs, classified by system organ class and preferred term, serious AEs (SAEs) and AEs leading to permanent treatment discontinuation. The period from the first dose of tofogliflozin up to 7 days after the last dose of tofogliflozin during 52 weeks was analysed for safety results concerning the tofo‐tofo group. The period from Week 16 up to 7 days after the last dose of tofogliflozin was analysed for safety results of the pla‐tofo group. Vital signs and clinical labouratory data were descriptively summarized.
Efficacy analyses were performed on the modified intention‐to‐treat (mITT) population, defined as the randomized patients who were exposed to at least 1 dose of tofogliflozin or placebo, and who had both a baseline measurement and at least 1 post‐baseline measurement of any efficacy variable. Data after 2 days of the last tofogliflozin treatment or after initiation of rescue therapy were excluded from analyses for each variable. Mean values and changes in HbA1c, body weight, FPG, systolic and diastolic blood pressure, uric acid, lipid parameter, insulin dose from baseline to Week 52 and the responder rates of HbA1c at Week 52 were descriptively calculated. Because we aimed to assess long‐term efficacy with an exploratory approach, multiplicity adjustment was not performed. As we used conventional units for uric acid, total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides, SI units can be determined by multiplying the following conversion factors with the conventional results: uric acid (mg/dL to μmol/L), 59.48; total cholesterol, HDL cholesterol and LDL cholesterol (mg/dL to mmol/L), 0.0259; triglycerides (mg/dL to mmol/L), 0.0113.
Sample‐size calculation is reported elsewhere.8 All analyses were performed using SAS® version 9.2 (SAS Institute, Cary, North Carolina) software.
3. RESULTS
3.1. Patient characteristics
Among the 320 patients with T2DM who were screened, 211 were randomized to the tofo‐tofo or pla‐tofo groups (n = 141 and 70, respectively) in 30 diabetic clinics (Figure S2). One patient in the tofo‐tofo group was randomized but not treated with tofogliflozin because of a pre‐treatment AE. A total of 210 patients received treatment as per randomization and were included in the ITT and the mITT population. During the double‐blind phase, 3 patients in the tofo‐tofo group and 2 in the pla‐tofo group discontinued treatment, leaving 137 patients in the tofo‐tofo group and 68 in the pla‐tofo group (opITT population). The reasons for discontinuation were consent withdrawal in the case of 1 patient and AEs (cold urticaria and worsening of glucose control) in the case of 2 patients in the tofo‐tofo group, and consent withdrawal in the case of 1 patient and selection criteria not met in the case of 1 patient in the pla‐tofo group. During the extension phase, 6 patients in the tofo‐tofo group and 2 in the pla‐tofo group discontinued treatment. Reasons for discontinuation were consent withdrawal in the case of 2 patients, AEs (peripheral arterial occlusive disease, genital infection and increased blood creatine phosphokinase) in the case of 3 patients, other reason(s) in the case of 1 patient in the tofo‐tofo group, and in the case of 1 patient each for consent withdrawal and an AE (drug eruption) in the pla‐tofo group.
Patients in the tofo‐tofo group were older and had a lower proportion of BMI ≥ 30 kg/m2 and longer duration of diabetes compared with the pla‐tofo group (Table 1). We observed no other notable differences in demographic and baseline characteristics. Mean treatment duration ± SD was 349.8 days ±56.9 in the tofo‐tofo group and 247.7 days ±18.0 in the pla‐tofo group.
Table 1.
Baseline demographic and clinical characteristics of ITT population
| Characteristics | Tofo‐tofo (n = 140) | Pla‐tofo (n = 70) |
|---|---|---|
| Age, years | 59.1 ± 10.9 | 56.4 ± 10.0 |
| ≥65 years, n (%) | 58 (41.4) | 17 (24.3) |
| Male, n (%) | 89 (63.6) | 48 (68.6) |
| HbA1c, % | 8.53 ± 0.76 | 8.40 ± 0.65 |
| ≥8.0%, n (%) | 107 (76.4) | 50 (71.4) |
| eGFR, mL/min/1.73 m2 | 79.8 ± 19.8 | 79.5 ± 17.0 |
| ≥30 to <60 mL/min/1.73 m2, n (%) | 14 (10.0) | 9 (12.9) |
| ≥60 to <90 mL/min/1.73m2, n (%) | 88 (62.9) | 45 (64.3) |
| ≥90 mL/min/1.73m2, n (%) | 38 (27.1) | 16 (22.9) |
| Body weight, kg | 68.84 ± 13.24 | 72.24 ± 11.12 |
| BMI, kg/m2 | 25.79 ± 3.46 | 26.89 ± 3.88 |
| BMI, n (%) | ||
| <25 kg/m2 | 60 (42.9) | 24 (34.3) |
| ≥25 to <30 kg/m2 | 62 (44.3) | 29 (41.4) |
| ≥30 kg/m2 | 18 (12.9) | 17 (24.3) |
| FPG, mg/dL | 164.0 ± 47.2 | 162.4 ± 43.2 |
| PPG, mg/dL | 300.3 ± 68.6 | 302.6 ± 65.6 |
| Systolic BP, mmHg | 134.8 ± 16.4 | 134.7 ± 15.9 |
| Diastolic BP, mmHg | 76.4 ± 10.5 | 77.9 ± 11.0 |
| Duration of diabetes, years | 15.06 ± 9.39 | 12.39 ± 7.34 |
| Insulin treatment at screening, n (%) | ||
| Basal‐bolus | 34 (24.3) | 17 (24.3) |
| Bolus | 16 (11.4) | 8 (11.4) |
| Premix | 23 (16.4) | 11 (15.7) |
| Basal | 67 (47.9) | 34 (48.6) |
| Basal only | 30 (21.4) | 9 (12.9) |
| Basal combined with DPP‐4 inhibitors | 37 (26.4) | 25 (35.7) |
| Comorbidity, n (%) | ||
| Diabetic retinopathy | 67 (47.9) | 34 (48.6) |
| Diabetic sensory or motor neuropathya | 40 (28.6) | 13 (18.6) |
| Diabetic autonomic neuropathya | 9 (6.4) | 0 |
| Diabetic nephropathya | 62 (44.3) | 27 (38.6) |
| Cardiac disorders | 21 (15.0) | 7 (10.0) |
| Concomitant cardiovascular medication, n (%) | ||
| β‐blocking agents | 11 (7.9) | 9 (12.9) |
| Diuretics | 10 (7.1) | 4 (5.7) |
| Agents acting on the renin‐angiotensin system | 5 (3.6) | 8 (11.4) |
| Lipid‐modifying agents | 5 (3.6) | 5 (7.1) |
| Peripheral vasodilators | 1 (0.7) | 1 (1.4) |
| Vasoprotectives | 1 (0.7) | 1 (1.4) |
Abbreviations: BMI, body mass index; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycosylated haemoglobin; ITT, intention‐to‐treat; PPG, post‐prandial plasma glucose; SD, standard deviation.
Patients who received tofogliflozin in both double‐blind and extension phases were referred to as “tofo‐tofo group”, and patients who received placebo in double‐blind phase and tofogliflozin in extension phase were referred to as “pla‐tofo group”. Data are mean ± SD unless otherwise indicated.
As reported by the attending physician.
3.2. Long‐term safety
Table 2 summarizes safety results during the 52‐week on‐treatment period of the tofo‐tofo group and the extension phase of the pla‐tofo group. No deaths occurred throughout the study. In addition, we observed no major differences in occurrence of AEs during the 52‐week and 36‐week peeriods.
Table 2.
Safety results during the 52‐week on‐treatment period in tofo‐tofo group and the extension phase in pla‐tofo group
| Overall summary | Tofo‐tofo(n = 140)a | Pla‐tofo (n = 68)b |
|---|---|---|
| Any TEAE | 126 (90.0) | 50 (73.5) |
| Any TEAE possibly related to study treatment | 77 (55.0) | 24 (35.3) |
| TEAE leading to death | 0 | 0 |
| Any serious TEAE | 4 (2.9) | 5 (7.4) |
| Any serious TEAE possibly related to study treatment | 1 (0.7) | 0 |
| TEAE leading to permanent treatment discontinuation | 5 (3.6) | 1 (1.5) |
| Individual TEAEs occurring in > 3% patients in either group | ||
| Hypoglycaemia | 60 (42.9) | 20 (29.4) |
| Nasopharyngitis | 40 (28.6) | 13 (19.1) |
| Blood ketone body increased | 11 (7.9) | 4 (5.9) |
| Thirst | 10 (7.1) | 2 (2.9) |
| Pharyngitis | 6 (4.3) | 1 (1.5) |
| Polyuria | 6 (4.3) | 0 |
| Constipation | 5 (3.6) | 1 (1.5) |
| Gastroenteritis | 4 (2.9) | 4 (5.9) |
| Abdominal pain upper | 2 (1.4) | 3 (4.4) |
| Periarthritis | 1 (0.7) | 3 (4.4) |
| Weight decreased | 1 (0.7) | 3 (4.4) |
| Investigations c | ||
| Blood ketone body increased | 11 (7.9) | 4 (5.9) |
| Weight decreased | 1 (0.7) | 3 (4.4) |
| Beta 2 microglobulin urine increased | 1 (0.7) | 2 (2.9) |
| Blood creatine phosphokinase increased | 3 (2.1) | 2 (2.9) |
| Urine ketone body present | 3 (2.1) | 2 (2.9) |
| TEAEs of special interest | ||
| Hypoglycaemia | 61 (43.6) | 20 (29.4) |
| Hypoglycaemia | 60 (42.9) | 20 (29.4) |
| Hypoglycaemic unconsciousness | 1 (0.7) | 0 |
| AEs related to volume depletion | 14 (10.0) | 5 (7.4) |
| Thirst | 10 (7.1) | 2 (2.9) |
| Constipation | 5 (3.6) | 1 (1.5) |
| Dehydration | 0 | 1 (1.5) |
| Loss of consciousness | 0 | 1 (1.5) |
| Excessive urination | 10 (7.1) | 2 (2.9) |
| Polyuria | 6 (4.3) | 0 |
| Pollakiuria | 3 (2.1) | 2 (2.9) |
| Nocturia | 1 (0.7) | 0 |
| Skin and subcutaneous tissue disorders | 10 (7.1) | 7 (10.3) |
| Asteatotic eczema | 3 (2.1) | 0 |
| Eczema | 2 (1.4) | 2 (2.9) |
| Rash | 2 (1.4) | 0 |
| Urticaria | 2 (1.4) | 1 (1.5) |
| Cold urticaria | 1 (0.7) | 0 |
| Dermatitis | 1 (0.7) | 0 |
| Miliaria | 1 (0.7) | 1 (1.5) |
| Dermatitis contact | 0 | 1 (1.5) |
| Drug eruption | 0 | 1 (1.5) |
| Hand dermatitis | 0 | 1 (1.5) |
| Pruritus | 0 | 1 (1.5) |
| Genital Infection | 3 (2.1) | 0 |
| Vaginal infection | 2 (1.4) | 0 |
| Genital infection | 1 (0.7) | 0 |
| Urinary Tract Infection | 3 (2.1) | 1 (1.5) |
| Cystitis | 2 (1.4) | 1 (1.5) |
| Urinary tract infection | 1 (0.7) | 0 |
| Hypoglycaemia | ||
| Any hypoglycaemia | 61 (43.6) | 20 (29.4) |
| Severe hypoglycaemiad | 2 (1.4) | 1 (1.5) |
| Hypoglycaemic unconsciousness | 1 (0.7) | 0 |
| Documented symptomatic hypoglycaemiae | 38 (27.1) | 12 (17.6) |
| Plasma glucose <54 mg/dL (3.0 mmol/L) | 16 (11.4) | 5 (7.4) |
| Asymptomatic hypoglycaemiaf | 18 (12.9) | 10 (14.7) |
| Plasma glucose <54 mg/dL (3.0 mmol/L) | 8 (5.7) | 4 (5.9) |
| Probable symptomatic hypoglycaemia | 16 (11.4) | 4 (5.9) |
| Nocturnal hypoglycaemia | 15 (10.7) | 5 (7.4) |
| Daytime hypoglycaemia | 60 (42.9) | 19 (27.9) |
Abbreviation: TEAE, treatment emergent adverse event.
Patients who received tofogliflozin in both double‐blind and extension phases were referred to as “tofo‐tofo group”, and patients who received placebo in double‐blind phase and tofogliflozin in extension phase were referred to as “pla‐tofo group”.
The period from the first dose of the tofogliflozin up to 7 days after the last dose of the tofogliflozin during 52 weeks was analyzed for the safety results of the tofo‐tofo group. The period from the next date of Week 16 up to 7 days after the last dose of tofogliflozin was analyzed for the safety results of the pla‐tofo group.
Data are n (%).
The intention‐to‐treat population is defined as all patients randomized and exposed to at least one dose of the tofogliflozin in the double‐blind period.
The intention‐to‐treat population for the open‐label period was defined as subjects randomized to the pla‐tofo group who completed the double‐blind period and were exposed to at least one dose of the tofogliflozin in the open‐label period.
TEAEs classified as “Investigation” occurring in >2% patients in either group are summarized.
One patient with hypoglycaemic unconsciousness did not meet the definition of severe hypoglycaemia defined in the study protocol in terms of not receiving a third person's help. As a third person's help is indispensable in a patient with hypoglycaemic unconsciousness, regardless of whether receiving actual help or not, this hypoglycaemia is categorized as severe in this paper.
Hypoglycaemic events that were associated with typical hypoglycaemic symptoms with an accompanying plasma glucose ≤70 mg/dL (3.9 mmol/L).
Hypoglycaemic events that were not associated with typical hypoglycaemic symptoms with an accompanying plasma glucose ≤70 mg/dL (3.9 mmol/L).
In the 52‐week on‐treatment period (tofo‐tofo group), serious TEAEs including hypoglycaemic unconsciousness, coronary artery stenosis, spondylolisthesis and ankle fracture occurred in 4 patients (2.9%). Prolonged electrocardiogram QT, graded mild, was reported in 2 patients (1.4%). TEAEs leading to permanent treatment discontinuation were reported in 5 patients (3.6%), including genital infection, worsening of glucose control, peripheral arterial occlusive disease, cold urticarial and increased blood creatine phosphokinase. During the extension phase in the pla‐tofo group, serious TEAEs occurred in 5 patients (7.4%), including gastroenteritis in 2 patients and cellulitis, cardiac failure and myocardial infarction in 1 patient each. Prolonged electrocardiogram QT, graded mild, was reported in 1 patient (1.5%). A TEAE leading to permanent treatment discontinuation was reported in 1 patient (1.5%) with drug eruption, which was considered to be related to tofogliflozin.
Hypoglycaemia was the most common TEAE in both the tofo‐tofo and pla‐tofo groups (60 patients [42.9%] and 20 patients [29.4%], respectively). According to the insulin treatment regimen in the tofo‐tofo group during the 52‐week on‐treatment period, hypoglycaemia was observed in 22 (64.7%), 7 (43.8%), 8 (34.8%) and 24 patients (35.8%) treated with basal‐bolus, bolus, premix (low and high) and basal insulin, respectively. Table S1 shows the incidence rates of patients who experienced hypoglycaemia per 100 person‐years for the tofo‐tofo and pla‐tofo groups.
Table 2 summarizes the TEAEs of special interest in the 52‐week on‐treatment period in the tofo‐tofo group and the extension phase of the pla‐tofo group. In the tofo‐tofo group during the 52‐week on‐treatment period, 3 patients (2.1%) each experienced genital and urinary tract infections, 10 (7.1%) experienced excessive urination and 14 (10.0%) experienced AEs related to volume depletion. In the pla‐tofo group during the extension phase, no patient experienced genital infection, 1 (1.5%) experienced urinary tract infection, 2 (2.9%) experienced excessive urination and 5 (7.4%) experienced AEs related to volume depletion. No patients were diagnosed as having ketoacidosis. No acute renal failure nor cases of amputation occurred in this study.
3.3. Long‐term efficacy
Table 3 summarizes the 52‐week efficacy results for HbA1c, systolic and diastolic blood pressure, uric acid, total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides.
Table 3.
Efficacy results during the 52‐week on‐treatment period
| Tofo‐tofo (n = 140) | Pla‐tofo (n = 70) | |||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | Mean change from baseline to Week 52 ± SE | n | Mean ± SD | Mean change from baseline to Week 52 ± SE | |
| HbA1c, % | ||||||
| Baseline | 140 | 8.53 ± 0.76 | 70 | 8.40 ± 0.65 | ||
| Week 16 | 135 | 7.80 ± 0.85 | 69 | 8.80 ± 0.98 | ||
| Week 52 | 130 | 7.75 ± 0.91 | −0.76 ± 0.077 | 66 | 7.68 ± 0.82 | −0.73 ± 0.102 |
| Systolic BP, mmHg | ||||||
| Baseline | 140 | 134.8 ± 16.4 | 70 | 134.7 ± 15.9 | ||
| Week 16 | 135 | 130.1 ± 17.2 | 67 | 132.3 ± 16.5 | ||
| Week 52 | 130 | 129.3 ± 17.2 | −5.5 ± 1.14 | 64 | 128.6 ± 15.7 | −6.5 ± 2.12 |
| Diastolic BP, mmHg | ||||||
| Baseline | 140 | 76.4 ± 10.5 | 70 | 77.9 ± 11.0 | ||
| Week 16 | 135 | 74.5 ± 11.7 | 67 | 78.8 ± 10.4 | ||
| Week 52 | 130 | 74.4 ± 11.2 | −2.0 ± 0.67 | 64 | 76.0 ± 10.2 | −2.4 ± 1.29 |
| Uric acid, mg/dL | ||||||
| Baseline | 140 | 5.05 ± 1.25 | 70 | 5.23 ± 1.42 | ||
| Week 16 | 135 | 4.87 ± 1.24 | 67 | 5.30 ± 1.44 | ||
| Week 52 | 130 | 4.92 ± 1.34 | −0.14 ± 0.067 | 64 | 4.90 ± 1.22 | −0.27 ± 0.098 |
| Total cholesterol, mg/dL | ||||||
| Baseline | 140 | 203.3 ± 40.5 | 70 | 204.9 ± 32.4 | ||
| Week 16 | 135 | 206.7 ± 35.8 | 67 | 205.2 ± 31.5 | ||
| Week 52 | 130 | 208.9 ± 37.8 | 4.9 ± 2.30 | 64 | 204.1 ± 35.2 | 1.6 ± 3.15 |
| HDL cholesterol, mg/dL | ||||||
| Baseline | 140 | 57.0 ± 17.0 | 70 | 55.6 ± 13.9 | ||
| Week 16 | 135 | 59.7 ± 18.8 | 67 | 56.2 ± 14.1 | ||
| Week 52 | 130 | 59.8 ± 19.6 | 2.6 ± 0.84 | 64 | 58.2 ± 14.7 | 2.0 ± 0.86 |
| LDL cholesterol, mg/dL | ||||||
| Baseline | 140 | 121.4 ± 33.0 | 70 | 130.2 ± 26.9 | ||
| Week 16 | 135 | 125.6 ± 32.8 | 67 | 129.8 ± 26.7 | ||
| Week 52 | 130 | 129.5 ± 35.3 | 7.1 ± 1.92 | 64 | 129.2 ± 30.9 | 1.0 ± 2.54 |
| Triglycerides, mg/dL | ||||||
| Baseline | 140 | 177.0 ± 218.9 | 70 | 128.6 ± 65.6 | ||
| Week 16 | 135 | 145.6 ± 111.9 | 67 | 127.3 ± 80.9 | ||
| Week 52 | 130 | 132.0 ± 101.3 | −43.4 ± 18.61 | 64 | 114.2 ± 57.7 | −6.8 ± 5.34 |
Abbreviation: BP, blood pressure; HbA1c, glycosylated haemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation.
Patients who received tofogliflozin in both double‐blind and extension phases were referred to as “tofo‐tofo group”, and patients who received placebo in double‐blind phase and tofogliflozin in extension phase were referred to as “pla‐tofo group”.
The analysis included data obtained from the first dose of the tofogliflozin or placebo during the whole treatment period or up to the introduction of the rescue medication, 1 day after the last dose of the tofogliflozin or placebo, whichever is the earliest.
HbA1c values decreased during the first 8 weeks and this reduction was sustained throughout the study in the tofo‐tofo group; mean HbA1c after tofogliflozin administration ranged between (min‐max) 7.70% and 7.80%). In the pla‐tofo group, HbA1c values did not change during the first 16 weeks (placebo period). However, after initiating tofogliflozin treatment from Week 16, HbA1c decreased during the first 8 weeks and this reduction was sustained throughout the study period; mean HbA1c after tofogliflozin administration ranged between (min‐max 7.53% and 7.76%) (Figure 1). Mean HbA1c ± standard deviation (SD) at baseline were 8.53% ± 0.76 and 8.40% ± 0.65 in the tofo‐tofo and pla‐tofo groups, respectively. Mean HbA1c changes ± standard error (SE) from baseline to Week 16 were −0.72% ± 0.061 and +0.41% ± 0.096, and from baseline to Week 52 were −0.76% ± 0.077 and −0.73% ± 0.102 in the tofo‐tofo and pla‐tofo groups, respectively (Table 3).
Figure 1.
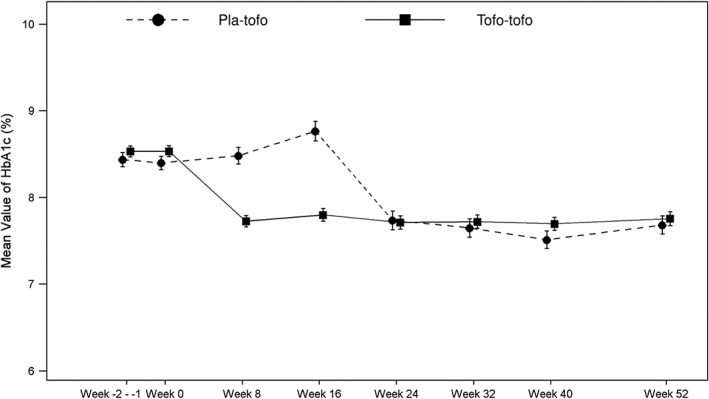
Plot of mean HbA1c value (%) by visit (mITT population). Patients who received tofogliflozin in both the double‐blind and extension phases were referred to as the ‘tofo‐tofo group’, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’. The graph includes data obtained from the first dose of tofogliflozin or placebo during the entire treatment period or up to the introduction of rescue medication, 1 day after the last dose of tofogliflozin or placebo, whichever is the earliest. Values are given as mean (%) ± standard error. Abbreviations: HbA1c, glycosylated haemoglobin; mITT, modified intention‐to‐treat; SE, standard error
FPG also decreased after the first 8 weeks of tofogliflozin treatment and was stable during the study in the tofo‐tofo group. After switching to tofogliflozin at Week 16, FPG decreased in the pla‐tofo group. Mean FPG ± SD at baseline, Week 16 and Week 52 was 164.0 mg/dL ± 47.2, 134.7 mg/dL ± 30.0 and 132.0 mg/dL ± 33.6 in the tofo‐tofo group and was 162.4 mg/dL ± 43.2, 167.1 mg/dL ± 47.2 and 136.7 mg/dL ± 39.1 in the pla‐tofo group, respectively. Mean change ± SE from baseline to Week 52 was −29.3 mg/dL ± 3.68 and −24.6 mg/dL ± 6.42 in the tofo‐tofo and pla‐tofo groups, respectively (Figure 2A).
Figure 2.
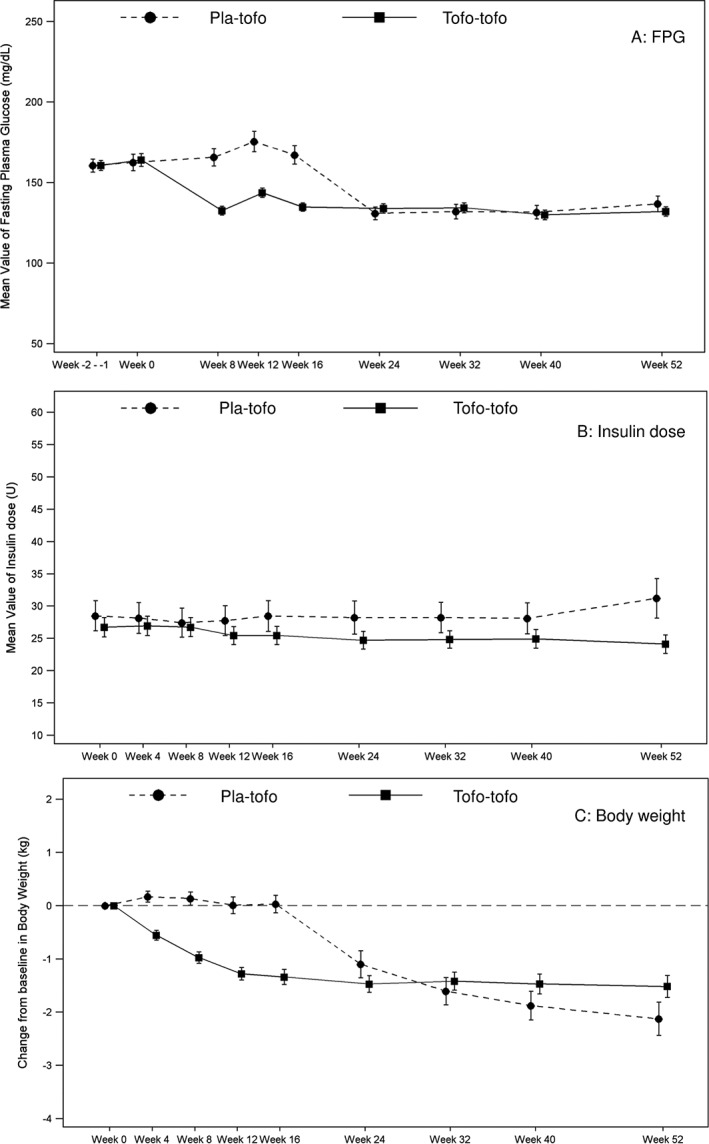
Plot of mean value of FPG and insulin dose, and plot of mean change in body weight (C) by visit (mITT population). Patients who received tofogliflozin in both the double‐blind and extension phases were referred to as the ‘tofo‐tofo group’, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’. (A–B) mean ± SE of FPG and insulin dose. (C) mean change ± SE in body weight. The graph includes data obtained from the first dose of tofogliflozin or placebo during the entire treatment period or up to introduction of the rescue medication, 1 day after the last dose of the tofogliflozin or placebo, whichever is the earliest. Abbreviations: FPG, fasting plasma glucose; mITT, modified intention‐to‐treat; SE, standard error
Mean insulin dose ± SD at baseline was 26.71 U ± 17.69 and 28.50 U ± 19.40 in the tofo‐tofo and pla‐tofo groups, respectively. Overall, the insulin dose slightly decreased from baseline to Week 52 (mean change ± SE, −1.27 U ± 0.615 and −0.58 U ± 0.915 in the tofo‐tofo and pla‐tofo groups, respectively) (Figure 2B).
As observed with the HbA1c and FPG levels, body weight reduced after tofogliflozin add‐on to insulin treatment in the tofo‐tofo and pla‐tofo groups and this reduction was maintained throughout the study. Mean body weight ± SD at baseline, Week 16 and Week 52 was 68.84 kg ± 13.24, 67.41 kg ± 13.02 and 67.73 kg ± 13.50 in the tofo‐tofo group and was 72.24 kg ± 11.12, 72.37 kg ± 11.57 and 70.29 kg ± 11.73 in the pla‐tofo group, respectively. Mean change ± SE from baseline to Week 52 was −1.52 kg ± 0.207 and −2.13 kg ± 0.313 in the tofo‐tofo and pla‐tofo groups, respectively (Figure 2C).
Mean changes in HbA1c, FPG, insulin dose and body weight from baseline to Week 52 by each insulin regimen (basal‐bolus, bolus, premix [low and high] and basal) are summarized in Table S2. Figure S3 shows mean HbA1c change ± SE from baseline through the follow‐up visits for each regimen. In the tofo‐tofo group, HbA1c mean change ± SE from baseline to Week 52 was −0.39% ± 0.116, −0.55% ± 0.187, −0.57% ± 0.155 and −1.03% ± 0.121 for patients with basal‐bolus, bolus, premix (low and high) and basal insulin, respectively. We observed a slight decrease in insulin doses in most regimens from baseline to Week 52 except in the basal‐bolus and basal regimens in the pla‐tofo group (Table S2).
In the tofo‐tofo group, the proportion of patients who achieved HbA1c < 7.0% at Week 52 was 15.0%, and those who achieved HbA1c ≤ 6.5% at Week 52 was 3.6%.
4. DISCUSSION
We examined long‐term safety and efficacy of tofogliflozin added to insulin over 52 weeks in T2DM patients in whom glycaemic control was inadequately controlled despite insulin use. The safety profiles of tofogliflozin were generally consistent with those reported in a 16‐week on‐treatment period study,8 and tofogliflozin treatment was well tolerated over the 52‐week period. No death occurred during this study. Hypoglycaemia was the most common AE in the tofo‐tofo and pla‐tofo groups. During the 52‐week on‐treatment period, several occurrences of urinary and genital tract infections were also reported as TEAEs in the tofo‐tofo group. Furthermore, improvements in glycaemic control, body weight, FPG, systolic and diastolic blood pressure, uric acid and triglycerides were generally maintained during the study period in the tofo‐tofo and pla‐tofo groups after tofogliflozin administration. This study has demonstrated the safety and efficacy of tofogliflozin as add‐on to insulin therapy in T2DM patients, offering a new therapeutic solution to diabetes management.
Hypoglycaemia was the most common AE in the tofo‐tofo group at Week 52 and at Week 16 (30.7% and 42.9%, respectively). Results for each insulin regimen showed that more than 60% of patients who used basal‐bolus insulin experienced hypoglycaemia. Compared with other OADs in general, the SGLT2 inhibitor class causes hypoglycaemia less frequently because of its insulin‐independent mechanism, which reduces renal glucose reabsorption.9 Previous studies have found increased frequencies of hypoglycaemia (35.0%‐59.0%) when SGLT2 inhibitors were added to insulin,10, 11, 12 and those proportions were generally consistent with those of our study. Thus, clinicians should pay careful attention to hypoglycaemia, especially when proceeding with combination treatment with basal‐bolus insulin and tofogliflozin.
Urinary tract and genital infections, the most common AEs of SGLT2 inhibitors,13 appeared in a small number of patients (< 3%) throughout this study. Similar frequencies were reported in a previous study of 52‐week tofogliflozin monotherapy, with groups receiving 20 and 40 mg tofogliflozin (1.6% and 0.8% for urinary tract infection, 0% and 1.6% for genital infection, 4.7% and 2.4% for cystitis, respectively).3 In this study, only 5 patients discontinued treatment in the tofo‐tofo group, including 1 patient who discontinued because of genital infection.
Other AEs, such as those related to volume depletion and excessive urination, have been observed in other studies of SGLT2 inhibitors added to insulin.11, 12, 14, 15 These AEs appeared also in our study. Although the typical AEs of SGLT2 inhibitors should be borne in mind, few serious AEs led to treatment discontinuation and no death was observed in the tofo‐tofo group, which indicates that tofogliflozin is well tolerated and the continuous use of tofogliflozin add‐on to insulin therapy may be a safe option for T2DM patients.
Ketoacidosis has been a controversial topic concerning use of an SGLT2 inhibitor because of the risk of ketoacidosis that is potentially induced by SGLT2 inhibitors16, 17, 18, 19, 20 and the related warning issued by the US Food and Drug Administration (FDA).21, 22 In this study, the ketone increase in blood and the ketone presence in urine were observed in both treatment groups, although no patients were diagnosed with diabetic ketoacidosis. Given that the risk of ketoacidosis may be a class effect, which was suggested by previous studies, and that ketones were observed in this study, continuous investigations concerning ketoacidosis and use of SGLT2 inhibitors, including tofogliflozin, should be explored further.
As stated in the package insert of tofogliflozin, because it may lead to a reduction in eGFR, regular checkups of renal function and careful attention to T2DM patients with renal dysfunction are required when using tofogliflozin. Acute renal failure was not observed in this study, which suggests that tofogliflozin added to insulin treatment did not worsen the renal function of patients with low eGFR (≥ 30‐< 60 mL/min/1.73 m2) at baseline.
The FDA alerted that canagliflozin, one of the SGLT2 inhibitors, increased the risk of amputation23 based on the results revealed by the CANVAS program, which reported an approximately 2‐fold increased risk of leg and foot amputations.20 In this study, no patients experienced amputations. Neither the clear mechanisms of amputation nor whether this is a class effect is known. Nevertheless, when using SGLT2 inhibitors, thorough consideration may be required to treat T2DM patients with potential predisposing factors for amputations, for example, history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers.
In terms of efficacy, we observed reduced levels of HbA1c, FPG, systolic and diastolic blood pressure, uric acid and triglycerides that were sustained throughout the study period in the tofo‐tofo group. We also observed these reductions in the pla‐tofo group after initiating tofogliflozin administration. Overall, the effect of tofogliflozin add‐on to insulin therapy was favourable, and mostly consistent with previous long‐term studies on other SGLT2 inhibitors added to insulin.10, 11, 12 In line with previous studies on long‐term SGLT2 inhibitors added to insulin treatment, a subtle reduction in insulin was observed in this study.12, 24, 25 The rather small improvement in glucose observed in this study may explain the slight reduction in insulin, as the majority presumably did not require insulin reduction (HbA1c responder rates <7.0% and ≤6.5% at Week 52, 15.0% and 3.6%, respectively, in the tofo‐tofo group). Additionally, the titration algorithm was not specified in the protocol, and changing the insulin dose was at the discretion of the investigator. Those factors may also have played a role in the subtle reduction in insulin observed in this study. These results suggest the importance of insulin adjustment that is appropriate for individuals, to ultimately achieve good glycaemic control and to prevent further comorbidities.
SGLT2 inhibitors, when used as monotherapy, or in combination therapy with OADs or insulin, have been shown to elevate LDL cholesterol.12, 26, 27, 28, 29, 30, 31 Likewise, in our study, LDL cholesterol levels were elevated, in both the tofo‐tofo and pla‐tofo groups, suggesting that it may be a class effect. The mechanisms of increase in LDL cholesterol with SGLT2 inhibitors are yet to be revealed, warranting further investigation.
Tofogliflozin add‐on to insulin therapy also reduced weight and has been demonstrated in studies of tofogliflozin and other SGLT2 inhibitors as add‐on to insulin therapy for both short and long terms.3, 4, 10, 14, 15 A study of dapagliflozin, a class of SGLT2 inhibitors, that measured body composition found that most weight reduction occurred by fat loss and that urinary glucose excretion was associated with weight reduction.32 Another study of tofogliflozin added to insulin also reported significant decreases in body fat but not in skeletal muscle mass or body fluid after 12 weeks of treatment, implying that weight reduction may have been attributable to fat loss.33 These results suggest that the continuous weight‐reduction effect of tofogliflozin in this study may be explained also by fat loss. Weight gain may worsen insulin resistance,34 which may necessitate higher doses of insulin. In addition, another study showed that each −1% reduction in HbA1c achieved with insulin was estimated to have an association of an approximate 2‐kg increase in weight during a 1‐year period of therapy.35 Under such circumstances, the effect of weight reduction with tofogliflozin plays a prominent role in combination therapy with insulin, especially for patients who require weight control.
The findings of this study require some careful considerations. First, the conclusion may be limited to the population studied and may not be entirely applicable to a worldwide T2DM general population. Second, the change in insulin dose was at the investigator's discretion during the extension phase. Thus, we cannot evaluate the full effect of tofogliflozin on changed insulin doses. Third, baseline characteristics between groups differed in terms of age, BMI and duration of diabetes. Finally, we collected the frequencies of AEs via patient diaries and telephone interviews, which mainly relied on patient discretion, and may have over or under estimated the frequencies of AEs.
In conclusion, tofogliflozin add‐on to insulin treatment induces some AEs typical of the SGLT2 inhibitor class, such as AEs related to volume depletion, excessive urination and genital and urinary tract infections. However, tofogliflozin is generally well tolerated. Furthermore, improved glycaemic control and reduced weight are well sustained for T2DM patients. This study demonstrates the safety and efficacy of tofogliflozin as add‐on to insulin therapy in T2DM patients, offering a new therapeutic solution to diabetes management.
CONFLICT OF INTEREST
Y. T. has received honoraria for speakers bureaus from Astellas Pharma Inc., AstraZeneca K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Company Limited, Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Company Ltd., Merck Sharp & Dohme K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Shionogi & Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Company Limited; and has received grants from Astellas Pharma Inc., AstraZeneca K.K., Bayer Yakuhin, Ltd., Daiichi Sankyo Company Limited, Dainippon Sumitomo Pharma Co., Ltd., Eli Lilly Japan K.K., Kowa Pharmaceutical Company Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Shionogi & Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Company Limited. M. T. and M. S. are employees of Sanofi K.K. R. G. is an employee of Kowa Company, Ltd. K. K. is an advisor to, and has received honoraria for lectures from as well as scholarship grants from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Sumitomo Dainippon Pharma, Fujifilm Pharma, Kissei Pharmaceutical, Kowa, MSD, Novartis Pharma, Ono Pharmaceutical, Sanofi K.K., Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Taisho Toyama Pharmaceutical and Daiichi Sankyo.
Supporting information
Figure S1 Study design. Patients who received tofogliflozin in both the double‐blind and extension phases were referred to as the ‘tofo‐tofo group’, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’. Patients were followed up for 3 days after Week 52.*Telephone interviews that assessed drug adherence and safety information. Abbreviations: FU, follow‐up; V, visit; W, Week.
Figure S2 Patient disposition. Patients who received tofogliflozin in both the double‐blind and the extension phases were referred to as the ‘tofo‐tofo group’, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’.
Figure S3 Plot of mean change in HbA1c from baseline by visit for each regimen. Patients who received tofogliflozin in both the double‐blind and extension phases were referred to as the ‘tofo‐tofo groupt, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’. The graph includes data obtained from the first dose of tofogliflozin or placebo during the entire treatment period or up to introduction of rescue medication, 1 day after the last dose of tofogliflozin or placebo, whichever is the earliest.
Values are given as mean change (%) ± standard error.
Table S1. Incidence rates of hypoglycemia per 100 person‐years (patients) for the tofo‐tofo and pla‐tofo groups.
Table S2. Mean changes in HbA1c, FPG, insulin dose, and body weight from baseline to Week 52 for each insulin regimen
ACKNOWLEDGMENTS
The authors thank all the physicians from 30 institutions who participated in this study. Medical writing assistance and publication support were provided by Clinical Study Support, Inc.; all their activities were funded by Sanofi K.K. and Kowa Company, Ltd.
Author contributions
Y. T. and K. K. contributed to the conception and design of the study, and participated in reviewing and editing the manuscript. M. T., M. S. and R. G. contributed to the design of the study protocol, managed the study and reviewed the manuscript. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Long‐term safety and efficacy of tofogliflozin as add‐on to insulin in patients with type 2 diabetes: Results from a 52‐week, multicentre, randomized, double‐blind, open‐label extension, Phase 4 study in Japan (J‐STEP/INS). Diabetes Obes Metab. 2018;20:1176–1185. https://doi.org/10.1111/dom.13213
Funding information This study was funded by Sanofi K.K. and Kowa Company, Ltd. who were responsible for the design and coordination of the study, monitoring clinical sites, collecting and managing data and performing all statistical analyses.
REFERENCES
- 1. Suzuki M, Honda K, Fukazawa M, et al. Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Pharmacol Exp Ther. 2012;341:692‐701. [DOI] [PubMed] [Google Scholar]
- 2. Poole RM, Prossler JE. Tofogliflozin: first global approval. Drugs. 2014;74:939‐944. [DOI] [PubMed] [Google Scholar]
- 3. Tanizawa Y, Kaku K, Araki E, et al. Long‐term safety and efficacy of tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open‐label, randomized controlled trials. Expert Opin Pharmacother. 2014;15:749‐766. [DOI] [PubMed] [Google Scholar]
- 4. Ikeda S, Takano Y, Cynshi O, et al. A novel and selective sodium‐glucose cotransporter‐2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:984‐993. [DOI] [PubMed] [Google Scholar]
- 5. Wulffelé MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care. 2002;25:2133‐2140. [DOI] [PubMed] [Google Scholar]
- 6. Sato S, Saisho Y, Kou K, et al. Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 diabetes: the EDIT randomized trial. PLoS One. 2015;10:e0121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 7. Approaches to glycemic treatment. Diabetes Care. 2016;39(suppl 1):S52‐S59. [DOI] [PubMed] [Google Scholar]
- 8. Terauchi Y, Tamura M, Senda M, Gunji R, Kaku K. Efficacy and safety of tofogliflozin in Japanese patients with type 2 diabetes mellitus with inadequate glycaemic control on insulin therapy (J‐STEP/INS): results of a 16 week randomized, double‐blind, placebo‐controlled multicentre trial. Diabetes Obes Metab. 2017;19:1397‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14:5‐14. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815‐1823. [DOI] [PubMed] [Google Scholar]
- 11. Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium‐glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403‐411. [DOI] [PubMed] [Google Scholar]
- 12. Araki E, Onishi Y, Asano M, Kim H, Yajima T. Efficacy and safety of dapagliflozin over 1 year as add‐on to insulin therapy in Japanese patients with type 2 diabetes: the DAISY (Dapagliflozin added to patients under InSulin therapY) trial. Diabetes Obes Metab. 2017;19:562‐570. [DOI] [PubMed] [Google Scholar]
- 13. Kim Y, Babu AR. Clinical potential of sodium‐glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes. 2012;5:313‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inagaki N, Harashima S, Maruyama N, Kawaguchi Y, Goda M, Iijima H. Efficacy and safety of canagliflozin in combination with insulin: a double‐blind, randomized, placebo‐controlled study in Japanese patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishihara H, Yamaguchi S, Nakao I, Okitsu A, Asahina S. Efficacy and safety of ipragliflozin as add‐on therapy to insulin in Japanese patients with type 2 diabetes mellitus (IOLITE): a multi‐centre, randomized, placebo‐controlled, double‐blind study. Diabetes Obes Metab. 2016;18:1207‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38:1680‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zinman B, Wanner C, Lachin JM, et al. EMPA‐REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 18. Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev. 2017. Nov;33(8). https://doi.org/10.1002/dmrr.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 21. Food and Drug Administration . FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015. Available at https://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf. Accessed December 4, 2017.
- 22. Food and Drug Administration . FDA drug safety communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. Available at https://www.fda.gov/downloads/Drugs/DrugSafety/UCM475487.pdf. Accessed December 4, 2017.
- 23. Food and Drug Administration . FDA drug safety communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR). 2017. Available at https://www.fda.gov/downloads/Drugs/DrugSafety/UCM558427.pdf. Accessed December 4, 2017.
- 24. Rosenstock J, Jelaska A, Zeller C, et al. EMPA‐REG BASALTM trial investigators. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:936‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S, Dapagliflozin 006 Study Group . Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124‐136. [DOI] [PubMed] [Google Scholar]
- 26. Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fadini GP, Bonora BM, Zatti G, et al. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: a randomized placebo‐controlled trial. Cardiovasc Diabetol. 2017;16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add‐on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154‐1160. [DOI] [PubMed] [Google Scholar]
- 29. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care. 2013;36:2508‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qiu R, Capuano G, Meininger G. Efficacy and safety of twice‐daily treatment with canagliflozin, a sodium glucose co‐transporter 2 inhibitor, added on to metformin monotherapy in patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2014;1:54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suzuki K, Mitsuma Y, Sato T, Anraku T, Hatta M. Comparison of combined Tofogliflozin and glargine, Tofogliflozin added to insulin, and insulin dose‐increase therapy in uncontrolled type 2 diabetes. J Clin Med Res. 2016;8:805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020‐1031. [DOI] [PubMed] [Google Scholar]
- 33. Kato M, Yamashita S, Tsutsui K, Kato N. Tofogliflozin add‐on therapy to insulin for poorly controlled Japanese, obese, type 2 diabetic patients. Prog Med. 2015;35:1067‐1075. [Google Scholar]
- 34. Al‐Goblan AS, Al‐Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yki‐Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758‐767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study design. Patients who received tofogliflozin in both the double‐blind and extension phases were referred to as the ‘tofo‐tofo group’, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’. Patients were followed up for 3 days after Week 52.*Telephone interviews that assessed drug adherence and safety information. Abbreviations: FU, follow‐up; V, visit; W, Week.
Figure S2 Patient disposition. Patients who received tofogliflozin in both the double‐blind and the extension phases were referred to as the ‘tofo‐tofo group’, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’.
Figure S3 Plot of mean change in HbA1c from baseline by visit for each regimen. Patients who received tofogliflozin in both the double‐blind and extension phases were referred to as the ‘tofo‐tofo groupt, and patients who received placebo in the double‐blind phase and tofogliflozin in the extension phase were referred to as the ‘pla‐tofo group’. The graph includes data obtained from the first dose of tofogliflozin or placebo during the entire treatment period or up to introduction of rescue medication, 1 day after the last dose of tofogliflozin or placebo, whichever is the earliest.
Values are given as mean change (%) ± standard error.
Table S1. Incidence rates of hypoglycemia per 100 person‐years (patients) for the tofo‐tofo and pla‐tofo groups.
Table S2. Mean changes in HbA1c, FPG, insulin dose, and body weight from baseline to Week 52 for each insulin regimen


