Abstract
Jellyfish have emerged as a source of next generation collagen that is an attractive alternative to existing sources, such as bovine and porcine, due to a plentiful supply and providing a safer source through lack of bovine spongiform encephalopathy (BSE) transmission risk and potential viral vectors, both of which could be transmitted to humans. Here we compare collagen implantable sponges derived for the first time from the Rhizostoma pulmo jellyfish. A further novelty for the research was that there was a comparison for sponges that were either uncrosslinked or crosslinked using 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide hydrochloride (EDC), and an assessment on how this affected resorption, as well as their biocompatibility compared to bovine type I collagen sponges. The scaffolds were prepared and examined using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) and scanning electron microscopy (SEM). The samples were implanted in adult male Wistar rats for in vivo experimentation. Both crosslinked and uncrosslinked jellyfish collagen sponges showed a significant reduction in histopathology scores over the course of the study, whereas the bovine collagen sponge scores were not significantly reduced. Both jellyfish collagen sponges and the bovine sponge were tolerated well by the hosts, and a recovery was visible in all samples, suggesting that R. pulmo jellyfish‐derived collagen could offer compelling biocompatibility with wound healing applications. We also demonstrate that noncrosslinked samples could be safer with better resorption times than crosslinked samples. © 2017 The Authors Journal of Biomedical Materials Research Part B: Applied Biomaterials Published by Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 1524–1533, 2018.
Keywords: jellyfish collagen, in vivo, regenerative medicine, collagen implantation, biomaterials
INTRODUCTION
Collagens are the main group of structural proteins to be found in the extracellular matrix (ECM) and are the most abundant protein found in animals, providing between 25 and 35% of the whole body protein count. Collagens share the characteristic triple helix structure of Gly‐X‐Y repeats, where X can be any amino acid and Y is often proline or hydroxyproline. The individual chains form a left‐handed helix, and the three chains wind around one another in a right‐handed super helix.1, 2 This structure makes the collagen fibers insoluble with high tensile strength.3 Collagen is a highly versatile biomaterial, which has a wide range of applications, and is particularly suited to a wide range of in vivo applications due to its low immunogenicity.4, 5, 6 These in vivo applications include wound dressings,7 artificial tissue and organ production,8 cartilage tissue regeneration,9, 10 drug delivery systems,11 and biomedical engineering.12
Collagen has been extracted from a range of sources, but is commonly obtained from bovine, porcine, and equine sources for in vivo use.13 However, these sources have problems with bovine spongiform encephalopathy (BSE), other transmittable spongiform encephalopathies (TSEs), and potential viral vectors that could be transmissible to humans.14, 15 More recently, jellyfish have emerged as a source of collagen that is an attractive alternative to existing sources due to a plentiful supply16 and a safer source through lack of BSE risk and potential viral vectors.17
This study aimed at assessing the biocompatibility of two dried jellyfish collagen implantable sponges, which either remained uncrosslinked or were crosslinked using 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide hydrochloride (EDC) and comparing it to the compatibility of control devices, namely a bovine type I collagen sponge implanted for 1, 2, 3, or 4 weeks in rats. EDC has been utilized as an alternative crosslinking agent for collagen to replace glutaraldehyde,18 which causes a number of problems when implanted including calcification of surrounding tissue.19 The study also investigated whether the use of EDC as a collagen crosslinking agent is necessary for adequate wound stability and its impact on sponge efficacy when implanted. To our knowledge this represents the first instance in the field of the testing of native collagen samples derived from R. pulmo being directly compared with bovine type I collagen sponges in vivo as well as investigating the effects of EDC crosslinking on jellyfish collagen performance in vivo. Some species of jellyfish have been previously trailed in vitro and in vivo 17, 20; however, it is important to assess jellyfish collagen on a species basis, and not to take for granted that all jellyfish collagen will behave uniformly. Differing jellyfish species have varying masses, water content, habitat temperature, environments as well as many other factors, and any individual factor could have an impact on the collagen characteristics and the potential immunogenicity of the collagen.21 Due to the makeup of nematocysts, namely their mini collagen fiber content,22, 23, 24 the toxicity of the species should also be considered, and it is for this reason that the R. pulmo jellyfish was used in these experiments, which has been shown to be mildly toxic to cells in vitro,25, 26 but does not cause fatalities in humans unlike the Chironex fleckeri found in waters surrounding Australia,27 and the Chironex yamaguchii recently discovered to have caused numerous deaths in Japan.28
METHODS
SDS PAGE analysis of collagen solution
Jellyfish collagen obtained from R. pulmo was provided by Jellagen, and protein purity was analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE). Briefly, 7.5 µL of 3 ng/mL collagen dissolved in acetic acid at 4°C was mixed with 2.5 µL NuPAGE® LDS Sample Buffer (4X) (Life Technologies, UK). This mixture was then heated at 70°C in an incubator to reduce the collagen.
SDS running buffer was prepared using 50 mL 20X NuPAGE® Tris Acetate SDS Running Buffer added to 950 mL deionized water. NuPAGE® Tris Acetate Mini Gels were loaded into the holding clamp frame (Jencons, UK) and the running buffer added accordingly: 200 mL inner chamber, 600 mL outer chamber. The samples were then loaded alongside repeats of reference Benchmark™ Unstained Protein Ladder (Novex, UK) and the gel ran at 150 V using a concord power pack. Observed current ran from starting 45 mA to final 27 mA. After running, the gel was removed and placed into a tray for staining.
The gel was stained using a Colloidal Blue staining kit (Invitrogen) based on work described by Neuhoff et al.29 The gel was initially stained using a mixture of Stainer A, composed of ammonium sulfate in a phosphoric acid solution, and Stainer B containing the Coomassie Brilliant Blue (CBB) G‐250 Stain create a colloidal suspension. Ultrapure water and methanol was added, dissolving the colloidal suspension, allowing complete diffusion of the dye. This solution was left to shake for a minimum of 3 h and then decanted and replaced with ultrapure water overnight to remove any dye not bound to the protein bands. The stained gels were then imaged and examined using ImageJ software.
Collagen sponge preparation
Freeze drying
The collagen solution was lyophilized to produce implantable collagen sponges for testing. To achieve this, samples were first aliquoted into 5 mL discs in six well plates (Corning, UK) and frozen at −20°C for 24 h, and then transferred to −80°C, as Lyostat analysis of the collagen solutions showed an onset of collapse of −32.5°C (Carried out by Biopharma Technologies, UK). The samples were transferred to a freeze drier (Scanvac CoolSafeTM) for drying. Once pressure was at 100 µbar and shelf temperature was measured at −30°C, the primary drying stage of the process was carried out for 100 h. Following this, shelf temperature was raised to +20°C for 15 h for the secondary drying stage. Samples were then removed from the drier and weighed before being stored in sealed containers with silica pouches at 4°C until use.
When dry, small fragments were examined using scanning electron microscopy (SEM) by coating in a 5 nm layer of chromium before being examined by SEM using a Hitachi S4800 FEG SEM at an acceleration voltage of 1 kV and emission current of 9.8 µA. Micrographs were taken with a magnification of 180× using the lower detector with images being analyzed using ImageJ software.
Crosslinking of implants
In order to produce the crosslinked samples 12 of the dried sponges were crosslinked by immersing them in a 1% solution of EDC (Sigma Aldrich) in ethanol (Fisher Scientific, UK) for 2 h. The crosslinking solution was then removed from the sponges and they were then soaked in water for 24 h. These sponges were cut to provide 48 crosslinked samples and 48 noncrosslinked samples. Control bovine type I collagen samples were purchased as ready‐made sponges from Cell Systems (Germany). These were cut to provide 20 bovine type I control sponge samples. Samples were provided to KWS Biotest (Bristol, UK) who performed the in vivo rat study and provided results for further analysis. All samples were soaked for 24 h in ethanol to sterilize and lyophilized.
Sterilization
To check sterility of the sample solution prior to lyophilization and implantation, samples were taken from stock solutions and from sponges which were redissolved in 0.5 M AcOH and were plated on agar petri dishes using 1 mL of solution at a concentration of 8.25 mg/mL. The plates containing collagen were incubated at either 20 or 37°C to check for microbial growth on the plates over a 24 and 72 h time period. Further to this, 5 mL of sample was prepared as above and was added to 45 mL of maximum recovery diluent (MRD) and incubated for 24 h before plating out in the same manner as before. Any potential growth was detected from these protocols using visual analysis of the presence or absence of colonies. The use of solutions and sponges past this point was carried out in sterile conditions in a Class II hood, including all ethanol soaking sterilization protocols.
In vivo experimental outline
All animal experiments comply with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Thirty‐two adult male Wistar rats were randomly allocated to experimental groups and allowed to acclimatize for 1 week. Collagen sponges were assigned to one of three groups, jellyfish collagen crosslinked sponges (JEL #01) where n = 12, jellyfish collagen noncrosslinked sponges (JEL #02) where n = 12 and control bovine type I collagen (CON) where n = 5. On day 0, each animal was implanted with up to three devices (collagen sponges). Implantation was performed under isoflurane anesthesia. The back skin was clipped then disinfected. A rostro caudal incision was performed in the back skin, starting at least two centimeters from the neck. Subcutaneous pockets were opened by blunt dissection, on each side of the midline. The distance between each device and the midline incision was no less than two centimeters. The distance between each implant on a given side was no less than two centimeters. The position of the implants was randomized between animals. On day 7 (group 1), day 14 (group 2), day 21 (group 3), and day 28 (group 4), animals were culled and the implants, together with the surrounding connective tissues, were dissected out. Gross morphological observations were made and pictures were taken when applicable. Connective tissues were stored in tissue fixative until histopathological analysis. A semiquantitative scoring system was used to evaluate the tissue response and sponge resorption.
Treatment groups and dosages
Animals were split into four experimental groups, representing termination dates in 7‐day intervals. All animals were implanted with sponges on day 0. In each group, three animals were implanted with JEL #01 and JEL #02 implants only, while five animals in each group received all implants (JEL #01, JEL #02, and CON). This is shown in Table 1.
Table 1.
Assignment of Animals to Experimental Groups With Termination Dates. Animals Were Split Into Four Experimental Groups, Differentiated by Termination Date, As Shown in the Table. 8 JEL#01 and JEL#02 Were Present in Each Group, Whereas There Were Only Five CON in Each Group, Meaning Three Animals in Each Group Only Had the Two Jellyfish Collagen Implants
| Group | Experimental Group | Sponge Implantation (Day 0) | Termination |
|---|---|---|---|
| 1 n = 8 | Subchronic 1 week | JEL #01 n = 8, JEL #02 n = 8, CON n = 5 | Day 7 |
| 2 n = 8 | Subchronic 2 weeks | JEL #01 n = 8, JEL #02 n = 8, CON n = 5 | Day 14 |
| 3 n = 8 | Subchronic 3 weeks | JEL #01 n = 8, JEL #02 n = 8, CON n = 5 | Day 21 |
| 4 n = 8 | Subchronic 4 weeks | JEL #01 n = 8, JEL #02 n = 8, CON n = 5 | Day 28 |
Nonspecific clinical observations
From day 0 until the end of the experiment, animals were checked daily for nonspecific clinical signs to include abnormal posture (hunched), abnormal coat condition (piloerection), and abnormal activity levels (reduced or increased activity).
Bodyweights
From day 0 until the end of the experiment, animals were weighed three times per week. Data were graphed (mean ± SEM). Raw and analyzed data are provided.
Gross morphological observations
At necropsy, gross morphological observations were made when applicable. The midline incision was observed as well as the connective tissue surrounding each implant. Gross signs of inflammation such as redness and/or neovascularization are reported. When applicable, representative pictures were taken.
Histopathology
Histopathology examinations were conducted under a double blind study design by an external histopathologist (KWS Biotest, Bristol) without knowledge of the experimental protocol and the differences between the four groups and three sites sampled to avoid bias between jellyfish and control samples.
Samples of residual implant (where identifiable) and surrounding connective tissue were processed, embedded in paraffin wax and sections were prepared. Sections were stained with hematoxylin and eosin (H&E) or with Masson's trichrome stains in order to visualize any cellular infiltrate and structural changes as well as the presence of collagen, respectively. One section per sample was analyzed for each of the stains. Tissue morphology and pathological changes were investigated, which monitored the presence of inflammatory cells (neutrophils, eosinophils, lymphocytes, macrophages, and/or giant cells) which surrounded or were within the implant. Where these were clearly identifiable, fibrous encapsulation, neovascularization, and/or necrosis were recorded. For each sample, a total histopathology score was calculated by adding the inflammation score, the fibrosis score, the necrosis score, and the neovascularization score. Each criterion was scored by a qualified histopathologist, blind to the experimental design, using a semiquantitative scoring system on a five‐point scale: (0) absence, (1) slight, (2) moderate, (3) marked, and (4) severe. The amount and distribution of collagen within the implant was assessed and measured quantitatively by the presence of the bright red staining of collagen sponge within each sample when using H&E stain. Samples were also scored for signs of sponge resorption using the same semiquantitative scale. Data were graphed (mean ± SEM). Raw and analyzed data are provided. Histopathology scores were analyzed by Kruskal‐Wallis test for nonparametric data followed by Dunn's post‐test for multiple comparisons between experimental groups. Total histopathology scores were calculated by adding the inflammation, fibrosis, necrosis and neovascularization scores.
RESULTS
SDS PAGE
SDS PAGE analysis of the collagen samples showed distinctive collagen α1 and α2 bands at ∼105 and ∼92 kDa, respectively; β chain expression as well as the presence of γ chains. These are shown in lanes 7–10 of Figure 1.
Figure 1.
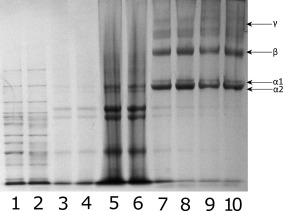
SDS PAGE of collagen extracted from R. pulmo showing collagen bands present in solution. Lanes 1 and 2: Benchmark high molecular weight protein ladder. Lanes 3–6: noncollagenous proteins removed from solution prior to testing. Lanes 7–10: Collagen solution containing α1, α2, β, and γ collagen chains.
The jellyfish derived collagen that was examined using SDS PAGE showed the distinct α1 and α2 chains associated with acid solubilized collagen, as well as β and γ chains, which indicates the presence of both acid soluble collagen monomeric chains, as well as a portion of semisoluble collagen in solution which was not cleaved by digestion or removed when samples were centrifuged prior to testing for SDS PAGE.
Freeze drying
Drying of collagen samples was conducted and residual moisture analysis showed between 2 and 5% in all samples. SEM micrographs of a small section of collagen sponge as shown in Figure 2 demonstrate that collagen sponge structure is assembled into sheets with fibers between the sheets, giving an open pore structure.
Figure 2.
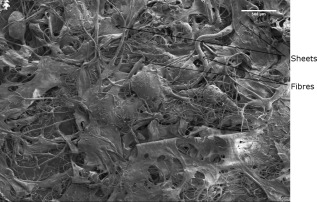
SEM micrograph of the collagen sponge showing a sheet structure with the presence of fibers. Acceleration voltage of 2 kV and emission current of 8.2 µA, magnification 30× taken using a mixture of upper and lower detectors of the Hitachi S4800 FEG SEM.
In vivo experimental results
Nonspecific clinical observations
From day 0 until the end of the experiment, animals were checked daily for nonspecific clinical signs. Animals did not show any nonspecific signs such as abnormal posture (hunched), abnormal coat condition (piloerection), or abnormal activity levels (reduced or increased activity).
Bodyweights
Bodyweight loss was not observed in any of the experimental groups for the duration of the study, as demonstrated in Figure 3 for each group of weeks 1–4.
Figure 3.
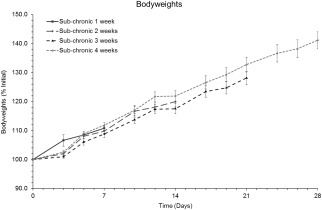
Bodyweights. Data are presented as mean ± SEM of percentages of the initial (day 0) bodyweights.
Gross morphological observations
Prior to implantation, JEL #01 matrices were observed to have a very soft consistency and appeared wet whereas JEL #02 and CON matrices appeared dry.
One week after implantation, swelling was visible under the skin of some of the animals. On day 11 and 12, three animals were culled in order to identify the cause of the swelling. In each of the three animals, a large pocket filled with blood was observed to cause the swelling. The pocket initiated from the area where implants JEL #01 had been inserted. Microbiological analysis of samples taken from these animals showed absence of bacterial contamination. The presence of the blood was attributed to a tissue reaction to the implant and/or to trace amounts of ethanol remaining in the porous matrices. Animals were monitored at weekly intervals for the visible presence of the implants, vascularization or tissue thickening. These observations are outlined in Table 2.
Table 2.
Summary of Gross Morphological Observations, Taken at Weekly Intervals. Data Represents the Number of Specimens Which Displayed Visible Signs of Implant, Vascularization, and Tissue Thickening. Visible Signs of These Decreased Throughout the Experiment in All Three Implants
| Day | Implant | Observations | Occurrence |
|---|---|---|---|
| Day 7 | JEL #01 | Implant still visible | 87.5% (7/8) |
| Vascularization | 62.5% (5/8) | ||
| Tissue thickening | 12.5% (1/8) | ||
| JEL #02 | Implant still visible | 87.5% (7/8) | |
| Vascularization | 75.0% (6/8) | ||
| Tissue thickening | 25.0% (2/8) | ||
| Control | Implant still visible | 100% (5/5) | |
| Vascularization | 60.0% (3/5) | ||
| Tissue thickening | 20.0% (1/5) | ||
| Day 14 | JEL #01 | Implant still visible | 60.0% (3/5) |
| Vascularization | 20.0% (1/5) | ||
| Tissue thickening | 20.0% (1/5) | ||
| JEL #02 | Implant still visible | 60.0% (3/5) | |
| Vascularization | 20.0% (1/5) | ||
| Tissue thickening | 20.0% (1/5) | ||
| Control | Implant still visible | 60.0% (3/5) | |
| Vascularization | 0% (0/5) | ||
| Tissue thickening | 40.0% (2/5) | ||
| Day 21 | JEL #01 | Implant still visible | 37.5% (3/8) |
| Vascularization | 12.5% (1/8) | ||
| Tissue thickening | 25.0% (2/8) | ||
| JEL #02 | Implant still visible | 12.5% (1/8) | |
| Vascularization | 50.0% (4/8) | ||
| Tissue thickening | 25.0% (2/8) | ||
| Control | Implant still visible | 20.0% (1/5) | |
| Vascularization | 60.0% (3/5) | ||
| Tissue thickening | 20.0% (1/5) | ||
| Day 28 | JEL #01 | Implant still visible | 0% (0/8) |
| Vascularization | 12.5% (1/8) | ||
| Tissue thickening | 0% (0/8) | ||
| JEL #02 | Implant still visible | 0% (0/8) | |
| Vascularization | 0% (0/8) | ||
| Tissue thickening | 0% (0/8) | ||
| Control | Implant still visible | 20.0% (1/5) | |
| Vascularization | 20.0% (1/5) | ||
| Tissue thickening | 0% (0/5) |
Histopathology
Many of the sections lacked any evidence of the presence of the collagen implant, which has a distinctive bright red appearance when H&E stain is used, but this could have been because the area of tissue containing any remaining sponge was not sampled.
The least resorption of the collagen implants was seen in group 1 on day 7, where what appeared to be an entire sponge was often present within a cystic cavity lined by granulomatous inflammation and granulation tissue. This is shown in Figure 4.
Figure 4.
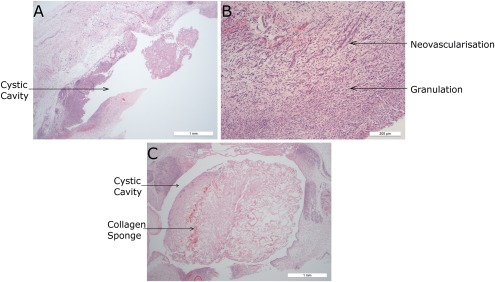
Histology sections from group 1 (HE stains). (a) JEL #01 cystic cavity containing portion of collagen sponge and lined by inflammation and fibrosis. (b) JEL #02 area of granulation with prominent neovascularization. (c) CON collagen sponge within a cystic cavity surrounded by circumferential granulomatous inflammation and fibrosis.
In group 2, on day 14, there was greater evidence of sponge resorption, with areas of residual sponge infiltrated by and surrounded by macrophages and multinucleate giant cells. JEL #02 appeared to show cystic cavities surrounding the collagen sponge in some animals, whereas this was not present in observed JEL #01 and CON samples. This is shown in Figure 5.
Figure 5.
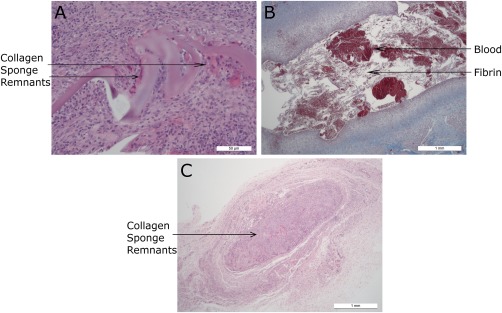
Histology sections from group 2. (a) JEL #01 (HE stain) focus of granulomatous inflammation and fibrosis centered on remnants of collagen sponge. (b) JEL #02 (Masson's stain) cystic cavity filled with blood and fibrin and lined by granulomatous inflammation and granulation tissue. (c) CON (HE stain): Discrete granulomatous focus centered on residual fragments of collagen sponge.
Only one animal in group 3 (on day 21) had evidence of resorbing the collagen sponge, but other animals in this group had evidence of subcutaneous hemorrhage and resorption of blood by hemosiderophages. A CON sample was sectioned showing a blood filled cystic cavity present surrounding the sponge. These results are shown in Figure 6. JEL #02 sections from this group were found to not contain any of the collagen sponges and so they are not shown.
Figure 6.
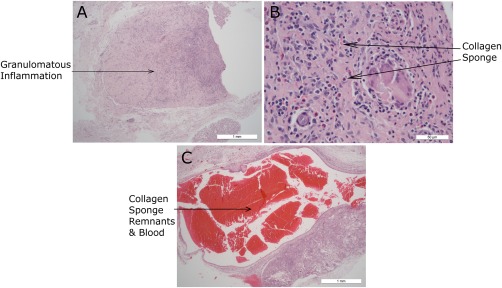
Histology sections from group 3 (HE stain). (a) JEL #01 focus of granulomatous inflammation and fibrosis. Fragments of collagen sponge are adjacent to the focus (b) JEL #01 granulomatous inflammation with a cluster of giant cells surrounding foreign material. Eosinophils and lymphoplasmocytic cells are scattered throughout. (c) CON blood filled cystic cavity with narrow lining adjacent to discrete granulomatous focus centered on remnants of collagen sponge.
Some animals in group 4 (on day 28) had even greater resorption of sponge with relatively small residual foci of inflammation and sponge remnants—but a greater number of animals in this group had no changes in the sampled tissues at all. This is shown in Figure 7.
Figure 7.
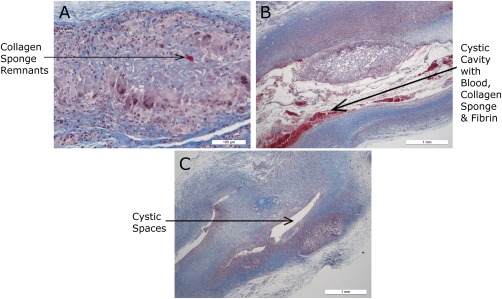
Histology sections from group 4 (Masson's stain). (a) JEL #01 very small discrete focus within subcutis containing multinucleate giant cells surrounding residual fragments of collagen sponge. (b) JEL #02 cystic cavity containing a fragment of residual collagen sponge, blood, and fibrin; surrounded by granulomatous inflammation and fibrosis. (c) CON small remnant of collagen sponge imbedded in a diffuse region of inflammation and fibrosis with cystic spaces.
Histopathology scores
There was no significant difference between experimental groups: the histopathology scores observed around JEL #01, JEL #02, and CON implants did not differ on a given day. The histopathology scores observed around the implants decreased with time. The difference in scores between day 7 and day 28 was statistically significant for JEL #01 and JEL #02 implants (p < 0.05). This is shown in Figure 8.
Figure 8.
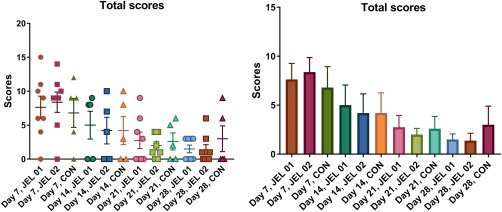
Total histopathology scores: (a) Scatterplot. Horizontal bars represent mean; vertical bars represent SEM. (b) Total histopathology scores presented as mean ± SEM. Total scores reduced over time, with JEL #01 and JEL #02 showing a significant difference in scores between day 7 and day 28 (p < 0.05).
Inflammation scores did not differ between experimental groups on a given experimental day or between experimental days for a given experimental group. Fibrosis scores did not differ between experimental groups on a given experimental day or between experimental days for a given experimental group. Necrosis scores were low on day 7 and returning to zero by day 14 for the JEL #01 and CON groups, and by day 21 for the JEL #02 group. Scores did not differ between experimental groups on a given experimental day or between experimental days for a given experimental group. This is shown in Figure 9.
Figure 9.
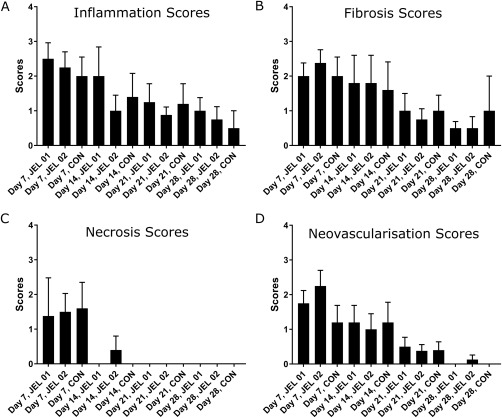
Mean inflammation, fibrosis, necrosis, and neovascularization scores ±SEM. (a) Inflammation scores in all three groups are significantly lower at day 28 than day 7 (p < 0.05). There were no differences between the experimental groups. (b) Fibrosis scores in JEL #01 and JEL #02 are significantly lower at day 28 than day 7 (p < 0.05). No significant difference is shown in the control group. (c) Necrosis scores in all JEL #01 and control groups are zero by day 14, however JEL #02 still has some necrosis present. (d) Neovascularization scores in all three groups are significantly lower at day 28 than day 7 (p < 0.05). There were no significant differences between the experimental groups.
Statistical methods
All graphs use ± standard error of the mean to indicate statistical significance. Histopathology scores were analyzed by Kruskal–Wallis test for nonparametric data followed by the Dunn's post‐test for multiple comparisons with a p values of <0.05 being considered statistically significant.
DISCUSSION AND CONCLUSIONS
Discussion
Subcutaneous implantation of JEL #01 or JEL #02 collagen sponges induced histopathological changes including inflammation, fibrosis, necrosis, and neovascularization. The severity of the histopathological changes was greater on the earliest time point studied (day 7). The severity of the histopathological changes decreased as soon as day 14 and kept decreasing until the end of the experiment on day 28. Histological changes throughout the experiment were very similar throughout the three test groups, with inflammation and fibrosis being visible throughout the time period, with both JEL #01 and JEL #02 both showing significant decreases throughout, whereas CON did not display a significant drop in fibrosis. All samples displayed no necrosis by the end of the study, and only JEL #02 showed any neovascularization at day 28, however all three groups were significantly lower.
These results show good acceptance of the jellyfish collagen implants by the host. Lack of necrosis shows that there are no toxins or infection that is causing cell death, while fibrosis and neovascularization are indicative of healing30 and inflammation is dropping throughout the study, as the foreign body is broken down and reabsorbed. The significant reduction in both JEL #01 and JEL #02 histopathology scores between weeks 4 and 1 suggest good bioequivalence and biocompatibility. These histopathological results are generally in line with similar studies conducted. Granulation and fibrosis was visible in studies conducted by Anselme et al.,31 and Alpaslan et al.,32 at around day 7. At around 30 days, both studies observed a sizeable reduction in the collagen present and fibrous tissue surrounding the remaining fragments. Both of these studies, however, continued past day 30 and ended at day 4532 and after 3 months.31 The trend of collagen reduction continued over these timeframes and it seems likely that this would be the case for jellyfish collagen.
The changes induced by the implants did not differ significantly in severity from the changes induced by control bovine collagen matrices. This is encouraging, as bovine collagen is well known to be of low antigenicity and tolerated well, and it is incorporated into a wide range of medical applications and devices13, 33, 34 and means that the jellyfish collagen implants are showing suitability for the same medical uses.
The jellyfish derived collagen that was examined using SDS PAGE showed the distinct α1 and α2 chains associated with acid solubilized collagen, as well as β and γ chains, which indicates the presence of a portion of semi‐insoluble collagen in suspension. This may in part be why the non‐crosslinked collagen was able to remain structurally complete during and following implantation. The banding pattern observed in Figure 1 is supported by other extracted marine collagens such as the skin of striped catfish, which also display clear α1, α2, β, and γ band patterns in equivalent locations.35 This conforms to both mammalian collagens and collagens from cartilaginous fish, which display the same banding pattern at similar molecular weights.36, 37
This study was carried out using sponges which were produced and stored using lab based techniques and settings, involving creating sponges in nonsterile conditions and then using an ethanol sterilization step. This led to trace ethanol being present in some implants when implanted, and caused large pockets of swelling to be present on some animals. Samples were tested for sterility before implantation and found not to be infected, so this can be confidently ruled out as the cause of the swelling pockets. Three animals were culled on days 11 and 12 to investigate this swelling, and it was found to be a large pocket of blood which was attributed to trace ethanol being present in the samples. To improve on this, it would be recommended for future sample preparation to be carried out with more rigorous focus on sterilization of the samples prior to implantation, using a technique such as filtration, gamma irradiation or E beam technologies. It is also acknowledged that this study used a small test population, which was further reduced by the need to cull three animals early, and that the number of control samples was not equal to jellyfish collagen samples.
Jellyfish collagen extracted from Rhopilema asamushi has been shown to denature at temperatures above 28.8°C,38 and collagen from R. pulmo denatures at 28.9°C which can be raised to 33°C by crosslinking with EDC.39 Crosslinking of collagen sponges, including the use of EDC has been shown to induce a pro inflammatory response, including macrophage activation and an increase of pro inflammatory cytokine release, despite a lack in understanding as to the mechanisms in which this occurs.40 The JEL #01 samples which remained uncrosslinked served to demonstrate that despite this temperature instability, no significant disadvantage was shown to exist in this study, and that they could be more biocompatible and better suited for in vivo applications due to their enhanced ability to be absorbed and integrated with the surrounding tissue. To improve observation of minor factors such as this, and to determine whether the presence of the semi‐insoluble collagen affected the stability of the uncrosslinked sponges, a higher test population should be used to improve statistical analysis, and more quantitative biological and genetic testing could be carried out on the surrounding tissues after treatment has occurred. Alternative crosslinking reagents/methods could also be used to optimize mechanical properties while maintaining good biocompatibility.
Research on the in vivo immunological effects of collagen extracted from jellyfish has previously been carried out, with data suggesting that jellyfish collagen stimulates the production of Ig by mouse lymphocytes from the spleen and Peyer's patch.41 Unfortunately, this group obtained collagen by heating the jellyfish extract to 121°C which would denature any native collagen into gelatin and give incomparable data to native extracted collagen used in this study. Available literature on pure, native collagen as used in this study is relatively sparse, however one of the few studies that can be compared against is Song et al.17 who found that jellyfish collagen sponges produced an immune response comparable to bovine collagen and gelatin. The results from our study support this finding, and further also show that both jellyfish collagen samples showed a significant reduction in histopathology scores whereas the bovine collagen did not, suggesting that perhaps jellyfish collagen was tolerated slightly better. Further research into the immunological pathways which are upregulated in the presence of R. pulmo collagen should be conducted. Song et al.17 did not compare between crosslinked and un crosslinked samples, which we demonstrate can play a large part in the degree of resorption, as well as the risks of the formation of cystic cavities, which could be dangerous to certain individuals.
Conclusions
In summary, both jellyfish and bovine collagen samples were accepted by the hosts, and did not appear to induce any severe immunogenic response. As such, a recovery was visible in all animals throughout the experiment. Jellyfish collagen samples JEL #01 and JEL #02 displayed a significantly lower histopathology score from start to end of the study, whereas the bovine collagen control did not. Collagen implantable sponges were derived for the first time from the R. pulmo jellyfish and showed successful incorporation in the host. Comparison was made for sponges that were either uncrosslinked or crosslinked using EDC, and demonstrated for the first time that R. pulmo collagen showed good resorption and biocompatibility in both crosslinked and uncrosslinked states. In conclusion, this suggests that jellyfish collagen is a promising biomaterial for medical applications, which is tolerated at least equally as well as bovine collagen.
ACKNOWLEDGMENTS
The work carried out in this manuscript was partially funded by Innovate UK (project number 710665), who had no involvement in study design, data collection, analysis or interpretation, or in either the writing or decision to submit the article for publication. On declaration of interest, JPW, AJP, and AMS are employed by Jellagen Pty Ltd which produces collagen extracted from jellyfish. No co‐authors have any financial interest related to the contents of this manuscript as the prototype device is not being produced for sale.
How to cite this article: Widdowson JP, Picton AJ, Vince V, Wright CJ, Mearns‐Spragg A. 2018. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J Biomed Mater Res Part B 2018:106B:1524–1533.
REFERENCES
- 1. Gorgieva S, Kokol V. Collagen‐vs. gelatin‐based biomaterials and their biocompatibility: Review and perspectives. Biomater Appl Nanomedicine 2011;17–51. [Google Scholar]
- 2. Myllyharju J, Kivirikko KI. Collagens and collagen‐related diseases. Ann Med 2001;33:7–21. [DOI] [PubMed] [Google Scholar]
- 3. Gelse K, Pöschl E, Aigner T. Collagens—Structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531–1546. [DOI] [PubMed] [Google Scholar]
- 4. Maeda M, Tani S, Sano A, Fujioka K. Microstructure and release characteristics of the minipellet, a collagen‐based drug delivery system for controlled release of protein drugs. J Control Release 1999;62:313–324. [DOI] [PubMed] [Google Scholar]
- 5. Sinha VR, Trehan A. Biodegradable microspheres for protein delivery. J Control Release 2003;90:261–280. [DOI] [PubMed] [Google Scholar]
- 6. Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B Appl Biomater 2004;71:343–354. [DOI] [PubMed] [Google Scholar]
- 7. Nehrer S, Breinan HA, Ramappa A, Young G, Shortkroff S, Louie LK, Sledge CB, Yannas IV, Spector M. Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials 1997;18:769–776. [DOI] [PubMed] [Google Scholar]
- 8. Kubota, Y , Kleinman, HK , Marin, GR , Lawley TJ, Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary like structures. J Cell Biol. 1988;107:1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoyer B, Bernhardt A, Lode A, Heinemann S, Sewing J, Klinger M, Notbohm H, Gelinsky M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater 2014;10:883–892. [DOI] [PubMed] [Google Scholar]
- 10. Sewing J, Klinger M, Notbohm H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two‐ and three‐dimensional collagen matrices. J Tissue Eng Regen Med. 2015;11:916–925. [DOI] [PubMed] [Google Scholar]
- 11. Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev 2003;55:1631–1649. [DOI] [PubMed] [Google Scholar]
- 12. Derkus B, Arslan YE, Bayrac AT, Kantarcioglu I, Emregul KC, Emregul E. Development of a novel aptasensor using jellyfish collagen as matrix and thrombin detection in blood samples obtained from patients with various neurodisease. Sensors Actuators, B Chem 2016;228:725–736. [Google Scholar]
- 13. Silvipriya KS, Krishna Kumar K, Bhat AR, Dinesh Kumar B, John A, Lakshmanan P. Collagen: Animal sources and biomedical application. J Appl Pharm Sci 2015;5:123–127. [Google Scholar]
- 14. Asher DM. The transmissible spongiform encephalopathy agents: Concerns and responses of United States regulatory agencies in maintaining the safety of biologics. Dev Biol Stand 1999;100:103–118. [PubMed] [Google Scholar]
- 15. Lupi O. Prions in dermatology. J Am Acad Dermatol 2002;46:790–793. [DOI] [PubMed] [Google Scholar]
- 16. W Jennah. Are jellyfish taking over the world? J Aquac Mar Biol 2015;2:1–13. [Google Scholar]
- 17. Song E, Yeon Kim S, Chun T, Byun HJ, Lee YM. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006;27:2951–2961. [DOI] [PubMed] [Google Scholar]
- 18. Barnes CP, Pemble CW, Brand DD, Simpson DG, Bowlin GL. Cross‐linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng 2007;13:1593–1605. [DOI] [PubMed] [Google Scholar]
- 19. Jayakrishnan A, Jameela SR. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 1996;17:471–484. [DOI] [PubMed] [Google Scholar]
- 20. Sun X‐D, Yan H, Shan C, Ding S‐S, Li C, Dong J, Cheng X, Qian W‐P. Biomimetic double‐layered scaffolds composed of jellyfish collagen and chitosan for cartilage tissue engineering. J Biomater Tissue Eng 2014;4:1080–1806. [Google Scholar]
- 21. Peggy Hsieh Y‐H, Leong F‐M, Rudloe J. Jellyfish as food. Hydrobiologia 2001;451:11–17. [Google Scholar]
- 22. Nüchter T, Benoit M, Engel U, Özbek S, Holstein T. Nanosecond‐scale kinetics of nematocyst discharge. Curr Biol 2006;16:R316–R318. [DOI] [PubMed] [Google Scholar]
- 23. Bloom DA, Burnett JW, Alderslade P. Partial purification of box jellyfish (Chironex fleckeri) nematocyst venom isolated at the beachside. Toxicon 1998;36:1075–1085. [DOI] [PubMed] [Google Scholar]
- 24. Blanquet R, Lenhoff HM. A disulfide‐linked collagenous protein of nematocyst capsules. Science 1966;154:152–153. [DOI] [PubMed] [Google Scholar]
- 25. Allavena A, Mariottini G, Carli A, Contini S, Martelli A. In vitro evaluation of the cytotoxic, hemolytic and clastogenic activities of Rhizostoma pulmo toxin(s). Toxicon 1998;36:933–936. [DOI] [PubMed] [Google Scholar]
- 26. Carli A, Bussotti S, Mariottini GL, Robbiano L. Toxicity of jellyfish and sea‐anemone venoms on cultured V79 cells. Toxicon 1996;34:496–500. [DOI] [PubMed] [Google Scholar]
- 27. Yanagihara AA, Shohet RV. Cubozoan venom‐induced cardiovascular collapse is caused by hyperkalemia and prevented by zinc gluconate in mice. PLoS One 2012;7:e51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis C, Bentlage B. Clarifying the identity of the Japanese Habu‐kurage, Chironex yamaguchii, sp nov (Cnidaria: Cubozoa: Chirodropida). Zootaxa 2009;2030:59–65. [Google Scholar]
- 29. Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G‐250 and R‐250. Electrophoresis 1988;9:255–262. [DOI] [PubMed] [Google Scholar]
- 30. Witte MB, Barbul A. General principles of wound healing. Surg Clin North Am 1997;77:509–528. [DOI] [PubMed] [Google Scholar]
- 31. Anselme K, Bacques C, Charriere G, Hartmann DJ, Herbage D, Garrone R. Tissue reaction to subcutaneous implantation of a collagen sponge. A histological, ultrastructural, and immunological study. J Biomed Mater Res 1990;24:689–703. [DOI] [PubMed] [Google Scholar]
- 32. Alpaslan C, Alpaslan GH, Oygur T. Tissue reaction to three subcutaneously implanted local hemostatic agents. Br J Oral Maxillofac Surg 1997;35:129–132. [DOI] [PubMed] [Google Scholar]
- 33. Holmes DF, Gilpin CJ, Baldock C, Ziese U, Koster AJ, Kadler KE. Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization. Proc Natl Acad Sci U S A 2001;98:7307–7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahmanian‐Schwarz A, Held M, Knoeller T, Stachon S, Schmidt T, Schaller HE, Just L. In vivo biocompatibility and biodegradation of a novel thin and mechanically stable collagen scaffold. J Biomed Mater Res Part A 2014;102:1173–1179. [DOI] [PubMed] [Google Scholar]
- 35. Singh P, Benjakul S, Maqsood S, Kishimura H. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem 2011;124:97–105. [Google Scholar]
- 36. Zhang Z, Li G, Shi B. Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. J Soc Leather Technol Chem 2006;90:23–28. [Google Scholar]
- 37. Kittiphattanabawon P, Benjakul S, Visessanguan W, Kishimura H, Shahidi F. Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem 2010;119:1519–1526. [Google Scholar]
- 38. Nagai T, Worawattanamateekul W, Suzuki N, Nakamura T, Ito T, Fujiki K, Nakao M, Yano T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem 2000;70:205–208. [Google Scholar]
- 39. Addad S, Exposito JY, Faye C, Ricard‐Blum S, Lethias C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar Drugs 2011;9:967–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delgado LM, Bayon Y, Pandit A, Zeugolis DI. To cross‐link or not to cross‐link? Cross‐linking associated foreign body response of collagen‐based devices. Tissue Eng Part B Rev 2015;21:298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morishige H, Sugahara T, Nishimoto S, Muranaka A, Ohno F, Shiraishi R, Doi M. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology 2011;63:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]


