Abstract
Patients with high socioeconomic status (SES) have better cancer outcomes than patients with low SES. This has also been shown in Sweden, a country with tax‐financed health care aiming to provide care on equal terms to all residents. The association between income and educational level and diagnostics and treatment as outlined in national guidelines and prostate cancer (Pca) and all‐cause mortality was assessed in 74,643 men by use of data in the National Prostate Cancer Register of Sweden and a number of other health care registers and demographic databases. In multivariable logistic regression analysis, men with high income had higher probability of Pca detected in a health‐check‐up, top versus bottom income quartile, odds ratio (OR) 1.60 (95% CI 1.45–1.77) and lower probability of waiting more than 3 months for prostatectomy, OR 0.77 (0.69–0.86). Men with the highest incomes also had higher probability of curative treatment for intermediate and high‐risk cancer, OR 1.77 (1.61–1.95) and lower risk of positive margins, (incomplete resection) at prostatectomy, OR 0.80 (0.71–0.90). Similar, but weaker associations were observed for educational level. At 6 years of follow‐up, Pca mortality was modestly lower for men with high income, which was statistically significant for localized high‐risk and metastatic Pca in men with no comorbidities. All‐cause mortality was less than half in top versus bottom quartile of income (12% vs. 30%, p < 0.001) among men above age 65. Our findings underscore the importance of adherence to guidelines to ensure optimal and equal care for all patients diagnosed with cancer.
Keywords: prostate cancer, clinical cancer register, socioeconomic status
Short abstract
What's new?
Even in Sweden, with publicly financed health care, higher socioeconomic status gives people a leg up when it comes to surviving cancer. Why? Here, the authors attempt to tease out associations between income, diagnosis, treatment and mortality. Men with higher incomes, they found, were more likely to have PCa detected in a routine checkup, had shorter wait times for prostatectomy and survived more often. Some of this can be attributed to better general health among wealthier men, but high SES men were also more likely to receive treatment even when the cancer was high risk, and guidelines did not recommend curative treatment.
In many countries, cancer outcomes are better in women and men with high compared to low socioeconomic status (SES).1, 2 This association has also been observed in Sweden, a country with a tax‐financed national health care system aiming to provide care on equal terms to all residents.3, 4, 5
In a previous study from our group, men with high SES were more likely to receive radical prostatectomy for high‐risk prostate cancer (Pca), and had lower Pca mortality compared to men with low SES with the same severity of disease.6 However, to date, there are little data on associations between SES and other aspects of Pca care, including waiting times, patterns of work‐up, diagnostics and treatment as well as outcome.7, 8
The aim of this study was to examine associations between indicators of SES and quality indicators of Pca management, and Pca and all‐cause mortality by use of data in a nationwide, population‐based cohort in Sweden.
Materials and methods
Study population
We conducted a cohort study of men with Pca diagnosed between 2007 and 2014 and registered in the National Prostate Cancer Register of Sweden (NPCR).9, 10, 11 NPCR captures 98% of all incident Pca cases in Sweden since 1998 compared to the Swedish Cancer Register to which reporting is mandated by law. NPCR contains detailed information on primary treatment and cancer characteristics, including a modified version of the National Comprehensive Cancer Network (NCCN) Pca risk categories9: low‐risk (local clinical stage T1–2, Prostate‐specific antigen (PSA) <10 ng/mL and, Gleason score (GS) ≤6), intermediate‐risk (T1–2, Gleason score 7 and/or PSA 10 to <20 ng/ml), high‐risk (T3 and/or Gleason score 8–10 and/or PSA 20 to <50 ng/ml), regionally metastatic (T4 and/or N1 and/or PSA 50 to <100 ng/ml in the absence of distant metastases (M0 or Mx)) and distant metastases (PSA ≥100 ng/mL or M1). Further risk categories that we applied in specific analyses were very low‐risk (age <75, cT1, Gleason score (GS) ≤6, PSA <10 ng/ml, PSA density <0.15, number of biopsy cores positive for cancer ≤4, cancer extension at biopsy <8 mm) and very high‐risk (T4, 50 ≤ PSA < 200 ng/ml, any N, M0).9 In May 2016, NPCR was linked to the Prescribed Drug Registry, the Patient Registry, the Cause of Death Register, the Longitudinal database on socioeconomic factors (acronym in Swedish LISA) and the Register of Total Population and Population changes to generate Prostate Cancer data Base Sweden (PCBaSe) RAPID. As previously described, Charlson Comorbidity Index (CCI) was calculated based on discharge diagnoses in the Patient Registry by use of 17 groups of diseases with each diagnosis assigned a specific weight (1, 2, 3 and 6) and the sum of these weights resulted in three levels of CCI: 0 for no comorbidity, 1–2 for mild to moderate, and 3+ for severe comorbidity.12, 13
Information on educational level, annual disposable income, and marital status was retrieved from the LISA database. Men were categorized according to the highest attained level of education, into low (<10 years mandatory school), intermediate (10–12 years high school), and high (university or college). Annual disposable income was defined as personal income reported in the year preceding the Pca diagnosis, and assessed in quartiles. Marital status was also retrieved from the year before Pca diagnosis. A number of end‐points associated with quality of care were used including adherence to recommendations in the Swedish National guidelines for Pca issued in 2007 regarding treatment selection, execution of treatment, and Pca and all‐cause mortality was also assessed by use of data in The Cause of Death Registry.
Statistical analyses
Logistic regression models were used to estimate univariable and multivariable odds ratios (OR) and 95% confidence intervals (CI) for the events of interest. Adjustments were made for age at diagnosis, calendar year of diagnosis, CCI, region of residence and Pca risk category. In addition, cumulative incidence of Pca mortality and all‐cause mortality was calculated as competing risk stratified for age (below or above 65 years), CCI (0, ≥1) and education level and income. Men were at risk from diagnosis until death or until end of follow‐up at December 31, 2014, whatever event that occurred first. Gray's test was used for comparing differences in mortality according to SES.
Results
Baseline characteristics
Of the 74,643 men in the study, a majority were below age 70 at date of diagnosis (54%), had CCI 0, that is, no known comorbid conditions (74%) and had a low or intermediate‐risk Pca (58%) (Table 1). Men in the highest income quartile had a higher proportion of low‐risk Pca compared to men in the lowest quartile of income (39% vs. 18%). Conversely, distant metastases were less common in men with highest income (6%) compared to men with lowest income (19%). In the full study group, educational level was low for 35% of the men, intermediate for 39%, and high for 25%, and 66% of men were married at the time of diagnosis. Median follow‐up was 3.3 years [IQR 1,5–5.3] and 101 men (<1%) were lost to follow‐up. Similar patterns were found according to educational level (Supporting Information, Table 1).
Table 1.
Baseline characteristics of the study cohort in Prostate Cancer data Base Sweden RAPID stratified by quartiles of disposable income
| All men* | Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|---|
| n = 74,643 | n = 18,660 | n = 18,659 | n = 18,659 | n = 18,659 | |
| Year of diagnosis | |||||
| 2007–2008 | 17,319 (23) | 5,858 (31) | 3,924 (21) | 4,164 (22) | 3,372 (18) |
| 2009–2011 | 29,439 (39) | 7,749 (42) | 7,455 (40) | 7,277 (39) | 6,954 (37) |
| 2012–2014 | 27,885 (37) | 5,053 (27) | 7,280 (39) | 7,218 (39) | 8,333 (45) |
| Age | |||||
| <65 years | 22,703 (30) | 2,877 (15) | 2,491 (13) | 7,766 (42) | 9,568 (51) |
| 65–69 years | 17,679 (24) | 3,148 (17) | 4,293 (23) | 4,936 (26) | 5,299 (28) |
| 70–74 years | 13,542 (18) | 3,935 (21) | 4,639 (25) | 2,889 (15) | 2,077 (11) |
| 75+ years | 20,719 (28) | 8,700 (47) | 7,236 (39) | 3,068 (16) | 1,715 (9) |
| Charlson comorbidity index | |||||
| 0 | 54,956 (74) | 11,873 (64) | 12,616 (68) | 14,734 (79) | 15,727 (84) |
| 1 | 9,175 (12) | 3,104 (17) | 2,756 (15) | 1,913 (10) | 1,402 (8) |
| 2+ | 10,512 (14) | 3,683 (20) | 3,287 (18) | 2,012 (11) | 1,530 (8) |
| Risk category | |||||
| Low risk | 20,838 (28) | 3,358 (18) | 4,073 (22) | 6,122 (33) | 7,282 (39) |
| Intermediate risk | 22,528 (30) | 4,659 (25) | 5,385 (29) | 6,072 (33) | 6,411 (34) |
| High risk | 16,186 (22) | 5,078 (27) | 4,839 (26) | 3,560 (19) | 2,708 (15) |
| Regionally metastatic | 4,313 (6) | 1,592 (9) | 1,229 (7) | 866 (5) | 626 (3) |
| Distant metastases | 8,944 (12) | 3,489 (19) | 2,613 (14) | 1,637 (9) | 1,204 (6) |
| Missing | 1,834 (2) | 484 (3) | 520 (3) | 402 (2) | 428 (2) |
| Primary treatment | |||||
| Radical prostatectomy | 21,218 (28) | 2,741 (15) | 3,562 (19) | 6,544 (35) | 8,366 (45) |
| Radiotherapy | 11,516 (15) | 2,428 (13) | 2,963 (16) | 3,419 (18) | 2,706 (15) |
| Active surveillance | 12,773 (17) | 2,074 (11) | 2,712 (15) | 3,660 (20) | 4,326 (23) |
| Watchful waiting | 8,594 (12) | 2,957 (16) | 2,882 (15) | 1,648 (9) | 1,107 (6) |
| Antiandrogens | 5,385 (7) | 1,923 (10) | 1,892 (10) | 964 (5) | 606 (3) |
| GnRH agonists | 15,157 (20) | 6,537 (35) | 4,648 (25) | 2,424 (13) | 1,548 (8) |
| Reason for PCa diagnosis | |||||
| Health check‐up | 32,231 (43) | 5,589 (30) | 6,844 (37) | 9,033 (48) | 10,762 (58) |
| LUTS | 26,092 (35) | 8,053 (43) | 7,414 (40) | 5,951 (32) | 4,673 (25) |
| Symptoms | 13,993 (19) | 4,484 (24) | 3,842 (21) | 3,105 (17) | 2,561 (14) |
| Missing data | 2,257 (3) | 518 (3) | 551 (3) | 551 (3) | 636 (3) |
| Education level | |||||
| Low | 26,146 (35) | 10,527 (56) | 7,836 (42) | 5,017 (27) | 2,766 (15) |
| Intermediate | 29,112 (39) | 6,104 (33) | 7,666 (41) | 8,407 (45) | 6,935 (37) |
| High | 18,771 (25) | 1,614 (9) | 3,081 (17) | 5,187 (28) | 8,889 (48) |
| Missing data | 614 (1) | 415 (2) | 76 (0) | 48 (0) | 69 (0) |
| Marital status | |||||
| Never married | 8,334 (11) | 2,739 (15) | 1,767 (9) | 2,161 (12) | 1,667 (9) |
| Divorced/Widower | 17,159 (23) | 4,897 (26) | 5,034 (27) | 3,863 (21) | 3,365 (18) |
| Married | 49,142 (66) | 11,023 (59) | 11,858 (64) | 12,635 (68) | 13,626 (73) |
| Missing data | 8 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) |
Abbreviation: GnRH: gonadotropin releasing hormone agonist, LUTS: lower urinary tract symptoms. Quartile of disposable income: Q1 lowest – Q4 highest. Risk categories: low‐risk (T1–2, Prostate‐specific antigen (PSA) <10 ng/mL and Gleason score (GS) ≤6), intermediate‐risk (T1–2, Gleason score 7 and/or PSA 10 to <20 ng/ml), high‐risk (T3 and/or Gleason score 8–10 and/or PSA 20 to <50ng/ml), regionally metastatic (T4 and/or N1 and/or PSA 50 to <100ng/ml in the absence of distant metastases (M0 or Mx)), and distant metastases (PSA ≥100 ng/mL or M1).
p values in Chi square test < 0.001 for all variables.
*6 (<1%) men have missing income data.
Men in the highest income quartile were more likely to receive a Pca diagnosis after PSA testing as a part of a health check‐up than men in the lowest quartile, odds ratio (OR) 1.60 (95% CI = 1.45–1.77), less likely to receive a diagnosis of advanced Pca (OR 0.57; 0.54–0.60), of waiting >3 months to radical prostatectomy (OR 0.77; 0.69–0.86), and of having a waiting time to start of radiotherapy exceeding the median (OR 0.81; 0.68–0.96) (Fig. 1 a). However, men with the highest incomes and very low‐risk Pca were not more likely to receive active surveillance, the recommended treatment for very low‐risk Pca, than men with lowest incomes (Fig. 1 b). In contrast, men with high education were more likely to receive active surveillance than men with low education, OR 1.28 (1.06–1.55).
Figure 1.
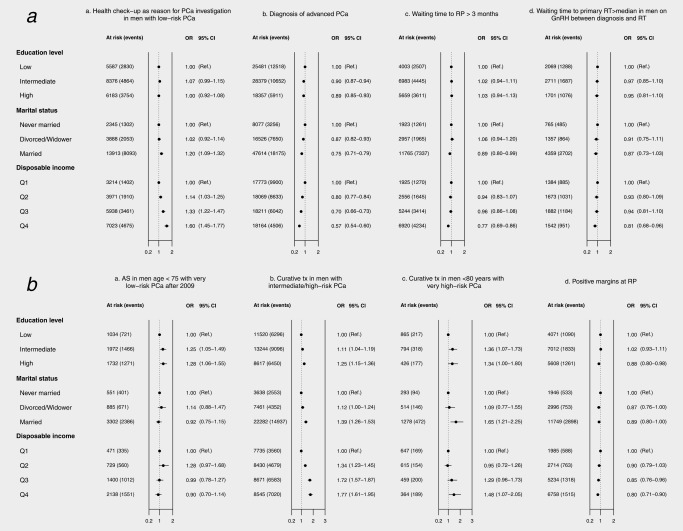
(a) Odds ratios (OR) and 95% confidence intervals (95% CI) for adherence to quality indicators in national guidelines according to educational level and income. (b) Treatment strategies and treatment execution.
Pca: prostate cancer; AS: active surveillance, curative tx: curative treatment (either radical prostatectomy or radiotherapy), GnRH: gonadotropin releasing hormone agonist, RP: radical prostatectomy, RT: radiotherapy; Educational level: low = compulsory school, <10 years; intermediate = upper secondary school, 10–12 years; high = college or university, >12 years; Quartile of disposable income: Q1 lowest – Q4 highest; Risk categories: very low‐risk (T1c, Gleason score (GS) ≤6, Prostate‐specific antigen (PSA) <10 ng/ml, PSA density < 0.15, number of biopsy cores positive for cancer ≤4, cancer extension at biopsy <8mm), low‐risk (T1‐2, PSA <10 ng/mL and GS ≤6), intermediate‐risk (T1‐2, Gleason score 7 and/or PSA 10 to <20 ng/ml), high‐risk (T3 and/or GS 8–10 and/or PSA 20 to <50ng/ml), very high risk (T4, PSA 50 to <200 ng/ml, any N stage, M0), regionally metastatic (T4 and/or N1 and/or PSA 50 to <100 ng/ml in the absence of distant metastases (M0 or Mx), and distant metastases (PSA ⋛100 ng/ml or M1); Advanced prostate cancer includes the risk categories of high‐risk, locally advanced, regionally metastatic, and distant metastases.
Men with high income were also more likely to receive curative treatment for intermediate and high‐risk Pca, OR 1.77 (1.61–1.95), more likely to receive curative treatment for very high‐risk Pca, OR 1.48 (1.07–2.05), and less likely to have positive surgical margins at prostatectomy (incomplete resection of the cancer on histopathological assessment of prostatectomy specimen), OR 0.80 (0.71–0.90).
There was a statistically significantly lower Pca mortality after 6 years of follow‐up in men with CCI 0 of all ages with highest income compared to lowest in the risk categories high‐risk and metastatic disease and in men above age 65 also among those with intermediate risk and regionally metastatic disease (Fig. 2). Similar, but smaller differences were also observed in men with CCI 1 and higher and also according to educational level (Supporting Information, Figs. 1–3). All‐cause mortality was substantially lower in men with high SES, for example, in men above age 65 with CCI 0 and low‐risk Pca, all‐cause mortality was two‐fold lower in men with highest compared to lowest income (12% vs. 30%, p < 0.001).
Figure 2.
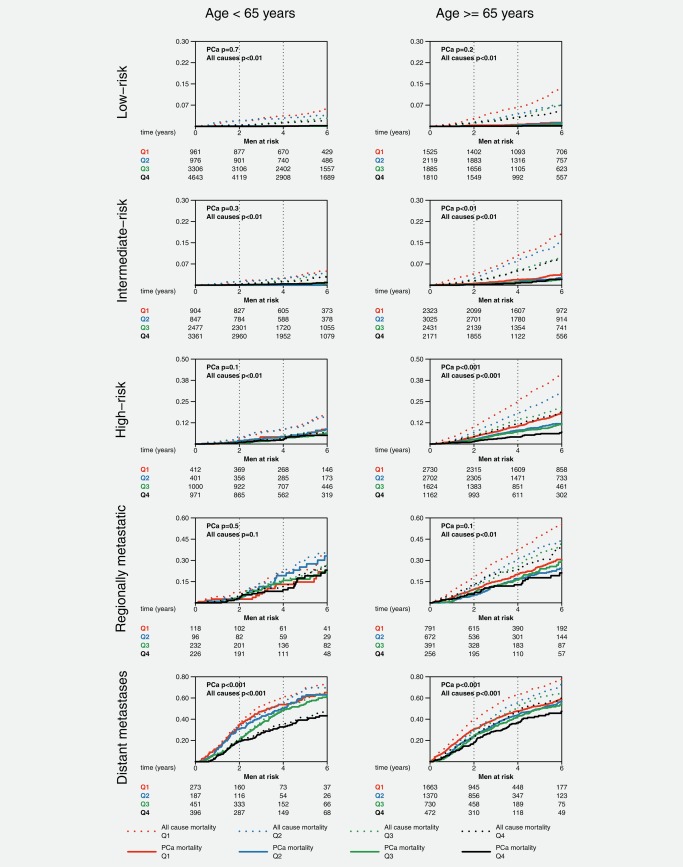
Prostate cancer and all‐cause mortality by income, age and prostate cancer risk category in men with Charlson Comorbidity Index 0.
Pca: prostate cancer; Quartile of disposable income: Q1 lowest – Q4 highest; Risk categories: very low‐risk (T1c, Gleason score (GS) ≤6, Prostate‐specific antigen (PSA) <10 ng/ml, PSA density < 0.15, number of biopsy cores positive for cancer ≤4, cancer extension at biopsy <8mm), intermediate‐risk (T1‐2, GS 7 and/or PSA 10 to <20 ng/ml), high‐risk (T3 and/or GS 8–10 and/or PSA 20 to <50ng/ml), and very high risk (T4, PSA 50 to <200 ng/ml, any N stage, M0); p from Gray's test.
Discussion
In this population‐based register study in Sweden of men diagnosed with Pca between 2007 and 2014, there were statistically significantly higher probability of low or intermediate‐risk cancer characteristics and higher diagnostic and treatment intensity in men with high compared to low SES assessed by income and educational level, with the strongest differences observed for income. There was modestly lower Pca mortality in men with high SES whereas all‐cause mortality was substantially lower in men with high SES.
Strengths of our study included the nationwide setting encompassing 75,000 men with Pca with virtually complete follow‐up.9, 11 In addition to comprehensive information on SES, Pca management and outcomes, data on several potential confounders were available, including comorbidity and marital status. In contrast to earlier studies of this topic, we had access to individual level information on SES indicators. Several limitations need mentioning. The use of income and educational level as indicators of SES do not cover all aspects of SES in relation to health. Income is affected by retirement, with less marked differences after retirement, and as a majority of men in this study were above age of retirement this likely attenuated the association between income and pattern of care and mortality. High educational level is associated with high health awareness and ability to navigate the health care system. Both income and education have been widely used in previous studies on social gradients in health care delivery and outcomes. In our study, these measures yielded similar risk estimates with somewhat stronger associations for income. In the absence of data, we were unable to address the effects of other factors such as life style, health beliefs and awareness, and health care seeking behavior. To assess comorbidity, an index based on conditions requiring hospital admissions identified in the in‐patient register was used, which likely underestimated the comorbidity burden.
Socioeconomic differences in disease management and outcomes have been reported in studies conducted in a large variety of settings.16, 17 Mechanisms contributing to social gradients in cancer management and prognosis remain incompletely understood, but have been discussed in relation to characteristics of the disease, the host and the health care system.18 Cancer characteristics include both biological features and stage at diagnosis with more advanced disease at presentation more common in disadvantaged groups often hypothesized as the main driver of socioeconomic inequalities in survival.19 In addition, results from our previous study showed differences in cancer survival according to SES also within a specific risk category.20
Host characteristics are also likely to affect outcomes. Comorbidity burden is generally higher in men with low SES. A poor general health is associated with frailty and impaired host resistance, precluding intensive treatment. Furthermore, life style factors that compromise health including smoking, physical inactivity and obesity are more prevalent in women and men with low SES. Health care‐seeking behavior and patient–clinician interactions are also factors associated with SES that influence management and outcomes. Finally, adherence to treatment and acceptance of surgical risks may differ by SES.21
Timely access to and provision of high quality cancer care is a key determinant for cancer outcomes. In our study, men with high SES, and in particular men with high income, had significantly shorter waiting times, more frequently received treatment with curative intent, and had better short‐term surgical outcomes. Furthermore, men with high SES and intermediate and high‐risk Pca were more likely to receive curative treatment as recommended by prostate cancer guidelines6, 22, 23 in line with findings in previous studies, for example, men with high SES in the UK and the US were more likely to undergo surgery.24, 25 More men with high SES and very high‐risk Pca in our study received treatment with curative intent than men with low SES, despite the absence of guidelines recommending this treatment.
One aspect that countered the benefit of the more active attitude toward Pca detection and treatment is that more men with high SES received an overdiagnosis, that is, a diagnosis of a cancer that would never have caused any symptoms even without treatment.
There were modest differences in Pca mortality according to SES after 6 years of follow‐up with a lower mortality in men with high income, with the strongest difference in men above 65 with no other comorbidities.6 Given the long disease trajectory in men with localized Pca, 6 years of follow‐up is a too short time period to assess outcome for men with low‐ and intermediate‐risk Pca. As expected and corroborating results from previous studies, all‐cause mortality was much lower in men with high compared to low SES.14, 15
Conclusions
Consistent differences according to SES in presentation, waiting times, treatment selection and execution and Pca and all‐cause mortality were found in men with Pca in Sweden, a country with a tax‐financed health care system aiming to provide equal care to all residents. While the reasons for these inequalities in cancer care according to SES remain unknown, these findings underscore the importance of adherence to guidelines to ensure optimal and equal care for all patients diagnosed with cancer.
Supporting information
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Table
CONFLICT OF INTEREST STATEMENT: The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the article.
This project was made possible by the continuous work of the National Prostate Cancer Register (NPCR) of Sweden, Pär Stattin (chairman), Anders Widmark, Camilla Thellenberg Karlsson, Ove Andrén, Ann‐Sofi Fransson, Magnus Törnblom, Stefan Carlsson, Marie Hjälm‐Eriksson, David Robinson, Mats Andén, Johan Stranne, Jonas Hugosson, Ingela Franck Lissbrant, Maria Nyberg, René Blom, Lars Egevad, Calle Waller, Eva Johansson, Fredrik Sandin and Karin Hellström.
References
- 1. Woods LM, Rachet B, Coleman MP. Origins of socio‐economic inequalities in cancer survival: a review. Ann Oncol 2006;17:5–19. doi:10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 2. Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 2009;20:417–35. doi:10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berglund A, Holmberg L, Tishelman C, et al. Social inequalities in non‐small cell lung cancer management and survival: a population‐based study in central Sweden. Thorax 2010;65:327–33. doi:10.1136/thx.2009.125914. [DOI] [PubMed] [Google Scholar]
- 4. Eaker S, Halmin M, Bellocco R, et al. Social differences in breast cancer survival in relation to patient management within a National Health Care System (Sweden). Int J Cancer 2009;124:180–7. doi:10.1002/ijc.23875. [DOI] [PubMed] [Google Scholar]
- 5. Cavalli‐Björkman N, Lambe M, Eaker S, et al. Differences according to educational level in the management and survival of colorectal cancer in Sweden. Eur J Cancer 2011;47:1398–406. doi:10.1016/j.ejca.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 6. Berglund A, Garmo H, Robinson D, et al. Differences according to socioeconomic status in the management and mortality in men with high risk prostate cancer. Eur J Cancer 2012;48:75–84. doi:10.1016/j.ejca.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 7. Larsen SB, Brasso K, Christensen J, et al. Socioeconomic position and mortality among patients with prostate cancer: influence of mediating factors. Acta Oncol (Madr) 2016;1–6. doi:10.1080/0284186X.2016.1260771. [DOI] [PubMed] [Google Scholar]
- 8. Cheng I, Witte JS, McClure LA, et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control 2009;20:1431–40. doi:10.1007/s10552-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the national prostate cancer register of sweden and prostate cancer data base Sweden 2.0. Int J Epidemiol 2013;42:956–67. doi:10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 10. NPCR . National Prostate Cancer Register of Sweden n.d. http://www.npcr.se (accessed February 1, 2017).
- 11. Tomic K, Berglund A, Robinson D, et al. Capture rate and representativity of The National Prostate Cancer Register of Sweden. Acta Oncol 2015;54:158–63. doi:10.3109/0284186X.2014.939299. [DOI] [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 13. Berglund A, Garmo H, Tishelman C, et al. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol 2011;185:833–40. doi:10.1016/j.juro.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 14. Stringhini S, Carmeli C, Jokela M, et al. Socioeconomic status and the 25 x 25 risk factors as determinants of premature mortality: a multicohort study and meta‐analysis of 1.7 million men and women. Lancet (London, England) 2017; doi:10.1016/S0140-6736(16)32380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA 2010;303:1159–66. doi:10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frederiksen BL, Osler M, Harling H, et al. Do patient characteristics, disease, or treatment explain social inequality in survival from colorectal cancer?. Soc Sci Med 2009;69:1107–15. doi:10.1016/j.socscimed.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 17. Mackenbach JP, Stirbu I, Roskam A‐JR, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med 2008;358:2468–81. doi:10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 18. Byers TE, Wolf HJ, Bauer KR, et al. The impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer 2008;113:582–91. doi:10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 19. Liu L, Cozen W, Bernstein L, et al. Changing relationship between socioeconomic status and prostate cancer incidence. J Natl Cancer Inst 2001;93:705–9. [DOI] [PubMed] [Google Scholar]
- 20. Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med 2003;163:49–56. [DOI] [PubMed] [Google Scholar]
- 21. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009;339:b4817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swedish Socioeconomic Classification. Reports on statistical coordination. Stat Sweden 1982.
- 23. Heidenreich A, Bolla M, Joniau S, et al. Guidelines on prostate cancer. Eur Urol 2008;53:68–80. doi:10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 24. Krupski TL, Kwan L, Afifi AA, et al. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol 2005;23:7881–8. doi:10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 25. Lyratzopoulos G, Barbiere JM, Greenberg DC, et al. Population based time trends and socioeconomic variation in use of radiotherapy and radical surgery for prostate cancer in a UK region: continuous survey. BMJ 2010;340:c1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Figure 2
Supporting Information Figure 3
Supporting Information Table


